13.3
Impact Factor
Theranostics 2022; 12(6):2580-2597. doi:10.7150/thno.70277 This issue Cite
Research Paper
Antibacterial PDT nanoplatform capable of releasing therapeutic gas for synergistic and enhanced treatment against deep infections
1. State Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun, 130012, China
2. Department of Oral Implantology, Jilin Provincial Key Laboratory of Sciences and Technology for Stomatology Nanoengineering, Hospital of Stomatology, Jilin University, Changchun, 130021, China
#These authors contribute equally to this work.
Abstract
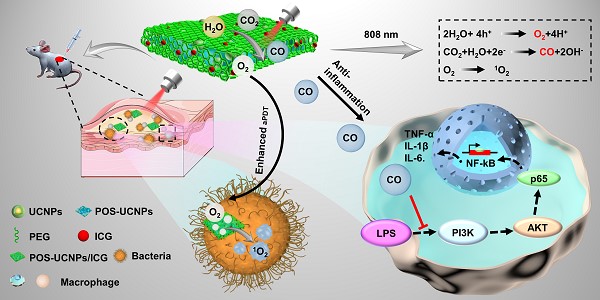
Antibacterial photodynamic therapy (aPDT) has emerged as an attractive treatment option for efficient removal of pathogenic bacteria. However, aPDT in deep tissue will encounter difficulties such as limited light penetration depth, insufficient oxygen (O2) supply and inability to eliminate inflammation introduced by bacteria, which hinders its clinical application. Herein, the near infrared (NIR) strategy of simultaneously generating O2 and CO was developed for aPDT based antibacterial therapy and mitigation of deep infection inflammation.
Methods: We prepared NIR-mediated multifunctional aPDT nanoplatform (POS-UCNPs/ICG) producing therapeutic gas of O2 and CO. The CO, O2 and ROS generation of the nanoplatform were characterized by dye probes, respectively. The antibacterial activity and anti-inflammation of POS-UCNPs/ICG were demonstrated in vitro and in vivo. In addition, the therapeutic effects in vivo were serially analyzed by immunofluorescence staining, Masson's staining, hematoxylin and eosin staining, colony formation units (CFU) and so on.
Results: NIR-mediated multifunctional aPDT nanoplatform was realized by combining the up-conversion nanoparticles (UCNPs) and partially oxidized SnS2 (POS) nanosheets (NSs) as well as indocyanine green (ICG). Using a single 808 nm light, aPDT can be achieved via ICG molecules, meanwhile, O2/CO can be generated by POS NSs through upconversion light excitation. During the aPDT process, O2 can enhance aPDT, while CO can regulate inflammation through the PI3K/NF-κB pathway. Therefore, POS-UCNPs/ICG groups had a highest percentage of healing area up to 91.55±1.26% in mouse abscess model.
Conclusion: Due to enhanced aPDT and anti-inflammatory collaborative therapy, the POS-UCNPs/ICG composites showed remarkably accelerated recovery in animal abscess models. Such NIR light responsive nanoplatform with optimized antibacterial capacity and immunomodulatory functions is promising for clinical therapeutics of bacteria-induced infections.
Keywords: antibacterial photodynamic therapy, anti-inflammation, carbon monoxide, reactive oxygen species, bacterial infections
 Global reach, higher impact
Global reach, higher impact