13.3
Impact Factor
Theranostics 2022; 12(6):2502-2518. doi:10.7150/thno.63824 This issue Cite
Research Paper
Hepatic PRMT1 ameliorates diet-induced hepatic steatosis via induction of PGC1α
1. The State Key Laboratory of Pharmaceutical Biotechnology, The University of Hong Kong, Hong Kong, China.
2. Department of Medicine, The University of Hong Kong, Hong Kong, China.
3. Joint Laboratory between Guangdong and Hong Kong on Metabolic Diseases, Guangdong Pharmaceutical University, Guangzhou, China.
4. Guangdong Research Center of Metabolic Diseases of Integrated Western and Chinese Medicine, Guangdong Pharmaceutical University, Guangzhou, China.
5. Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, China.
6. Department of Health Informatics and Technology, The Hong Kong Polytechnic University, Hong Kong, China.
7. Hong Kong Polytechnic University Shenzhen Research Institute, Shenzhen, China.
*First authors
Abstract
Rationale: Over-nutrition will lead to overexpression of PRMT1 but protein hypomethylation is observed in the liver of obese subjects. The dynamic alteration of the expression and methyltransferase activity of PRMT1 in the progression of fatty liver diseases remains elusive.
Methods: We used recombinant adeno-associated virus-mediated gene delivery system to manipulate the hepatic PRMT1 expression level in diet-induced obese mice to investigate the role of PRMT1 in hepatic steatosis. We further utilized a cohort of obese humans with biopsy-proven nonalcoholic fatty liver disease to support our observations in mouse model.
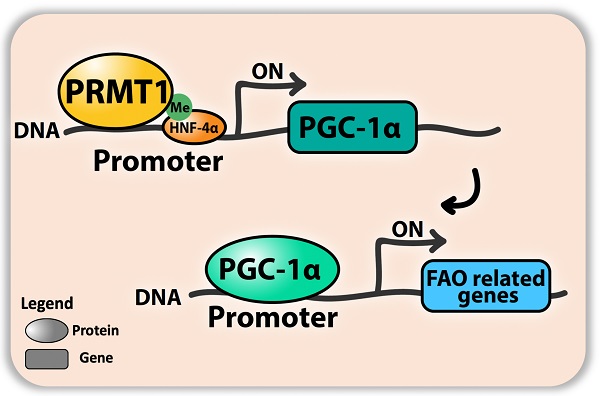
Results: We demonstrated that knockdown of PRMT1 promoted steatosis development in liver of high-fat diet (HFD) fed mice. Over-expression of wild-type PRMT1, but not methyltransferase-defective mutant PRMT1G80R, could alleviate diet-induced hepatic steatosis. The observation is conserved in the specimens of obese humans with biopsy-proven nonalcoholic fatty liver disease. Mechanistically, methyltransferase activity of PRMT1 was required to induce PGC-1α mRNA expression via recruitment of HNF-4α to the promoter of PGC-1α, and hence attenuated HFD-induced hepatic steatosis by enhancing PGC-1α-mediated fatty acid oxidation.
Conclusions: Our results identify that activation of the PRMT1/HNF-4α/PGC-1α signaling is a potential therapeutic strategy for combating non-alcoholic fatty liver disease of obese subjects.
Keywords: Non-alcoholic fatty liver disease (NAFLD), Diet-induced hepatic steatosis, PRMT1, PGC-1α, HNF-4α
 Global reach, higher impact
Global reach, higher impact