13.3
Impact Factor
Theranostics 2022; 12(5):2095-2114. doi:10.7150/thno.69465 This issue Cite
Review
Advances in aptamers against Aβ and applications in Aβ detection and regulation for Alzheimer's disease
1. Key Laboratory of Medical Molecule Science and Pharmaceutics Engineering, Ministry of Industry and Information Technology, Key Laboratory of Cluster Science of Ministry of Education, Beijing Key laboratory of Photoelectronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 100081, P. R. China.
2. Analysis & Testing Center, Beijing Institute of Technology, Beijing 100081, P. R. China.
3. State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Key Laboratory for Bio-Nanotechnology and Molecular Engineering of Hunan Province, Hunan University, Changsha 410082, P. R. China.
Received 2021-11-26; Accepted 2022-1-11; Published 2022-1-31
Abstract
Alzheimer's disease (AD) is an irreversible neurodegenerative disease, causing profound social and economic implications. Early diagnosis and treatment of AD have faced great challenges due to the slow and hidden onset. β-amyloid (Aβ) protein has been considered an important biomarker and therapeutic target for AD. Therefore, non-invasive, simple, rapid and real-time detection methods for AD biomarkers are particularly favored. With the development of Aβ aptamers, the specific recognition between aptamers and Aβ plays a significant role in AD theranostics. On the one hand, aptamers are applied to construct biosensors for Aβ detection, which provides possibilities for early diagnosis of AD. On the other hand, aptamers are used for regulating Aβ aggregation process, which provides potential strategies for AD treatment. Many excellent reviews have summarized aptamers for neurodegenerative diseases or biosensors using specific recognition probes for Aβ detection applications in AD. In this review, we highlight the crucial role of the design, classification and applications of aptamers on Aβ detection as well as inhibition of Aβ aggregation for AD.
Keywords: Alzheimer's disease, β-amyloid aggregation, Aptamer, Sensing and detection, Inhibition
Introduction
Alzheimer's disease (AD) has challenged society and public health with a huge burden, whose early diagnosis and treatment are difficult [1, 2]. Longer life expectancy has led to an increased number of AD cases in elders (>65 years old) approximately 115.4 million worldwide by the middle of this century [3]. AD accounts for more than 80% of all dementia cases globally and costs $290 billion for health care alone [4]. The symptom of AD is characterized by prominent amnestic cognitive impairment associated with a severe loss in speech express, visuospatial processing and execution [5]. Once the dementia is gradual onset and ongoing progression with obvious symptoms and signs, AD patients and their family bear economic pressure and emotional pain, ravaging their lives. Therefore, the early diagnosis and treatment of AD are particularly important. The pathological characteristic of AD is the accumulation of amyloid plaques and the formation of fibril entanglement by highly phosphorylated microtubule-related Tau protein filaments [6]. The main component of amyloid plaque is 39-43 amino acid β-amyloid (Aβ) peptide [7, 8]. According to the 'Aβ cascade hypothesis', the cytotoxic substances formed by the aggregation of misfolded Aβ are pathologically related to AD [9, 10]. The Aβ aggregation process is a spontaneous process in which Aβ monomers transforms from initial into oligomers, then assemble to form pro-fibrils, and finally aggregate into mature fibrils under certain conditions [11]. Genetic evidence, biochemical and histopathological observations all indicate that Aβ accumulation plays a key role in the pathogenesis of AD. Therefore, Aβ monomers and their aggregates have been widely regarded as the main biomarkers and therapeutic targets of AD [12, 13].
The diagnosis of AD mainly relies on clinical symptoms and traditional imageology examinations [14, 15]. However, due to its slow onset and insidiousness, the appearance of symptoms often lags behind the course of the disease [15]. The development of non-invasive, real-time, rapid and reliable strategies for AD is of great urgence for early diagnosis and treatment of AD [17]. Direct detection of Aβ levels and changes provides the most reliable and direct information for AD diagnosis [18, 19]. In recent years, biosensors have played an important role in the detection of Aβ monomers and aggregates. Researchers have constructed electrochemical sensors [20, 21], surface plasmon resonance (SPR) sensors [22], surface enhanced Raman (SERS) sensors [23, 24], colorimetric sensors [25, 26], etc. for the quantification of Aβ. The aptamer generally includes single-stranded nucleic acid aptamers obtained by systematic evolution of ligands by exponential enrichment (SELEX) and peptide aptamers, which has been widely used in biosensing [27-29], drug delivery [30], imaging, diagnosis and treatment of diseases [31, 32]. Since aptamers against Aβ were screened out, the specific interaction between aptamers and Aβ has attracted great attention in the area of Aβ detection. Benefit from the advantages of high specificity, easy to design, high cost-effectiveness and stability, aptamers have promoted the development and progress of Aβ sensing, which is significant for the early diagnosis of AD.
Meanwhile, researchers have also made many efforts to develop amyloid aggregation inhibitors in order to promote the therapy of AD. They have discovered kinds of Aβ aggregation-regulated molecules and nanomaterials by exploring the interaction between amyloid and antibodies [33-38], peptides [39-44], small molecules [45-51], aptamers [52-56], polymers [57-59], as well as nanomaterials [60-63]. Most of these inhibitors can regulate the aggregation process of Aβ in vitro or in vivo and reduce the cytotoxicity caused by Aβ aggregates to a certain extent, providing a valuable treatment strategy for delaying AD progression. Compared to other inhibitors, aptamers were considered as promising inhibitors for Aβ aggregation due to their features of easier modification, more cost-effectiveness, higher reproducibility, better biocompatibility, stability and specificity to Aβ [64, 65].
Recently, aptamers' application in the field of Aβ biosensing and Aβ aggregation has attracted increasing attention. The several previous reviews summarized aptamers for neurodegenerative diseases [66, 67] or biosensing strategies based on specific recognition probes (antibodies, peptides, aptamers and small molecules) for Aβ detection [68-73]. However, the latest advance of aptamers and their functions in Aβ aggregation were not reported systematically. Herein, we summarize and highlight the screening aptamers against Aβ, aptamers' applications in the Aβ detection and inhibition of Aβ aggregation, as well as their valuable prospects in AD diagnosis and treatment (Figure 1).
Representation of the main characteristics of aptamers in this review.
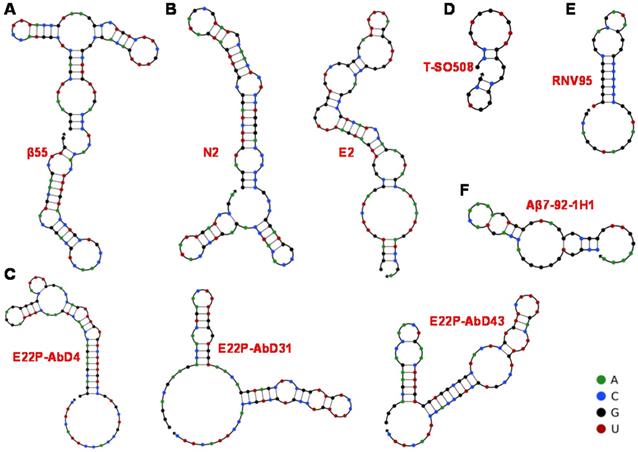
Predicted secondary structure of obtained aptamers by UNPACK analysis (http://www.nupack.org). The bases A, C, G and U are simply represented by green, blue, black and red, respectively. (A) The secondary structure prediction of β55 [74]. (B) The secondary structure prediction of N2 and E2 [53]. (C) The secondary structure of three RNA aptamers screened by Murakami et al [76]. (D) The secondary structure prediction of DNA aptamer T-SO508 [54]. (E) Computational prediction of the stem-loop structure of RNV95 [55]. (F) Predicted secondary structure DNA aptamer of Aβ7-92-1H1 [77].
The reported aptamers against Aβ
| Name | Classification | Target | Binding Affinity (Kd) | Ref. |
|---|---|---|---|---|
| β55 | RNA | Aβ40 fibril | 29 nM | [74] |
| N2, E2 | RNA | Aβ40 monomer | 21.6 µM; 10.9 µM | [53] |
| KM33, KM41 | RNA | Aβ40 fibril | - | [75] |
| E22P-AbD43 | RNA | Aβ42 dimer | 20 ± 6.0 nM | [76] |
| T-SO508 | DNA | Aβ40 oligomer | 25 nM | [54] |
| RNV95 | DNA | Aβ40 oligomer | 50-400 nM | [55] |
| Aβ-79-1H1 | DNA | Aβ42 monomer | 63.4 nM | [77] |
| c-abp2, n-abp4 | Peptide | Aβ42 oligomer | 35.80 ± 18.22 pM; 217.97 ± 27.01 pM | [78] |
Aptamers against Aβ
Since Aβ is the main biomarker of AD, aptamers against Aβ will be helpful to the early diagnosis and treatment of AD. At present, the obtained aptamers include several kinds of RNA aptamers, DNA aptamers and peptide aptamers, which are listed in Table 1. The most abundantly produced species Aβ40, and the more aggregation prone and toxic form Aβ42 are a simplification as the γ-secretase generates peptides from Aβ1-36 to Aβ1-43 [79]. Although the only difference between Aβ40 and Aβ42 is two extra residues at the C-terminus of Aβ42, they differ in the structure and property of aggregates, aggregation process and biological toxicity [80]. Therefore, the design, screening process, and target identification of Aβ40-specific aptamers and Aβ42-specific aptamers are discriminated. The applications of these aptamers mainly focus on Aβ detection and inhibition of Aβ aggregation. Moreover, most results of Aβ research field show aptamers with higher binding affinity may be contributed to lower detection limit and higher aggregation inhibition efficiency.
RNA aptamers against Aβ
The aptamers prepared through chemical synthesis are comparable to those of antibodies in the affinities and specificities, which are also allowed modifications and necessarily avoids biological contaminations. The first aptamer against Aβ was RNA aptamer. Ylera et al. used Aβ40 monomer as the target to screen for RNA aptamers through the affinity column method in 2002 [74]. A cysteine was first connected to the N-terminus of Aβ40. Then Aβ40 was covalently cross-linked to the beads through formed disulfide bonds on the sulfhydryl functionalized agarose beads. 18 RNA aptamers were obtained after eight rounds of screening. The aptamer β55 with the smallest Kd value (Kd = 29 nM) containing 107 bases was selected. The predicted secondary structure of β55 is shown in Figure 2A, which contains multiple stem-loop structures. 5-nm-diameter gold nanoparticles (AuNPs) were used to label β55 and incubated with Aβ40 in order to further explore the performance of β55. It was found that when β55 was incubated with Aβ40, β55 labeled with AuNPs was distributed along Aβ40 fibril rather than in the substrate. The morphology results showed that the RNA aptamer β55 did not bind to the Aβ40 monomer while bound to the Aβ40 fibril rich in β-sheet.
Furthermore, Takahashi et al. improved the screening procedure of aptamers against Aβ, where Aβ40 monomer conjugated with AuNP (Aβ-AuNP) as an oligomer model of Aβ was as the selection target [53]. After nine selection cycles, the obtained RNA aptamers N2 and E2 bound to both Aβ-AuNP and free Aβ monomer. The prediction results of their secondary structure are shown in Figure 2B. Fluorescence polarization method confirmed that the Kd values of N2 and E2 were 21.6 μM and 10.9 μM towards Aβ40, respectively. Benefit from the recognition of aptamers with Aβ monomer, N2 and E2 may be applied to Aβ detection and regulation of Aβ aggregation.
The obtained aptamers above were initially carried out using Aβ monomers with almost a random coil as targets. Numerous studies have indicated that the cytotoxicity of Aβ oligomer is higher than Aβ monomer and Aβ fibril. With the in-depth understanding of Aβ aggregation process and cytotoxicity of Aβ species, the interaction between Aβ oligomers with β-sheet structure and recognition molecules has attracted more and more attention. Besides, for the inhibition of Aβ aggregation, aptamers should bind to the monomeric and oligomeric forms of Aβ. Therefore, it is necessary to obtain aptamers targeting to Aβ oligomers. Rahimi et al. obtained RNA aptamers (KM33 and KM41) using Aβ40 oligomer as the target by nitrocellulose membrane filtration method [75]. However, these RNA aptamers did not recognize Aβ40 oligomer but bound to Aβ40 fibril, Aβ42 fibril and other types of amyloid fibrils (such as lysozyme amyloid fibril, islet amyloid fibril, etc.). To further determine the recognition performance of the RNA aptamer, they also used stable Aβ40 oligomers generated by the photochemical cross-linking method as targets to repeat the above-mentioned screening process [81]. The results also proved that KM33 and KM41 recognized amyloid fibrils instead of Aβ40 oligomers. The aptamer against Aβ40 oligomer was not obtained. However, researchers have made progress in the recognition of aptamers and Aβ species (including monomers, oligomers and fibrils) after unremitting efforts.
As a meaningful target for AD, Aβ42 is more prone to aggregation and deposition because of its stronger ability to assemble into toxic species compared to that of Aβ40 [82, 83]. Moreover, there is a significant inverse linear association between amyloid plaques and Aβ42 levels in AD patients. The RNA aptamers obtained above are all targeting Aβ40. Thanks to the advance of screening technology, aptamers targeting Aβ42 have also been screened out. A series of RNA aptamers (E22P-AbD4, E22P-AbD31 and E22P-AbD43) that recognize Aβ42 protofibril were obtained based on the E22P-Aβ42 dimer model (Figure 2C). Compared with fibrils formed by Aβ42 monomer or variant E22P-Aβ42, these RNA aptamers had a higher affinity for Aβ42 protofibril (Kd = 150 ± 11 nM). At the same time, the preferential binding of E22P-AbD43 to the protofibril was due to its higher affinity for the Aβ42 dimer (Kd = 20 ± 6.0 nM) than the less toxic Aβ40 aggregate, which may be related to the formation of the G-quadruplex structure [76]. As recognition molecules, RNA aptamers have already achieved the quantification of Aβ40/Aβ42 monomers and aggregates, possibly the inhibition of Aβ aggregation. However, specificity and inhibition efficiency still need to be improved because of the lower binding affinity between RNA aptamer and Aβ. Moreover, most RNA aptamers with longer sequences are unstable and susceptible to contamination, which may limit their applications in the complex biological systems.
DNA aptamers against Aβ
With the continuous progress and development of screening technology, more excellent Aβ-specific aptamers have been screened and widely applied. Besides, researchers have set their sights on DNA aptamers with lower chemical synthesis costs and more stable properties in order to enhance aptamer's specificity and applicability in the complex system. In 2012, Tsukakoshi et al. obtained 8 DNA aptamers bound to α-synuclein oligomers based on a competitive screening method. Surprisingly, these aptamers can also bind to Aβ40 oligomers (Figure 2D) [54].The Kd value (25 nM) of aptamer T-SO508 and Aβ40 oligomers was slightly lower than that of α-synuclein oligomers (68 nM). By comparing with the result of imprinting hybridization of the oligomer-specific antibody A11, it was speculated that the aptamer could recognize β-sheet structure of soluble amyloid oligomer. Presently, aptamer T-SO508 has been the most commonly used recognition molecule in Aβ40 oligomers detection. These researches also show that oligomer-binding aptamers may serve as powerful analytical tools for the development of drugs and diagnostic tests for neurodegenerative diseases.
It is difficult to discriminate Aβ monomer and oligomer due to Aβ monomer's high tendency to form Aβ aggregates in solution, along with the structural relevance of Aβ monomer and Aβ oligomer (especially low-molecular-weight oligomers). Therefore, the development of specific recognition molecules for low-molecular-weight oligomers seems more significant. The DNA aptamer RNV95 targeting Aβ40 low-molecular-weight oligomers were screened using magnetic bead-assisted SELEX (Figure 2E) by Chakravarthy et al. [55]. The structure prediction of RNV95 showed that it was a stable stem-loop structure (39-base). Moreover, the detection of tetrameric/pentameric low molecular weight Aβ aggregates in the hippocampal tissue of autopsy was conducted for investigating the recognition performance of RNV95 in the complex biological systems. The results showed that RNV95 could be applied to a wide range of affinity assays to detect Aβ oligomers.
Given the important role of Aβ42 in AD, for the first time, our group obtained three Aβ42 monomer-specific DNA aptamers based on magnetic bead-assisted in vitro screening [77]. Moreover, DNA aptamer Aβ7-92-1H1 with a length of 44 bases was obtained through further sequence optimization. The prediction result of its secondary structure in Figure 2F showed that DNA aptamer Aβ7-92-1H1 possessed the stem-loop structure. Besides, the Kd value of the aptamer binding to the Aβ42 monomer is 63.4 nM, which also showed high specificity of this aptamer for Aβ42 monomer. In our further investigation, Aβ7-92-1H1 could not bind to Aβ40 monomer, but recognize Aβ42 and Aβ40 aggregates (oligomer and fibril) with various affinities. In other words, the selectivity of Aβ7-92-1H1 for Aβ40 monomer need to be improved. But it is the kind of universal recognition ability with Aβ monomer and aggregates that is very suitable for the development of inhibitors for regulating Aβ aggregation. DNA aptamers T-SO508 and RNV95 are specific for Aβ oligomer, which probably suggest that the G-quadruplex form of DNA aptamer tends to preferentially bind to the β-structures of Aβ oligomer. They are more suitable for the construction of Aβ sensing platform. It is also difficult for them to distinguish between Aβ40 oligomer and Aβ42 oligomer. Most the obtained DNA aptamers with a shorter base sequence facilitate synthesis and design than RNA aptamers. Moreover, the higher affinity between DNA aptamer and Aβ may be beneficial to improve detection sensitivity and inhibition efficiency for further applications in clinical diagnosis and treatment of AD.
Peptide aptamers against Aβ
Peptides are small-molecule ligands with convenient synthesis and desirable biological properties such as good cell penetrability, low immunogenicity, high biocompatibility [84]. With the advance of high affinity and specificity screening and integrated novel nanotechnology for peptide screening and identification, peptide aptamers open a new avenue for rapid discovery of new peptide-based reagents for disease diagnostics and therapeutics [85, 86]. Recently, the peptide aptamers were developed as antibody alternatives through phage display [78]. Subsequently, the two peptide aptamers (c-abp2 and n-abp4) were confirmed to have excellent binding affinity to Aβ aggregates with high specificity by immunohistochemical staining and western blot using the brains of transgenic AD mice. This present study confirmed that newly developed amyloid-binding peptides could be used as novel probes for the detection of Aβ aggregates, which was helpful to the clinical diagnosis of AD in the future.
Although several aptamers against Aβ have been developed, the targets are still relatively single, mostly targeting Aβ oligomers, but not targeting all heterogeneous populations of Aβ. The specificity of aptamers (β55, KM33 and KM41) screened against Aβ monomers or oligomers did not meet expectations, which have stronger recognition for Aβ fibrils. It may be caused by easy aggregation characteristics and instability of Aβ in the solution. Therefore, the new and actual screening methods that can obtain more specific aptamers should be developed and emphasized vigorously, such as screening in vivo. In addition, DNA origami technology capable of precise programming can be considered to design of aptamers for improving the specificity of aptamers. Furthermore, the interaction mechanism of aptamers and many targets has been explored very clearly, such as thrombin and its nucleic acid aptamers [87, 88]. But the interaction mechanism between aptamers and Aβ has not been investigated clearly, limiting the further advance of aptamers. In our previous work, we employed single-molecule force spectroscopy technology to initially investigate interactions of the aptamer with Aβ40 monomer, Aβ40 oligomer and Aβ40 fibril before and after respective application of electric field to Aβ40 samples [89]. The basic investigation of interaction mechanism between aptamers and Aβ also showed that aptamer could be used as an effective tool to clearly characterize the effects of electric field on Aβ40 monomer and Aβ40 aggregates at the single-molecule level. Therefore, the analysis of the interaction mechanism of aptamers and Aβ will be beneficial to aptamers' applications in AD theranostics.
The applications of aptamer in Aβ detection
Biosensors have attracted much attention in Aβ detection with the advantages of simplicity, strong specificity and high sensitivity [90, 91]. Compared with antibodies, aptamers possess the characteristics of flexible design and satisfactory stability as recognition probes of Aβ detection. Most obtained aptamers against Aβ were successfully used for detecting Aβ monomer or aggregates. The constructed sensors based on aptamers include electrochemical sensors, fluorescence sensors and other optical sensors. Some biosensors have been used in complex biological systems for the quantification of Aβ aggregates, which are increasingly important and useful for the early diagnosis of AD.
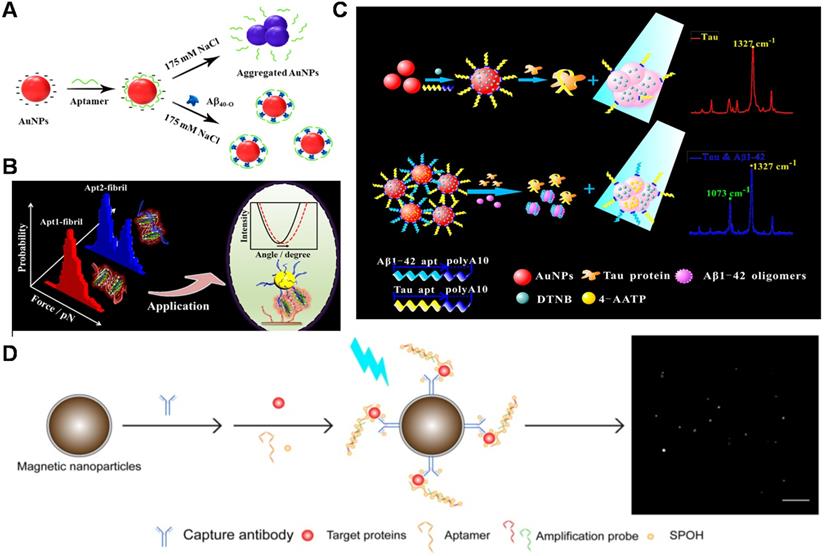
Electrochemical sensors
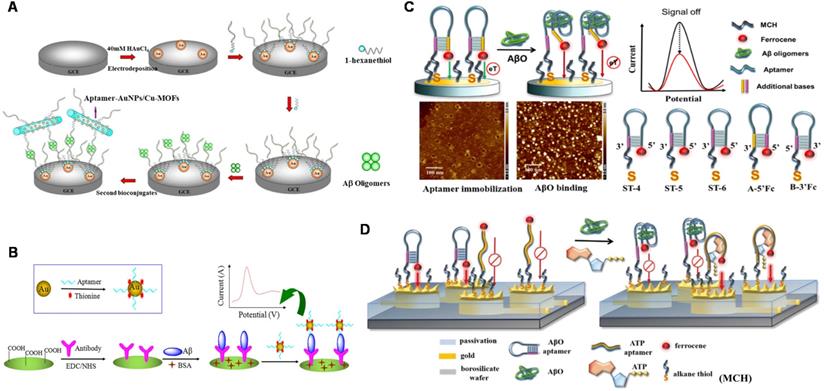
Electrical detection has beneficial features such as low-cost instrumentation, ease of miniaturization, and superior sensitivity (down to femtomolar levels) in the label-free detection method, which have provoked extensive research on electrical sensing of AD biomarkers [92, 93]. At present, a number of electrochemical Aβ sensors have been developed for detecting Aβ species, interactions with recognition molecules, and its aggregation kinetics in relevant fluids including cerebrospinal fluid (CSF), serum and plasma [94-95]. A facile electrochemical aptamer-based sensor (aptasensor) for Aβ oligomers detection based on aptamer-tagged gold nanoparticles/Cu-metal-organic framework (AuNPs/Cu-MOFs) as signal probes and aptamer-tethered gold nanoflowers (AuNFs) as capture probes (Figure 3A). The designed aptasensor exhibits wide linear range from 1 nM to 2 µM, with a low detection limit (LOD) of 450 pM [96]. The evaluation of Aβ oligomers in artificial CSF provided valuable information for the early diagnosis of AD. Deng et al. developed an electrochemical aptasensor for detecting Aβ40 oligomer to overcome the antibody-based sensor's shortcomings [97]. LOD of 93 pM was achieved. The electrochemical aptasensor could work in artificial CSF and human serum, which meets the demands of clinic determination of Aβ40 oligomer. Besides, the antibody-aptamer sandwich method was developed for detecting Aβ oligomers (Aβ40 oligomer and Aβ42 oligomer) with LOD of 100 pM (Figure 3B). This method also realized the detection of Aβ oligomers in artificial CSF, which provided important information for AD diagnosis [98]. Compared to the mentioned above electrochemical aptasensor, this sensor based on antibody-aptamer sandwich could be used to monitor Aβ40 aggregation. A new electrochemical biosensor was constructed for the quantification of Aβ42 oligomers based on the sandwich method of molecularly imprinted polymers and DNA aptamers. The biosensor obtained the improved detection sensitivity (LOD: ~0.27 pM) [99]. In present, Liao et al. developed a novel dual-amplification strategy based on exonuclease III-assisted DNA recycling and rolling circle amplification (RCA) for more sensitive detection of Aβ oligomer [100]. This method quantified Aβ40 oligomer down to 39 fM with a dynamic range from 0.1 pM to 10 nM.
The schematic diagram of the electrochemical sensor used to detect Aβ40 and Aβ42 oligomers. (A) Electrochemical aptasensor for Aβ oligomers detection based on AuNPs/Cu-MOFs and AuNFs. Adapted with permission from Ref. [96], copyright 2018 Royal Society of Chemistry. (B) Detection of Aβ40 and Aβ42 oligomers based on antibody-nucleic acid aptamer sandwich assay method. Adapted with permission from Ref. [98], copyright 2016 Spring Nature. (C) Detection of Aβ40 oligomers based on a hairpin structure. Adapted with permission from Ref. [109], copyright 2019 American Chemical Society. (D) A multi-electrode array chip modified based on 3D nanostructures used for the simultaneous detection of ATP and Aβ40 oligomers. Adapted with permission from Ref. [112], copyright 2020 Royal Society of Chemistry.
Electrochemiluminescent (ECL) assay has gradually drawn increasing interest in biomedical analysis owing to the perfect coalition of unique advantages for both electrochemical methods and chemiluminescent spectroscopy in recent years [101, 102]. Compared to traditional electrochemical sensors, ECL sensors generally own higher sensitivity and a wider detection range. In recent years, with the development of new nanotechnology, nanomaterials have been widely used to design various biosensors to achieve clinical diagnosis [103]. Combining these materials with highly specific aptamers enhances the selectivity, sensitivity and response characteristics [104]. Yin et al. developed an ECL aptasensor with signal enhancement by AuNP/MOF nanocomposite to effectively and conveniently detect Aβ42 oligomer for earlier diagnosis of AD [105]. The disposable biosensor with high performance, facile operation and low cost could quantify Aβ oligomer concentration ranging from 0.1 pM to 10 pM. A switchable electrochemical aptasensor based on triple helix switch coupling with AuNPs@CuMOF labeled signaling displaced-probe was proposed for ultra-sensitive quantification of Aβ42 oligomer [106]. The aptasensor exhibited excellent selectivity and sensitivity with the linear range from 0.5 fM to 500 fM and LOD of 0.25 fM. In order to develop a simple, cost-effective and sensitive biosensing strategy for advance the applications in AD diagnosis, Qin et al. presented a simple, label-free and signal-on ECL aptasensor for the detection of Aβ16 using luminol ECL enhancing mechanism based on in situ generation of reactive oxygen species (ROS) accelerated by Cu2+-Aβ complexes [107]. This signal-on ECL aptasensor has exhibited favorable analytical performance for Aβ16 monomer with LOD of 35 fM. Besides, more sensitive detection of Aβ peptide with a wider linear range of 10 fM-0.1 μM was obtained by hybridizing two-dimensional graphite-like carbon nitride (g-C3N4) with heme [108]. The proposed label-free ECL biosensor with prominent specificity, reproducibility and stability could be used to assess recovery of Aβ40 monomer in human serum, which offered a useful analytical method for quantifying Aβ peptides in a biological matrix.
Mayer's group realized ultra-low concentration detection of Aβ40 oligomers based on hairpin-based DNA aptamer probes [109]. As shown in Figure 3C, the sensitive quantitative analysis of Aβ40 oligomers was achieved (LOD: 2 fM) by adjusting the peak current of ferrocene by optimizing the length of the probe stem and the ferrocene end, which may help to promote the diagnosis and pathology of AD. For detecting Aβ40 oligomer in a wider linear range, the magnetic aptasensor based on electrochemiluminescence resonance energy transfer (ECL-RET) was fabricated based on the quenching effect from RET between Ru(bpy)32+ and gold nanorods (GNRs) acting as ECL-RET electron donor and acceptor, respectively [110]. A wide linear range from 1.0 × 10-5 to 100 ng/mL with LOD of ~0.9 fM was determined. Furthermore, this methodology with distinctive and desirable properties was applicable to analyze the Aβ content in real CSF samples with satisfactory results. Furthermore, a ratiometric ECL-RET aptasensor based on g-C3N4 and Ru@MOFs as energy donor-receptor pairs was also designed for Aβ40 oligomer detection, which achieved lower sensitivity in a wider linear range (1.0 × 10-5 - 500 ng/mL) [111].
According to the comparison among these developed electrochemical sensing platforms listed in Table 2, we can find that the sensitive detection of Aβ monomer or oligomer at the picomolar level was obtained. The widest linear range was across 7 orders of magnitude. Meanwhile, most biosensors have been used in complex biological systems for Aβ detection, which offer useful information and more possibility for the early diagnosis of AD. Considering that AD is usually related to mitochondrial dysfunction, mitochondrial dysfunction is closely related to decreased adenosine triphosphate (ATP) levels. Therefore, it is meaningful to detect ATP and Aβ40 oligomers simultaneously. A dual-aptamer functionalized 3D nanostructure equipped with a multi-electrode array chip was designed to simultaneously quantify ATP and Aβ40 oligomers (Figure 3D). This dual-AD-biomarkers detecting biosensor provided a potential tool for accurate AD diagnosis strategies [112].
Compared to the sensing platform based-Aβ antibody [113-115], the sensing method based-Aβ aptamer mostly has the advantages of lower cost, more flexible design, more convenient operation and more extensive scenes. Besides, with the help of distinguishing features of aptamers and a variety of nanomaterials with excellent properties, the newly developed electrochemical biosensors possess the advantages of high sensitivity and high selectivity, a wide linear range, convenient observation, good reproducibility as well as simple operation. For instance, silicon nanowire-based field-effect transistors are widely considered promising candidates for transducing electrochemical signals caused by various charged bio-objects due to their remarkable electrical and mechanical properties [116]. Recently, field-effect transistor sensors have been applied for portable Aβ detection with sub-femtomolar sensitivity and wider linear range at least three orders of magnitude [117, 118]. A micron-scale organic electrochemical transistor (OECT) integrated with a microfluidic platform was developed for the label-free detection of Aβ aggregates over eight orders of magnitude wide concentration range (from 2.21 fM to 221 nM) in 1 μL of human serum samples [119]. The electrochemical biosensing platform not only promotes rapid development of AD biomarkers detection but also lays the foundation for diagnosis and treatment of AD.
Fluorescence sensors
Fluorescence sensors have attracted much attention because of the advantages of high sensitivity and fast response speed in Aβ detection and analysis [120, 121], becoming the second mainstream method of Aβ quantitative analysis except for electrochemical sensors. Moreover, aptamers are easy for chemical synthesis, modification and design. Therefore, the fluorescence sensing platform developed by combining aptamers and fluorescent molecules provides an effective detection tool, which strongly promotes the advance of Aβ detection. A fluorescence method based on DNA aptamers-functionalized Fe3O4 and upconversion nanoparticles (UCNPs) was developed to detect Aβ40 and Aβ42 oligomers. Aptamer bound to Aβ oligomer with remarkable affinity and specificity. With the help of the magnetic separation, concentration effects of magnetic nanoparticles and high responsiveness of UCNPs, simple and sensitive detection of Aβ oligomers (LOD: 36 pM) could be achieved. It also can be applied for Aβ40 and Aβ42 oligomers detection in artificial CSF [122]. In view of the fact that dopamine nanomaterials inhibited the aggregation process of Aβ [123], Liu et al. first prepared bifunctional polydopamine nanospheres and then constructed a 'signal on' detection platform based on aptamers modified with fluorescent groups [124]. The platform could sensitively and specifically detect Aβ40 oligomers (LOD: 12.5 nM). Although the sensitivity of this sensing platform was lower, it could quantify Aβ40 oligomers in a wider detection range and also inhibit the Aβ40 aggregation process. More importantly, the bifunctional polydopamine nanospheres used as quenchers and inhibitors are expected to be applied in the diagnosis and treatment of AD.
Recently, a novel and simple 'off-to-on' fluorescence sensing platform for Aβ42 oligomer was developed based on molybdenum disulfide nanosheets (MoS2 NSs) adsorbed by FAM dye-labeled DNA aptamers (Figure 4A) [125]. A linear range from 0.01 to 20 μM and LOD of 3.1 nM could be achieved. The platform also quantified Aβ42 oligomers in the hippocampus and cortex of the transgenic AD mice. In addition, it was found that MoS2 NSs possessed therapeutic effects on inhibiting the aggregation of Aβ42 and degrading the pre-formed Aβ42 fibrils. Therefore, the strategy based on MoS2 NSs and DNA aptamers with the features of high sensitivity, specificity, good biocompatibility and effective anti-aggregation ability provided broad prospects in AD-related research. With the extensive and in-depth application of various sensing technologies in Aβ detection, a DNase-driven three-dimensional DNA Walker nanoprobe with the advantages of great sensitivity, high specificity, and convenience was designed for highly sensitive quantification of Aβ40 oligomers (LOD: 22.3 pM) [126]. As shown in Figure 4B, the presence of Aβ40 oligomers triggered the DNAzyme walking strand to cleave the fluorophore -labeled substrate strand modified on the AuNPs surface and release fluorophore -labeled DNA fragment, causing the recovery of fluorescent signal. The whole process took place autonomously and continuously, which generated a large amount of fluorophore fluorescence, achieving a signal enhancement effect. This enzyme-free signal amplification strategy not only accurately located and visualized the distribution of Aβ40 oligomers in living cells but also realized real-time imaging of Aβ oligomers in AD mice and effectively distinguished the AD mice from the wild-type mice. A quadrivalent cruciform DNA nanostructure-mediated cascaded catalyzed hairpin assembly (CHA) amplifier was designed for detecting Aβ40 oligomer (LOD: 0.69 pM) (Figure 4C). Benefit from the improved biological stability, reaction kinetics, and hybridization efficiency, the proposed strategy performed well in real samples [127].
In addition to directly detection of Aβ monomers and their aggregates as recognition elements, aptamers are also used as imaging reagents to target Aβ monomers and aggregates to obtain imaging of amyloid plaques in vivo. Besides, fluorescence imaging is considered as the most reliable diagnostic tool to distinguish AD patients from normal controls by in vivo detecting Aβ plaques in human brains for monitoring the neurodegenerative progress of AD. Farrar et al. demonstrated for the first time that aptamer probe β55 could image amyloid plaques in isolated AD brain tissue and in vivo transgenic mice [128]. As shown in Figure 4D, β55 staining of amyloid plaques was observed in the isolated AD brain tissue slices, while only very weak plaques were observed for the control aptamer β55rc. Meanwhile, the co-localization of β55-positive plaques and thioflavin-S stained plaques was also realized. More interestingly, it was also found that the oligomeric structure around the dense core of amyloid plaques was visualized through the binding of β55 and Aβ oligomers. It is noted that many small molecular fluorescence probes have also been designed and developed for selective detection and imaging of Aβ aggregates including oligomers and fibrils/plaques both in vitro and in vivo. For example, DANIR 8c possessed good binding affinity to Aβ aggregates and good brain pharmacokinetic property, which can be applied for in vivo NIR imaging of Aβ plaque [129]. Besides, these probes also exhibit additional therapeutic functions such as inhibitory effect on Aβ aggregation and potent neuroprotective effect. Compared to the fluorescence sensors based on aptamers, these probes with favorable Aβ-imaging properties present more potential as a blood-brain barrier (BBB)-permeable and Aβ-targeting vehicle to carry an effective imaging agent or a drug into the human brain [130], although the specificity and sensitivity need to be improved. The possibility of being degraded by nucleases in vivo and the weaker capability of BBB penetration limit aptamer's applications in AD theranostics. But the characteristics of aptamers with easy to synthesis, design and modification allow to be coupled with fluorescent probes. Therefore, the combination provides a promising strategy for targeting Aβ high specifically, visualizing amyloid aggregates and reducing amyloid plaques in animal models.
The reported biosensors based on aptamers for Aβ detection
| Detection mechanism | Biomarkers/Functions | Samples tested | Detection range | LOD | Ref. |
|---|---|---|---|---|---|
| Electrochemical aptasensor | Aβ40/42 oligomers | Artificial CSF | 1 nM-2 µM | 450 pM | [96] |
| Electrochemical aptasensor | Aβ40 oligomers | Artificial CSF, serum | 0.1 nM-1 μM | 93 pM | [97] |
| Antibody-aptamer sandwich method | Aβ40/42 oligomers/ monitoring aggregation | Artificial CSF | 0.5-30 nM | 100 pM | [98] |
| Molecularly imprinted polymers-aptamer sandwich method | Aβ42 oligomers | Serum | 5 pg/mL-10 ng/mL | 1.22 pg/mL | [99] |
| Electrochemical aptasensor based on dual amplification | Aβ40 oligomers | Artificial CSF, serum | 0.1 pM to 10 nM | 39 fM | [100] |
| ECL aptasensor enhanced by AuNP/MOF nanocomposite | Aβ42 oligomers | Serum | 0.1 pM-10 pM | 71 fM | [105] |
| Switchable electrochemical aptasensor based on triple helix switch | Aβ42 oligomers | Artificial CSF | 0.5 fM-500 fM | 0.25 fM | [106] |
| Signal-on ECL aptasensor based on ROS generation | Aβ16 monomers | Serum | 0.1 pM to 10 nM | 35 fM | [107] |
| ECL biosensor based on g-C3N4-heme composite | Aβ40 monomers | Serum | 10 fM-0.1 μM | 3.25 fM | [108] |
| Hairpin-based DNA aptamer sensor | Aβ40 oligomers | Artificial CSF | 0.1-10 pM | 2 fM | [109] |
| Magnetic aptasensor based on ECL-RET | Aβ40 oligomers | Real CSF | 10 fg/mL-100 ng/mL | 4.2 fg/mL | [110] |
| Ratiometric ECL-RET aptasensor based on g-C3N4 and Ru@MOFs | Aβ40 oligomers | Serum | 10 fg/mL-500 ng/mL | 3.9 fg/mL | [111] |
| Dual-aptamer functionalized 3D nanostructure with a multi-electrode array chip | ATP and Aβ40 oligomers | Artificial CSF | 1 pM-0.2 μM (Aβ); 0.01 nM-1 μM (ATP) | 2 pM for ATP; 0.3 pM for Aβ | [112] |
| Micron-scale organic electrochemical transistor | Aβ aggregates | Serum | 2.21 fM-221 nM | 2.21 fM | [119] |
| Fluorescence sensor based on aptamers-functionalized Fe3O4 and UCNPs | Aβ40/42 oligomers | Artificial CSF | 0.2-15 nM | 36 pM | [122] |
| Signal on fluorescence sensor based on bifunctional polydopamine nanospheres | Aβ40 oligomers/ inhibiting aggregation | - | 20 nM-10 μM | 12.5 nM | [124] |
| Off-to-on fluorescence sensor based on MoS2 NSs and dye-labeled DNA aptamers | Aβ42 oligomers/ inhibiting aggregation, degrading fibrils | Hippocampus, cortex of transgenic AD mice | 0.01-20 μM | 3.1 nM | [125] |
| DNase-driven three-dimensional DNA Walker nanoprobe | Aβ40 oligomers | Living cells, AD mice | 0.1-1.0 nM | 22.3 pM | [126] |
| Fluorescence sensor based on “DD-A” FRET and CHA amplifier | Aβ40 oligomers | Artificial CSF, serum | 1 pM-100 nM | 0.69 pM | [127] |
| Fluorescence imaging based on aptamer probe β55 | Aβ plaques | AD brain tissue, AD mice | - | - | [128] |
| Aptasensor based on IRS and nanoporous anodic aluminum oxide | Aβ42 oligomers | - | 0.5-50 μg/mL | 0.02 μg/mL | [132] |
| Colorimetric sensor based on AuNPs aggregation | Aβ40 oligomers | Artificial CSF | 1-600 nM | 0.56 nM | [133] |
| Light-up colorimetric sensor based on non-thiolated aptamers | Aβ40 oligomers | Real CSF | 35-700 nM | 10 nM | [134] |
| New ELISA based on aptamer-antibody sandwich | Aβ40/42 oligomers | Artificial CSF | 0.02-25 nM | 50 pM | [135] |
| Dual aptamers-based SPR sensor | Aβ40 oligomers/ fibrils | Artificial CSF, real CSF | 0-10 pM | 0.2 pM for oligomer; 0.05 pM for fibril | [137] |
| SERS sensor based on different Raman dye-encoded polyA aptamer-AuNPs | Tau proteins and Aβ42 oligomers | Artificial CSF | 1 fM-3 nM (Tau); 1-10 μM (Aβ) | 0.42 fM for Tau; 0.37 pM for Aβ | [138] |
| TIRFM-EMCCD imaging system based on antibody-aptamer sandwich | Aβ42 monomer, Tau441 and p-tau181 | Real CSF and serum | 0-1 pM | 8.4 fM for Aβ42; 4.3 fM for Tau441; 3.6 fM for p-tau181 | [139] |
Schematic diagram of fluorescence sensor for detecting Aβ. (A) A fluorescent detection platform based on dye-labeled DNA aptamers and molybdenum disulfide nanosheets used for Aβ42 oligomer detection. Adapted with permission from Ref. [125], copyright 2020 Royal Society of Chemistry. (B) The three-dimensional DNA walker nanoprobe drove by DNase for detecting Aβ40 oligomers. Adapted with permission from Ref. [126], copyright 2020 American Chemical Society. (C) The fluorescence detection of Aβ oligomers based on donor donor-acceptor ('DD-A') fluorescence resonance energy transfer (FRET) and CHA amplifier. Adapted with permission from Ref. [127], copyright 2021 American Chemical Society. (D) β55 staining of amyloid plaques in AD brain tissue. Confocal images of red and green channels in frozen slices of AD brain tissue stained with biotinylated β55 (i) and β55rc (ii); (iii) Co-localized fluorescence image of biotinylated β55 (red) and Thioflavin-S (green) in the positive plaques of AD brain tissue. The scale bar is 50 mm. Adapted with permission from Ref. [128], copyright 2014 Public Library of Science.
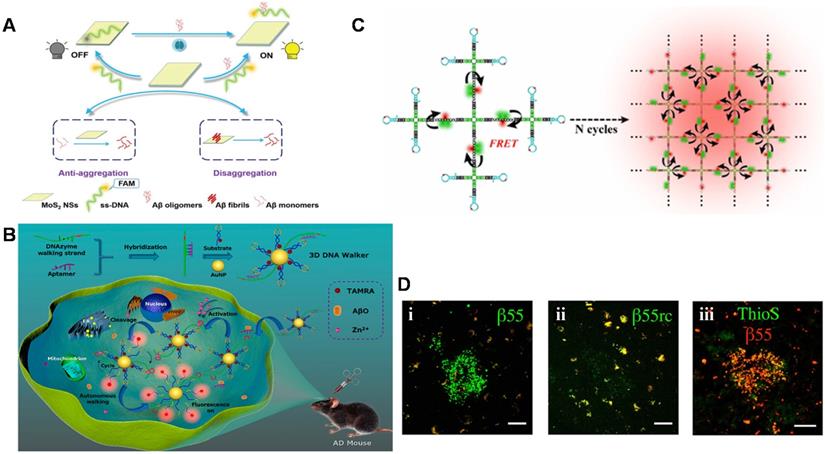
Fluorescence sensors could provide direct, rapid information for more sensitive quantification of Aβ, especially in the complex biological systems. However, expensive fluorescent labels and the requirements of large-scale equipment made some restrictions in Aβ detection. In addition to using various amplification technologies to further improve the sensitivity of fluorescence sensing, the stability of biosensors for Aβ detection in the complex system (CSF, serum and brain tissue) should also be guaranteed. On this basis, more sensitive and real-time detection of Aβ species will provide more valuable feedback for AD diagnosis and treatment. Anyway, the construction of fluorescence sensing platforms has provided an outstanding prospect for the early diagnosis development of AD.
Other optical sensors
In general, optical sensing platforms measure the analyte induced changes in signals, such as absorbance, fluorescence, surface plasmon resonance, and Raman scattering. Except the above-mentioned fluorescence sensors, other optical sensing methods have also played an important role in the detection of Aβ. Unlike fluorescence sensing platforms for Aβ detection that require expensive labels, they serve for label-free, rapid and portable analysis on-site and are developed based on actual application scenarios. For example, SPR sensing platform stands out for its sensibility, real-time and label-free nature, and has been developed for aiding in AD diagnosis. In this section, we focus on the fundamental sensing principles and methodologies to achieve the sensitivity requirements for AD biomarkers throughout optical sensing platforms, mainly including the interference reflectance spectroscopy (IRS)-based sensing, colorimetric sensing, ELISA, SPR sensing and SERS sensing.
As one of the optical sensors, IRS-based biosensor is based on white light interference at thin nano/microporous film surfaces [131]. The biosensors possess the advantages of low-cost, high sensitivity and simple installation, which may play a beneficial role in Aβ detection. The aptamer sensor based on nanoporous anodic aluminum oxide and interference reflectance spectroscopy was constructed for the first time, achieving highly sensitive detection of Aβ42 oligomers [132]. The detection range of this method was from 0.5 to 50 μg/mL (LOD: 0.02 μg/mL). With the continuous advancement of clinical requirements for real-time, portable and rapid detection, more and more sight has been focused on rapid and visual detection technology. Colorimetric sensors have attracted much attention because of their easy operation, low cost, and direct reading of the results with the naked eye. Zhu et al. designed a colorimetric sensor for quantitative analysis of Aβ40 oligomers based on the phenomenon of AuNPs aggregation induced by high salt [133]. The AuNPs stabilized by the aptamer resisted aggregation induced by high salt in the absence of Aβ40 oligomers. The AuNPs were induced to aggregate, and the absorption spectrum changes significantly due to the recognition of the aptamer and Aβ40 oligomers. The method could be used for detecting Aβ40 oligomers in artificial CSF. The performance of most biosensors is usually interfered with by high salt environment of CSF, which severely limits their applications. Therefore, there is an urgent need to develop a method to achieve sensitive and selective AD biomarker detection under the high salt condition. A light-up colorimetric sensor based on non-thiolated aptamers was constructed (Figure 5A), which achieved a simple, low-cost, high-sensitivity and highly reproducible detection of low molecular weight Aβ40 oligomers under high salt concentration (175 mM NaCl) conditions [134]. The colorimetric sensor provides a very favorable opportunity for Aβ detection in the complex system, which shows great potential to develop into point-of-care testing (POCT).
Detection methods of AD biomarker. (A) Quantification of Aβ40 oligomers based on a light-up colorimetric sensor. Adapted with permission from Ref. [134], copyright 2018 American Chemical Society. (B) Interaction of aptamers with Aβ40 aggregates and the construction of dual Apt-based SPR sensor. The only unimodal distribution of the force histogram was displayed for the interactions of Apt2-Aβ40 fibril. Especially, the interaction force of Apt1-Aβ40 fibril showed a double distinguishing Gaussian fitting. Adapted with permission from Ref. [137], copyright 2020 American Chemical Society. (C) Schematic illustrations of PAapt-AuNPs conjugate-based SERS biosensor for simultaneous detection of Aβ42 oligomers and Tau protein using different Raman dye-coded PAapt-AuNPs conjugates. Adapted with permission from Ref. [138], copyright 2019 American Chemical Society. (D) Schematic diagram of simultaneous detection of multiple AD biomarkers. Adapted with permission from Ref. [139], copyright 2019 Ivyspring International Publisher.
Enzyme-linked immunosorbent assay (ELISA) is an optical analytic platform with signals detected by absorption and/or fluorescence. A new ELISA strategy was developed using aptamers as probes for efficient detection of Aβ oligomers [135]. With the acid of capture probe aptamers with higher binding affinity than antibodies and nanomaterials (graphene oxide and AuNPs), the capturing efficiency of Aβ oligomers was enhanced. Therefore, this sandwich pattern of aptamer-Aβ oligomer-antibody helped to reach the detection at 50 pM. Compared to antibody-aptamer sandwich assay, dual-aptamers sandwich assay possesses advantages in avoiding the limitation of steric hindrance and epitope [136]. Recently, our group found that two aptamers (aptamer1 and aptamer2) could bind to amyloid aggregates simultaneously through investigating the interactions of aptamers with Aβ aggregates by single-molecule force spectroscopy technology (Figure 5B). Based on the valuable findings, a novel sensitive dual aptamers-based SPR sensor was fabricated for the amplification detection of Aβ40 aggregates [137]. The dual aptamers-based SPR sensor not only avoided the limitation of steric hindrance and epitope but also employed simple operation as well as inexpensive recognition probes. A detection limit as low as 0.2 pM for Aβ40 oligomer and 0.05 pM for Aβ40 fibril could be achieved. Moreover, the established sensor was also successfully applied to detect Aβ40 aggregates in artificial CSF and undiluted real CSF. The work broadens the application of aptamer in the diagnosis of neurodegenerative diseases.
The simultaneous evaluation of multiple biomarkers (Aβ, Tau, phosphorylated Tau and so on) is essential for accurate screening and diagnosis of AD. A reliable and convenient SERS sensing platform was constructed for simultaneously detecting Aβ42 oligomers and Tau proteins for the first time by connecting different Raman dye-encoded polyA aptamer-AuNPs (PAapt-AuNPs) complexes (Figure 5C), which was significant for accurate prediction and diagnosis of AD. When the target (Aβ42 oligomer and Tau protein) was present, the aptamer specifically recognized and captured the target. Then the recognition mediated the AuNPs aggregation and the plasma coupling effect, thereby enabling the target detection in 'turn on' mode. SERS strategy created a useful and universal platform for low-cost and fast detecting multiple protein biomarkers (15 min) and related clinical diagnosis [138]. In addition, Chan et al. built a total internal reflection fluorescence microscopy electron-multiplying charge-coupled device (TIRFM-EMCCD) imaging system (Figure 5D), and used the antibody-aptamer sandwich method to simultaneously detect Aβ42 monomer (LOD: 8.4 fM), Tau441 (LOD: 4.3 fM) and p-tau181 (LOD: 3.6 fM) [139]. Compared to the antibody-antibody method, this detection system was afforded with one-third the cost and higher sensitivity for detecting multiple biomarkers in CSF and serum samples because of the amplified DNA assembly with fluorescent labels.
The level of Aβ in body fluids (including CSF and blood plasma) of AD patients decreases because of the massive accumulation of Aβ in pathological deposits. Clinical researches have indicated that the concentration of Aβ42 in CSF of AD patients is below 500 pg/mL, which is ~40% lower than those of healthy individuals [140, 141]. The average level of Aβ42 in the plasma of AD patients is 38.2 pg/mL, which is ~20% lower than those of the healthy control. In contrast, the concentrations of Aβ40 in CSF are similar in AD patients (~7.20 ng/mL) and age-matched healthy controls (~6.80 ng/mL), which is ~30 times than those in the plasma [142, 143]. Taken overall, most of developed biosensing systems with their own characteristics have met the quantification of Aβ in body fluids. Compared to the constructed electrochemical methods for Aβ detection, the fluorescence and optical methods are more direct and rapid. However, the sensitivity of them is relatively low, and the cost of equipment is also higher through the description and comparison of the above-mentioned different sensors based on Aβ aptamer. The analytic tools based on electrical signal transduction have advantageous features such as superior sensitivity, good portability, simple integration with electronics and low cost. Further developments of miniaturized and real-time platforms (including capillary, smartphone-based portable optical sensing and paper-based sensing chips) are needed for the detection of AD biomarkers using low cost and portable instruments. Under such circumstances, the sensing platform will better serve the diseases screening, especially in underdeveloped areas where medical conditions are scarce. The fruitful research results from various biosensing platforms have presented the great prospects of aptamers in vitro and in vivo applications. AD diagnosis has still faced challenges due to the complex environment in vivo and the interaction of Aβ with other biomolecules (such as Tau protein) though the sensitive sensing of Aβ based on aptamers has been achieved successfully. So, it is particularly important to detect Aβ in the complex environment of the body. Besides, the detection of interaction of Aβ with recognition molecules and other biomolecules should also be highlighted. The solution of these questions will also provide a significant and valuable reference for Aβ aggregation, which also gives us sufficient confidence to realize early diagnosis and treatment of AD.
The applications of aptamer in the inhibition of Aβ aggregation
Although there is no consensus on the exact nature of amyloid toxicity, the inhibition of amyloid protein aggregation and removal of amyloid plaques have been still considered as useful treatment methods. Inhibiting amyloid protein aggregation plays a very important role in the study of Aβ aggregation in vitro and in guiding the search for effective AD therapy, which has become one of the potential and promising strategies for AD treatment. At present, only a few aptamers have been used as inhibitors of the Aβ aggregation and the treatment of AD, but provided a new strategy with specificity, high-efficiency, low immunogenicity and biosafety for the regulation of amyloid aggregation and eventually AD treatment. Moreover, aptamers are easily associated with nanomaterials with unique optical properties and good biocompatibility. This kind of nano-scaled reagent could step across BBB and then achieve a more effective effect on inhibition of Aβ aggregation in vivo.
Aptamers and their combination with nanomaterials used for inhibiting Aβ aggregation. (A) TEM images of Aβ40 aggregates after 19 h of incubation in the absence of RNA aptamer and the presence of N2 and E2 RNA aptamers. Scale bar = 100 nm. Adapted with permission from Ref. [53], copyright 2009 Royal Society of Chemistry. (B) AFM images of Aβ42 monomer incubated in the absence or presence of Aβ7-92-1H1 (Aβ42 monomer/Aβ-Apt = 5:1) for different incubation times. The size of each image is 2 µm. Adapted with permission from Ref. [77], copyright 2020 American Chemical Society. (C) Inhibition of Zn2+-induced Aβ aggregation and TEM image of fresh Aβ42, Aβ42 incubated with Zn2+ and Aβ42 incubated with Zn2+ and molecular modulator. Adapted with permission from Ref. [56], copyright 2019 American Chemical Society. (D) Histochemical analysis of AppNL‑G‑F/NL‑G‑F mouse brains using RNA aptamers. Representative micrographs were obtained after treatment with E22P-AbD4, -AbD31, and -AbD43 (400 nM). High-magnification images (scale bar = 50 µm) of the area (scale bar = 500 µm) inside the rectangles of the hippocampus are shown within each picture. Arrowheads indicate diffuse staining. Adapted with permission from Ref. [146], copyright 2020 American Chemical Society. (E) Schematic illustration of Aβ-targeting, CD-mediated photomodulation modality for spatiotemporal inhibition of Aβ aggregation in vivo (Left picture). Exemplary ThS-stained coronal section images of mouse brain treated by Apta@CDs with or without illuminations (Right picture). Dashed lines indicate the expected Apta@CDs distribution area and a light path along with the brain, scale bar: 0.5 mm. Adapted with permission from Ref. [153], copyright 2020 American Chemical Society.
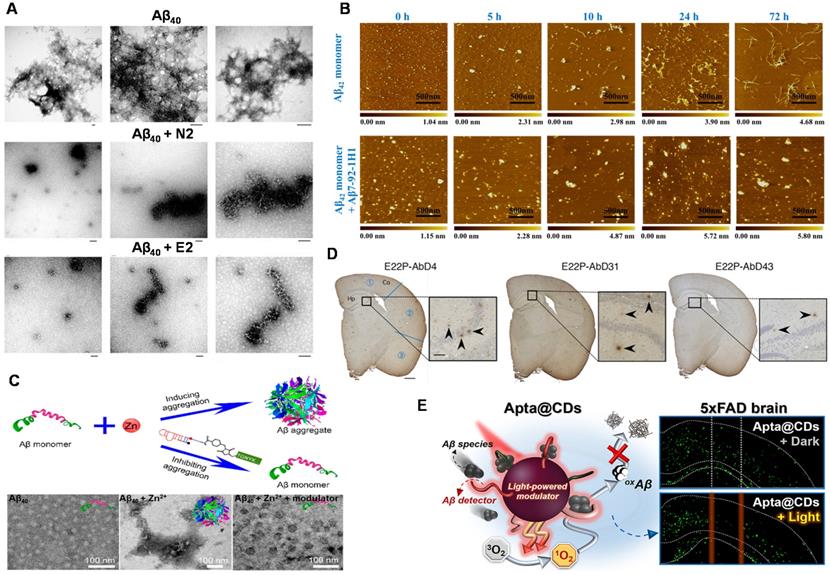
Aptamers used as inhibitors
Takahashi et al. investigated the effect of Aβ40 RNA aptamers (N2, E2) on the fibrosis process of Aβ40 [53]. Figure 6A showed that Aβ40 aggregated formed obvious Aβ40 fibrils when Aβ40 was incubated alone. However, there was no Aβ40 fibril was observed after Aβ40 was incubated with N2 (or E2). The results showed that N2 and E2 inhibited the fibrosis process of Aβ40, which was potential for the prevention and treatment of AD. At the same time, the influence of aptamer N2 on the aggregation process of Aβ40 was further investigated [52]. They found that no fibrils were present when N2 was incubated with Aβ40, which again proved that N2 has an inhibition effect on Aβ40 aggregation. This work may contribute the rapid diagnosis and inhibition of Aβ aggregation in the clinical applications.
The stability of RNA aptamer and its applicability in complex systems are key challenges for the development of Aβ aggregation inhibitors. Besides, the exhaustive inhibition mechanism of Aβ aptamer has not been explored thoroughly. Therefore, in order to develop a new aptamer for inhibiting the Aβ aggregation with low cost, easy synthesis, and high efficiency, researchers have also carried out a series of necessary explorations. Therefore, our group developed the DNA aptamer (Aβ7-92-1H1) and used them as a potent inhibitor for regulating Aβ aggregation (Figure 6B). Notably, the inhibition effect of Aβ7-92-1H1 on the Aβ42 oligomer aggregation was more obvious than that on the Aβ42 monomer aggregation, which may be attributed to different binding behaviors of Aβ7-92-1H1 with Aβ42 monomer and Aβ42 oligomer. The analysis of SPR suggested that the binding affinity of Aβ7-92-1H1 with Aβ42 oligomer was stronger than that of Aβ7-92-1H1 with Aβ42 monomer [77]. This work not only provided a promising platform with high efficiency for manipulating Aβ aggregation but also promoted the application of aptamer in the field of AD treatment.
In the process of studying AD pathology, researchers found that metal ions (Zn2+, Cu2+, etc.) facilitate the aggregation of Aβ, which plays an important role in the progress of AD [144, 145]. Therefore, the inhibition of metal-induced Aβ aggregation is significant and necessary. A dual-aptamer-conjugated molecular modulator (composed of Zn2+ specific aptamer and Aβ42 specific RNA aptamer β55) was designed to detect metal ions and inhibit metal ion-mediated Aβ42 aggregation (Figure 6C). The molecular modulator selectively targeted Aβ42 species and blocked Zn2+ due to the specific recognition capability of aptamers. Therefore, the aggregation of Aβ42 was inhibited due to the inhibition of intramolecular interaction between Aβ42 monomer and Aβ42 monomer. More importantly, the generation of Zn2+-triggered Aβ42 aggregation was also inhibited because of the trapping of Zn2+ around Aβ42. In addition, the molecular modulator was added to the Aβ42 solution containing Zn2+. No Aβ42 aggregates were observed in the solution, indicating that the molecular modulator successfully inhibited the aggregation of Aβ42 [56].
Intervention of Aβ aggregation in the early stages could inhibit the oligomerization, gathering more clinical implications for the treatment of AD. Murakami et al. found that the RNA aptamer E22P-AbD43 significantly inhibited the initial nucleation stage of the Aβ-dimer in human neuroblastoma cells, and it also significantly reduced the neurotoxicity of Aβ42 [76]. In addition, compared with senile plaques composed of amyloid fibrils, E22P-AbD43 preferentially recognized diffuse aggregates in the AD mouse model, which derived from protofibrils or higher-order oligomers with curvilinear structures. The result showed that aptamer E22P-AbD43 was a promising tool for studying AD treatment. Furthermore, this team also used ion mobility mass spectrometry to determine that RNA aptamers changed the size of Aβ42 oligomers [146]. It further revealed that aptamer E22P-AbD43 and Aβ42 monomer (or dimer) formed a complex to inhibit the oligomerization and fibrosis of Aβ42, which illustrated the utility of aptamers as therapeutic agents for AD. Different from the visualized imaging analysis for presenting aptamers' inhibition effect, histochemical analysis of stained mouse brains was employed and emphasized (Figure 6D). This transformation reflects the progress of exploratory researches on the inhibitory effect from in vitro to in vivo. Although the investigation of targeted inhibition in organisms is not in-depth and insufficient, it points out the forward direction of research on inhibitor of Aβ aggregation in the future.
Combination of targeted inhibition and other therapeutics
Given that a single-mode treatment is weak in the treatment of AD, combination therapy will be the trend. Besides, the combination of targeted inhibition and physical therapy is a very effective precision medical method. Presently, great efforts have been committed to alleviating Aβ burden as an aspiring treatment option for AD. To suppress the formation of toxic Aβ aggregates, photochemical modulation of Aβ aggregation using light has recently been proposed as an antiamyloidosis strategy [147-150]. Some nanomaterials play a very important role as effective photodynamic therapeutic agents due to their outstanding optical properties [151, 152]. However, the extension of this modality into animal studies has been rarely researched. Recently, You et al. reported that Aβ-binding DNA aptamer-functionalized carbon dots (Apta@CDs) target Aβ species more effectively than bare CDs in vitro and colocalize with Aβ aggregates ex vivo and in vivo in brain tissues (Figure 6E). Notably, Apta@CDs served as an effective photomodulating nanoagent under red light emitting diode (LED) irradiation, denaturing Aβ peptides, impeding the formation of β-sheet rich Aβ aggregates and attenuating Aβ-associated cytotoxicity [153]. Moreover, the strategy achieved effective suppression of Aβ aggregation in vivo, which significantly reduced the Aβ burden at the targeted sites in the brain of 5xFAD mice by ∼40%. This work demonstrated the distinctive therapeutic potential of photomodulating CDs for light-driven suppression against Aβ self-assembly and related neurotoxicity.
The above researches not only show that the aptamers are ideal inhibitors with high efficiency and low immunogenicity for inhibiting Aβ aggregation but also provided a new and promising strategy with low cost and low toxicity for regulating amyloid aggregation. Besides, the combination between aptamers and special functional nanomaterials is great benefit in vivo application of aptamer. Such advances bring more expectation for evaluating different therapies efficiency. The efficacious combined strategy of targeted inhibition and other therapeutics will promote the development of more robust treatments and the search for the cure.
Conclusions and perspectives
Aptamers are simultaneously used as recognition molecules and aggregation inhibitors which showed outstanding advantages in AD diagnosis and treatment. On the one hand, aptamers have contributed valuable services to Aβ biosensing, which provides the most direct and useful information for the early diagnosis of AD. On the other hand, aptamers have played an important role in the inhibition of Aβ aggregation, which provides a promising way for the treatment of AD. These fruitful researches have expanded the application market of aptamers to a certain extent and brought hope to patients suffering from neurodegenerative diseases. Aptamers have not been widely used in the field of Aβ aggregation regulation for AD treatment, and there is no broad and deep research, through some aptamers have been used as drugs or drug conjugates [154, 155]. Recently, Aβ antibody Aduhelm (aducanumab) have been approved by FDA to treat AD patients, which gives us full confidence to believe that the drug based on Aβ aptamer will have a bright prospect. In order to make better use of aptamer's advantages and functions, the following issues should be given more attention.
Firstly, the accelerated development of specific recognition probes, especially aptamers for accurate discriminating and detecting Aβ monomers and Aβ oligomers, protofibrils/fibrils/plaques with well-defined β-sheet structure should be emphasized and valued. Presently, the obtain of aptamers with high-specificity and high-selectivity still has faced challenges, which hindered and troubled the judgment for progression and treatment effect of AD. The single molecular technology and computational simulation technology provide opportunities for the discovery and development of innovative Aβ-targeting aptamer probes. Moreover, with the development and advance of artificial base [156], functional nucleic acid [157] and DNA assembly [158, 159], the abilities of target recognition, response and delivery can be achieved by the combination of novel functional elements or nanostructure and Aβ-specific aptamers with excellent characteristics, which can give rise to a variety of fascinating novel applications for AD diagnosis and treatment.
Secondly, it is vital to deeply and comprehensively understand the pathological relationship between the main biomarker (Aβ) of AD and different biomarkers (e.g. Tau, cholesterol, IgG or IgM indices). Especially, related studies have reported that AD is related to the increase of total tau protein and phosphorylated tau protein levels and the decrease of Aβ level in CSF [160]. Besides, more and more attention has been focused on the interaction between Aβ and Tau protein. For instance, the simultaneous detection of Aβ and Tau contributes to analyze the role of them in AD pathology and accurate diagnosis of AD. The aptamer-based microfluidic/microarray sensing system with multi-target and high-throughput provides a promising strategy. The complex synergy of multiple molecular mechanisms makes AD diagnosis and treatment face huge challenges. The exploration of pathological relationships will be helpful and valuable to make the therapeutic strategies work using Aβ as the target. The complexity of the pathogenesis of AD indicates that the progression of AD is not driven by a single target but a multiple pathogenic pathway controlled by multiple targets. A single target detection or single inhibition of a certain pathway may not achieve the desired effect. Therefore, multi-targeting techniques capable of simultaneously detecting several pathological biomarkers are likely a working strategy for complex pathologies, diagnostics and treatment of AD. Biosensors allow multiple and extremely sensitive biomarkers quantification at low cost and present a high potential for portability, being an ideal point-of-care diagnosis approach, which are promising tools for aiding on AD early diagnosis.
Thirdly, nanotechnology should be considered and reasonably introduced into AD diagnosis and treatment system. AD therapies like photothermal and photodynamic therapies mainly rely on nanomaterial/nanostructure, which achieve considerable effects in regulating amyloid aggregates, efficiently mitigating amyloid neurotoxicity and facilitating the removal of amyloid plaques. However, dose dependence, biocompatibility and induced immune response may be the main factors that reduce the therapeutic effect in vivo. Because aptamers are small size, none or low immunogenicity, specifically targeting misfolded proteins and preventing aggregating, they are ideal materials for clinical applications [161]. The limitations and challenges of aptamers in the complex internal environment are based on issues of the susceptibility to nucleases, route of administration, BBB penetration, efficiency and applicability [162, 163]. Fortunately, various approaches have been proposed to overcome these limitations such as aptamer-chemical modification, aptamer-nanomaterial conjugation. Benefit from advantage of nanomaterials, photothermal, photodynamic, chemotherapy and other therapies could be easily combined to the therapy based on aptamers. For instance, black phosphorous nanomaterials with photothermal and photodynamic properties are low biological toxicity and capable of chelating metal ions. Aptamer-black phosphorous nanocomposite can be favorable candidates for AD treatment. Accordingly, aptamers will gain the special area in Alzheimer's detection, monitoring disease progression and evaluation of the efficacy of potential AD drugs. Furthermore, in recent years, various of advanced technologies, such as nanopore sensing [164], mass cytometry [165], Raman-flow cytometry [166] have achieved high-precision analysis of the target at single-molecule or single‐cell level. It is believed that the combination of a variety of technology will fully demonstrate powerful functions in the early diagnosis and treatment evaluation of AD in the future.
Finally, the interdisciplinary joint efforts of pathologists, biologists, chemists, computer scientists, and materials scientists will accelerate the pace of early diagnosis and treatment of AD. Thanks to researchers' unremitting efforts to the achievement of great research findings in analyzing AD pathology, interrogating Aβ aggregates, developing new recognition probes, quantifying Aβ and inhibiting Aβ aggregations, which have given us great confidence to firmly believe that the early diagnosis and treatment of AD is optimistic.
Abbreviations
AD: Alzheimer's disease; Aβ: β-amyloid; SPR: surface plasmon resonance; SERS: surface enhanced Raman; SELEX: systematic evolution of ligands by exponential enrichment; AuNPs: gold nanoparticles; CSF: cerebrospinal fluid; Aptasensor: aptamer-based sensor; LOD: low detection limit; RCA: rolling circle amplification; ECL: electrochemiluminescent; MOF: metal-organic framework; AuNFs: gold nanoflowers; ROS: reactive oxygen species; g-C3N4: graphite-like carbon nitride; ECL-RET: electrochemiluminescence resonance energy transfer; GNRs: gold nanorods; ATP: adenosine triphosphate; OECT: organic electrochemical transistor; UCNPs: upconversion nanoparticles; MoS2 NSs: molybdenum disulfide nanosheets; CHA: cascaded catalyzed hairpin assembly; DD-A: donor donor-acceptor; FRET: fluorescence resonance energy transfer; IRS: interference reflectance spectroscopy; POCT: point-of-care testing; ELISA: Enzyme-linked immunosorbent assay; PAapt: polyA aptamer; TIRFM-EMCCD: total internal reflection fluorescence microscopy electron-multiplying charge-coupled device; BBB: blood-brain barrier; CDs: carbon dots; LED: light emitting diode.
Acknowledgements
This research was supported by National Natural Science Foundation of China (financial support from the National Key R&D Program of China (2018YFE0205400), National Natural Science Foundation of China (22074006, 21775031 and 22074032).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Eisele YS, Monteiro C, Fearns C, Encalada SE, Wiseman R.L, Powers ET. et al. Targeting protein aggregation for the treatment of degenerative diseases. Nat Rev Drug Discov. 2015;14:759-80
2. Blakemore DC, Castro L, Churcher I, Rees DC, Thomas AW, Wilson DM. et al. Organic synthesis provides opportunities to transform drug discovery. Nat Chem. 2018;10:383-94
3. Alberdi A, Aztiria A, Basarab A. On the early diagnosis of Alzheimer's disease from multimodal signals: a survey. Artif Intell Med. 2016;71:1-29
4. Alzheimer's Association. 2019 Alzheimer's disease facts and figures. Alzheimer's Dementia. 2019;15:321-87
5. Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT. et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7:34-54
6. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353-6
7. Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis, Science. 1992; 256: 184-5.
8. Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptide. Proc Natl Acad Sci U S A. 1987;84:4190-4
9. Aliyan A, Cook NP, Martí A A. Interrogating amyloid aggregates using fluorescent probes. Chem Rev. 2019;119:11819-56
10. Sahoo BR, Cox SJ, Ramamoorthy A. High-resolution probing of early events in amyloid-β aggregation related to Alzheimer's disease. Chem Commun. 2020;56:4627-39
11. Lee SJ, Nam E, Lee HJ, Savelieff MG, Mi HL. Towards an understanding of amyloid-beta oligomers: characterization, toxicity mechanisms, and inhibitors. Chem Soc Rev. 2017;46:310-23
12. Qiang W, Yau WM, Lu JX, Collinge J, Tycko R. Structural variation in amyloid-β fibrils from Alzheimer's disease clinical subtypes. Nature. 2017;541:217-21
13. Zhu B, Jiang LL, Huang T, Zhao YJ, Liu TF, Zhong YW. et al. ER-associated degradation regulates Alzheimer's amyloid pathology and memory function by modulating γ-secretase activity. Nat Commun. 2017;8:1472
14. Ke PC, Zhou R, Serpell LC, Riek R, Knowles TPJ, Lashuel HA. et al. Half a century of amyloids: past, present and future. Chem Soc Rev. 2020;49:5473-509
15. Nasica-Labouze J, Nguyen PH, Sterpone F, Berthoumieu O, Buchete NV, Coté S. et al. Amyloid β protein and Alzheimer's disease: when computer simulations complement experimental studies. Chem Rev. 2015;115:3518-63
16. Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V. et al. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature. 2018;554:249-54
17. Tana HY, Zhouc K, Yan JW, Sun H, Pistolozzi M, Cui MH. et al. Dual-functional red-emitting fluorescent probes for imaging beta-amyloid plaques and viscosity. Sens Actuators B Chem. 2019;298:126903-9
18. Qin JL, Cho M, Lee YW. Ultrasensitive detection of amyloid-β using cellular prion protein on the highly conductive Au nanoparticles-poly(3,4-ethylene dioxythiophene)-poly(thiophene-3-acetic acid) composite electrode. Anal Chem. 2019;91:11259-65
19. Kim K, Kim MJ, Kim WK, Su YK, Chan BP. Clinically accurate diagnosis of Alzheimer's disease via multiplexed sensing of core biomarkers in human plasma. Nat Commun. 2020;11:119
20. Jia Y, Yang L, Feng RP, Ma HM, Fan DW, Yan T. et al. MnCO3 as new electrochemiluminescence emitter for ultrasensitive bioanalysis of β-amyloid-42 oligomers based on site-directed immobilization of antibody. ACS Appl Mater Interfaces. 2019;11:7157-63
21. Wustoni S, Wang S, Alvarez JR, Hidalgo TC, Nunes SP, Inal S. An organic electrochemical transistor integrated with a molecularly selective isoporous membrane for amyloid-beta detection. Biosens Bioelectron. 2019;143:111561-6
22. Yang JK, Hwang IJ, Cha MG, Kim HI, Yim DB, Jeong DH. et al. Reaction kinetics-mediated control over silver nanogap shells as surface-enhanced raman scattering nanoprobes for detection of Alzheimer's disease biomarkers. Small. 2019;15:1900613-24
23. Guerrini L, Arenal R, Mannini B, Chiti F, Pini R, Matteini P. et al. SERS detection of amyloid oligomers on metallorganic-decorated plasmonic beads. ACS Appl Mater Interfaces. 2015;7:9420-8
24. Buell AK, Esbjorner EK, Riss PJ, White DA, Aigbirhio FI, Toth G. et al. Probing small molecule binding to amyloid fibrils. Phys Chem Chem Phys. 2011;13:20044-52
25. Zhou YL, Liu LT, Dong MT, Xu MT. Simple colorimetric detection of amyloid beta-peptide (1-40) based on aggregation of gold nanoparticles in the presence of copper ions. Small. 2015;11:2144-9
26. Xia N, Zhou BB, Huang NB, Jiang MS, Zhang JB, Liu L. Visual and fluorescent assays for selective detection of beta-amyloid oligomers based on the inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Biosens Bioelectron. 2016;85:625-32
27. Hou HF, Jin Y, Wei H, Ji WL, Xue YF, Hu JB. et al. A generalizable and noncovalent strategy for interfacing aptamers with a microelectrode for the selective sensing of neurotransmitters in vivo. Angew Chem Int Ed Engl. 2020;59:18996-9000
28. Cheng R, Tao MJ, Shi ZL, Zhang XF, Jin Y, Li BX. Label-free and sensitive detection of T4 polynucleotide kinase activity via coupling DNA strand displacement reaction with enzymatic-aided amplification. Biosens Bioelectron. 2015;73:138-45
29. Liu M, Khan A, Wang ZF, Liu Y, Yang GJ, Deng Y. et al. Aptasensors for pesticide detection. Biosens Bioelectron. 2019;130:174-84
30. Niu WJ, Chen XG, Tan WH, Veige AS. N-Heterocyclic carbene gold (I) complexes conjugated to a leukemia specific DNA-aptamer for targeted drug delivery. Angew Chem Int Ed Engl. 2016;55:8889-93
31. Ren X, Gelinas AD, Carlowitz IV, Janjic N, Pyle AM. Structural basis for IL-1α recognition by a modified DNA aptamer that specifically inhibits IL-1α signaling. Nat Commun. 2017;8:810
32. Pang XH, Cui C, Wan S, Jiang Y, Zhang LL, Xia L. et al. Bioapplications of cell-SELEX-generated aptamers in cancer diagnostics, therapeutics, theranostics and biomarker discovery: a comprehensive review. Cancers. 2018;10:47-64
33. Monsonego A. Immunotherapeutic approaches to Alzheimer's disease. Science. 2003;302:834-8
34. Guerrero-Munoz MJ, Castillo-Carranza DL, Krishnamurthy S, Paulucci-Holthauzen AA, Sengupta U, Lasagna-Reeves CA. et al. Amyloid-β oligomers as a template for secondary amyloidosis in Alzheimer's disease. Neurobiolo Dis. 2014;71:14-23
35. Chen ZL, Singh P, Wong J, Horn K, Strickland S, Norris EH. An antibody against HK blocks Alzheimer's disease peptide β-amyloid-induced bradykinin release in human plasma. Proc Natl Acad Sci U S A. 2019;116:22921-3
36. Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173-7
37. Hock C, Konietzko U, Streffer JR, Tracy J, Nitsch RM. Antibodies against β-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547-54
38. Zhang XN, Huai YY, Cai J, Song CL, Zhang YJ. Novel antibody against oligomeric amyloid-β: insight into factors for effectively reducing the aggregation and cytotoxicity of amyloid-β aggregates. Int Immunopharmacol. 2019;67:176-85
39. Li X, Wang WJ, Dong XY, Sun Y. Conjugation of RTHLVFFARK to human lysozyme creates a potent multifunctional modulator for Cu2+-mediated amyloid β-protein aggregation and cytotoxicity. J Mater Chem B. 2020;8:2256-68
40. Wallin C, Hiruma Y, Warmlander SKTS, Huvent I, Jarvet J, Abrahams JP. et al. The neuronal tau protein blocks in vitro fibrillation of the amyloid-β (Aβ) peptide at the oligomeric stage. J Am Chem Soc. 2018;140:8138-46
41. Andreetto E, Yan LM, Tatarek-Nossol M, Velkova A, Frank R, Kapurniotu A. Identification of hot regions of the Abeta-IAPP interaction interface as high-affinity binding sites in both cross- and self-association. Angew Chem Int Ed Engl. 2010;49:3081-5
42. Chakravarthy B, Ito S, Atkinson T, Gaudet C, Ménard M, Brown L. et al. Evidence that a synthetic amyloid-beta oligomer-binding peptide (ABP) targets amyloid-beta deposits in transgenic mouse brain and human Alzheimer's disease brain. Phys Chem Chem Phys. 2014;445:656-60
43. Huang F, Wang JZ, Qu AT, Shen LL, Liu JJ, Liu JF. et al. Maintenance of amyloid β peptide homeostasis by artificial chaperones based on mixed-shell polymeric micelles. Angew Chem Int Ed Engl. 2014;53:8985-90
44. Xiong N, Zhao YJ, Dong XY, Zheng J, Sun Y. Design of a molecular hybrid of dual peptide inhibitors coupled on AuNPs for enhanced inhibition of amyloid β-protein aggregation and cytotoxicity. Small. 2017;13:1601666-79
45. Jia LG, Wang WJ, Yan YX, Hu R, Liu F. General aggregation-induced emission probes for amyloid inhibitors with dual inhibition capacity against amyloid β-protein and α-synuclein. ACS Appl Mater Interfaces. 2020;12:31182-94
46. Ahmed R, VanSchouwen B, Jafari N, Ni XD, Ortega J, Melacini G. Molecular mechanism for the (-)-epigallocatechin gallate-induced toxic to nontoxic remodeling of Aβ oligomers. J Am Chem Soc. 2017;139:13720-34
47. Li M, Howson SE, Dong K, Gao N, Ren JS, Scott P. et al. Chiral metallohelical complexes enantioselectively target amyloid β for treating Alzheimer's disease. J Am Chem Soc. 2014;136:11655-63
48. Lorenzo A, Yankner BA. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci U S A. 1994;91:12243-7
49. Ren BP, Liu YL, Zhang YX, Cai YQ, Gong X, Chang Y. et al. Genistein: a dual inhibitor of both amyloid β and human islet amylin peptides. ACS Chem Neurosci. 2018;9:1215-24
50. Han X, Park J, Wu W, Malagon A, Wang LY, Vargas E. et al. A resorcinarene for inhibition of Aβ fibrillation. Chem Sci. 2017;8:2003-9
51. Pihlasalo S, Deguchi T, Virtamo M, Jacobino J, Chary K, Lopez-Picon FR. et al. Luminometric nanoparticle-based assay for high sensitivity detection of β-amyloid aggregation. Anal Chem. 2017;89:2398-404
52. Babu E, Mareeswaran PM, Sathish V, Singaravadivel S, Rajagopal S. Sensing and inhibition of amyloid-β based on the simple luminescent aptamer-ruthenium complex system. Talanta. 2015;134:348-53
53. Takahashi T, Tada K, Mihara H. RNA aptamers selected against amyloid β-peptide (Aβ) inhibit the aggregation of Aβ. Mol BioSyst. 2009;5:986-91
54. Tsukakoshi K, Abe K, Sode K, Ikebukuro K. Selection of DNA aptamers that recognize α-Synuclein oligomers using a competitive screening method. Anal Chem. 2012;84:5542-7
55. Chakravarthy M, Alshamaileh H, Huang H, Tannenberg R, Chen S, Worrall S. et al. Development of DNA aptamers targeting low-molecular-weight amyloid-β peptide aggregates in vitro. Chem Commun. 2018;54:4593-6
56. Wang J, Wang YQ, Hu XX, Zhu CL, Ma QQ, Liang L. et al. Dual-aptamer-conjugated molecular modulator for detecting bioactive metal ions and inhibiting metal-mediated protein aggregation. Anal Chem. 2019;91:823-9
57. Song Y, Moore EG, Guo Y, Moore JS. Polymer-peptide conjugates disassemble amyloid β fibrils in a molecular-weight dependent manner. J Am Chem Soc. 2017;139:4298-301
58. Zhao Y, Cai JQ, Liu ZC, Li YS, Zheng CX, Zheng YD. et al. Nanocomposites inhibit the formation, mitigate the neurotoxicity, and facilitate the removal of β-amyloid aggregates in Alzheimer's disease mice. Nano Lett. 2019;19:674-83
59. Sahoo BR, Genjo T, Nakayama TW, Stoddard AK, Ando T, Yasuhara K. et al. Alzheimer's amyloid-beta intermediates generated using polymer-nanodiscs. Chem Sci. 2019;10:3976-86
60. Andrikopoulos N, Li YH, Cecchetto L, Nandakumar A, Ros TD, Davis TP. et al. Nanomaterial synthesis, an enabler of amyloidosis inhibition against human diseases. Nanoscale. 2020;12:14422-40
61. Zhang M, Mao X, Yu Y, Wang CX, Yang YL, Wang C. Nanomaterials for reducing amyloid cytotoxicity. Adv Mater. 2013;25:3780-801
62. Li Y, Du Z, Liu XP, Ma MM, Yu DQ, Lu Y. et al. Near-infrared activated black phosphorus as a nontoxic photo-oxidant for Alzheimer's amyloid-β peptide. Small. 2019;15:1901116-21
63. Mondal S, Chowdhury SR, Shah M, Kumar V, Kumar S, Iyer PK. Nano particle assisted regulation of nucleation pathway of amyloid tetramer and inhibition of their fibrillation kinetics. ACS Appl Bio Mater. 2019;2:2137-42
64. Bing T, Shen LY, Wang JY, Wang LL, Liu XJ, Zhang N. et al. Aptameric probe specifically binding protein heterodimer rather than monomers. Adv Sci. 2019;6:1900143-51
65. Zhang LQ, Wan S, Jiang Y, Wang YY, Fu T, Liu QL. et al. Molecular elucidation of disease biomarkers at the interface of chemistry and biology. J Am Chem Soc. 2017;139:2532-40
66. Rahimi F. Aptamers selected for recognizing amyloid β-protein-a case for cautious optimism. Int J Mol Sci. 2018;19:668-87
67. Bouvier-Müller A, Ducongé F. Nucleic acid aptamers for neurodegenerative diseases. Biochimie. 2017;145:73-83
68. Kim K, Lee CH, Park CB. Chemical sensing platforms for detecting trace-level Alzheimer's core biomarkers. Chem Soc Rev. 2020;49:5446-72
69. Celia TR, García-Alonso FJ, Escosura-Muñiz A. Electrochemical biosensors based on nanomaterials for early detection of Alzheimer's disease. Sensors. 2020;20:4748-90
70. Jamerlan A, An SSA, Hulme J. Advances in amyloid beta oligomer detection applications in Alzheimer's disease. Trends Analyt Chem. 2020;129:115919-33
71. Zhang Y, Ren B, Zhang D, Liu Y, Zhang M, Zhao C. et al. Design principles and fundamental understanding of biosensors for amyloid-β detection. J Mater Chem B. 2020;8:6179-97
72. Carneiro P, Moraisb S, Pereira MDC. Biosensors on the road to early diagnostic and surveillance of Alzheimer's disease. Talanta. 2020;211:120700-14
73. Brazaca LC, Sampaio I, Zucolotto V, Janegitz BC. Applications of biosensors in Alzheimer's disease diagnosis. Talanta. 2019;210:120644-55
74. Ylera F, Lurz R, Erdmann VA, Furste JP. Selection of RNA aptamers to the Alzheimer's disease amyloid peptide. Biochem Bioph Res Co. 2002;290:1583-8
75. Rahimi F, Murakami K, Summers JL, Chen CB, Bitan G. RNA aptamers generated against oligomeric Aβ40 recognize common amyloid aptatopes with low specificity but high sensitivity. PloS One. 2009;4:e7694
76. Murakami K, Obata Y, Sekikawa A, Ueda H, Izuo N, Awano T. et al. An RNA aptamer with potent affinity for a toxic dimer of amyloid β42 has potential utility for histochemical studies of Alzheimer's disease. J Biol Chem. 2020;295:4870-80
77. Zheng Y, Wang P, Li SY, Geng XH, Zou LY, Jin MM. et al. Development of DNA aptamer as a β-amyloid aggregation inhibitor. ACS Appl Bio Mater. 2020;3:8611-8
78. Kim SH, Lee EH, Lee SC, Kim AR, Park HH, Son JW. et al. Development of peptide aptamers as alternatives for antibody in the detection of amyloid-beta 42 aggregates. Anal Biochem. 2020;609:113921-9
79. Ke PC, Sani MA, Ding F, Kakinen A, Javed I, Separovic F. et al. Implications of peptide assemblies in amyloid diseases. Chem Soc Rev. 2017;46:6492-531
80. Owen MC, Gnutt D, Gao M, Wärmländer SKTS, Jarvet J, Gräslund A. et al. Effects of in vivo conditions on amyloid aggregation. Chem Soc Rev. 2019;48:3946-96
81. Rahimi F, Bitan G. Selection of aptamers for amyloid β-protein, the causative agent of Alzheimer's disease. J Vis Exp. 2010;39:1-7
82. Findeis MA. The role of amyloid β peptide 42 in Alzheimer's disease. Pharmacol Ther. 2007;116:266-86
83. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353-6
84. Delehanty JB, Bradburne CE, Susumu K, Boeneman K, Mei BC, Farrell D. et al. Spatiotemporal multicolor labeling of individual cells using peptide-functionalized quantum dots and mixed delivery techniques. J Am Chem Soc. 2011;133:10482-9
85. Jia XQ, Guo MM, Han QJ, Tian YW, Yuan YF, Wang ZH. et al. Synergetic tumor probes for facilitating therapeutic delivery by combined-functionalized peptide ligands. Anal Chem. 2020;92:5650-5
86. Wang WZ, Hu ZY. Targeting peptide-based probes for molecular imaging and diagnosis. Adv Mater. 2018;30:1804827-34
87. Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 1992;355:564-6
88. Tasset DM, Kubik MF, Steiner W. Oligonucleotide inhibitors of human thrombin that bind distinct epitopes. J Mol Biol. 1997;272:688-98
89. Zheng Y, Wang Q, Yang XH, Nie WY, Zou LY, Liu XF. et al. Aptamer as a tool for investigating the effects of electric field on Aβ40 monomer and aggregates using single molecule force spectroscopy. Anal Chem. 2019;91:1954-61
90. Zhang N, Bing T, Shen LY, Song RS, Wang LL, Liu XJ. et al. Intercellular connections related to cell-cell crosstalk specifically recognized by an aptamer. Angew Chem Int Ed Engl. 2016;55:3914-8
91. Liu BW, Huang ZC, Liu JW. Polyvalent spherical nucleic acids for universal display of functional DNA with ultrahigh stability. Angew Chem Int Ed Engl. 2018;57:9439-42
92. Zhao GH, Wang YG, Li XJ, Yue Q, Dong X, Du B. et al. Dual quenching electrochemiluminescence strategy based on 3D metal-organic frameworks for ultrasensitive detection of amyloid-β. Anal Chem. 2019;91:1989-96
93. Qin JL, Cho M, Lee YW. Ferrocene-encapsulated Zn zeolitic imidazole framework (ZIF-8) for optical and electrochemical sensing of amyloid-β oligomers and for the early diagnosis of Alzheimer's disease. ACS Appl Mater Interfaces. 2019;11:11743-8
94. Chen WL, Gao G, Jin Y, Deng CY. A facile biosensor for Aβ40O based on fluorescence quenching of prussian blue nanoparticles. Talanta. 2020;216:120930-8
95. Zhang YT, Figueroa-Miranda G, Lyu Z, Zafiu C, Willbold D, Offenhaeusser A. et al. Monitoring amyloid-β proteins aggregation based on label-free aptasensor. Sensor Actuators B Chem. 2019;288:535-42
96. Zhou YL, Li CM, Li XQ, Zhu X, Ye BX, Xu MT. A sensitive aptasensor for detection of β-amyloid oligomers based on metal-organic frameworks as electrochemical signal probes. Anal Methods. 2018;10:4430-7
97. Deng CY, Liu H, Si SH, Zhu XJ, Tu QY, Xiang J. An electrochemical aptasensor for amyloid-β oligomer based on double-stranded DNA as “conductive spring”. Microchim Acta. 2020;187:239-46
98. Zhou YL, Zhang HQ, Liu LT, Li CM, Chang Z, Zhu X. et al. Fabrication of an antibody-aptamer sandwich assay for electrochemical evaluation of levels of β-amyloid oligomers. Sci Rep. 2016;6:35186-93
99. You M, Yang S, An Y, Zhang F, He PG. A novel electrochemical biosensor with molecularly imprinted polymers and aptamer-based sandwich assay for determining amyloid-β oligomer. J Electroanal Chem. 2020;862:114017-24
100. Liao XJ, Zhang CY, Shi ZQ, Shi HL, Qian Y, Gao FL. Signal-on and label-free electrochemical detection of amyloid β oligomers based on dual amplification induced hemin/G-quadruplex formation. J Electroanal Chem. 2020;878:114604-14
101. Ji JJ, Wen J, Shen YF, Lv YQ, Chen YL, Liu SQ. et al. Simultaneous noncovalent modification and exfoliation of 2D carbon nitride for enhanced electrochemiluminescent biosensing. J Am Chem Soc. 2017;139:11698-701
102. Muzyka K, Saqib M, Liu ZY, Zhang W, Xu GB. Progress and challenges in electrochemiluminescent aptasensors. Biosens Bioelectron. 2017;92:241-258
103. Nugroho FAA, Darmadi I, Cusinato L, Arce AS, Schreuders H, Bannenberg LJ. et al. Metal-polymer hybrid nanomaterials for plasmonic ultrafast hydrogen detection. Nat Mater. 2019;18:489-95
104. Lopez A, Liu JW. Nanomaterial and aptamer-based sensing: target binding versus target adsorption illustrated by the detection of adenosine and ATP on metal oxides and graphene oxide. Anal Chem. 2021;93:3018-25
105. Yin LX, Wang YJ, Tan R, Li HL, Tu YF. Determination of β-amyloid oligomer using electrochemiluminescent aptasensor with signal enhancement by AuNP/MOF nanocomposite. Microchim Acta. 2021;188:53-60
106. Wang XY. Li LY, Gu X, Yu BJ, Jiang M. Switchable electrochemical aptasensor for amyloid-β oligomers detection based on triple helix switch coupling with AuNPs@CuMOF labeled signaling displaced-probe. Microchim Acta. 2021;188:49-59
107. Qin HX, Gao X, Yang XY, Cao W, Liu SF. A label-free and signal-on electrochemiluminescence strategy for sensitive amyloid-beta assay. Biosens Bioelectron. 2019;141:111438-45
108. Zhang MJ, Chen ZX, Qin HX, Yang XY, Cao W, Liu SF. g-C3N4-heme bound to amyloid β peptides: in-situ generation of the secondary co-reactant for dual-enhanced electrochemiluminescence assay of amyloid β detection. Electrochim Acta. 2020;361:137096-104
109. Zhang YT, Figueroa-Miranda G, Zafiu C, Willbold DY, Offenhäusser A, Mayer D. Amperometric aptasensor for amyloid β oligomer detection by optimized stem-loop structures with an adjustable detection range. ACS Sens. 2019;4:3042-50
110. Ke H, Sha HF, Wang YF, Guo WW, Zhang X, Wang ZM. et al. Electrochemiluminescence resonance energy transfer system between GNRs and Ru(bpy)32+: application in magnetic aptasensor for β-amyloid. Biosens Bioelectron. 2018;100:266-73
111. Wang YF, Zhang Y, Sha HF, Xiong X, Jia NQ. Design and biosensing of a ratiometric electrochemiluminescence resonance energy transfer aptasensor between a g-C3N4 nanosheet and Ru@MOF for amyloid-β protein. ACS Appl Mater Interfaces. 2019;11:36299-306
112. Zhang YT, Figueroa-Miranda G, Wu CT, Willbold D, Offenhusser A, Mayer D. Electrochemical dual-aptamer biosensors based on nanostructured multielectrode arrays for the detection of neuronal biomarkers. Nanoscale. 2020;12:16501-13
113. Yi XY, Feng CT, Hu SQ, Li HF, Wang JX. Surface plasmon resonance biosensors for simultaneous monitoring of amyloid-beta oligomers and fibrils and screening of select modulators. Analyst. 2015;141:331-6
114. Abbasi HY, Tehrani Z, Devadoss A, Ali MM, Moradi-Bachiller S, Albani D. et al. Graphene based electrochemical immunosensor for the ultra-sensitive label free detection of Alzheimer's beta amyloid peptides Aβ(1-42). Nanoscale Adv. 2021;3:2295-304
115. Xu R, Wei D, Du B, Cao W, Fan DW, Zhang Y. et al. A photoelectrochemical sensor for highly sensitive detection of amyloid beta based on sensitization of Mn:CdSe to Bi2WO6/CdS. Biosens Bioelectron. 2018;122:37-42
116. Zadorozhnyi I, Hlukhova H, Kutovyi Y, Handziuk V, Naumova N, Offenhaeusser A. et al. Towards pharmacological treatment screening of cardiomyocyte cells using Si nanowire FETs. Biosens Bioelectron. 2019;137:229-35
117. Salehirozveh M, Dehghani P, Zimmermann M, Roy VAL, Heidari H. Graphene field effect transistor biosensors based on aptamer for amyloid-β detection. IEEE Sens J. 2020;20:12488-94
118. Kutovyi Y, Hlukhova H, Boichuk N, Menger M, Offenhausser A, Vitusevich S. Amyloid-beta peptide detection via aptamer-functionalized nanowire sensors exploiting single-trap. Biosens Bioelectron. 2020;154:112053-60
119. Koklu A, Wustoni S, Musteata VE, Ohayon D, Moser M, McCulloch I. et al. Microfluidic integrated organic electrochemical transistor with a nanoporous membrane for amyloid-β detection. ACS Nano. 2021;15:8130-41
120. Zhao Y, Li X, Yang Y, Si SH, Deng CY, Wu HY. A simple aptasensor for Aβ40 oligomers based on tunable mismatched base pairs of dsDNA and graphene oxide. Biosens Bioelectron. 2019;149:111840-5
121. Ren HX, Miao YB, Zhang YD. An aptamer based fluorometric assay for amyloid-β oligomers using a metal-organic framework of type Ru@MIL-101(AI) and enzyme-assisted recycling. Microchim Acta. 2020;187:114-21
122. Jiang LF, Chen BC, Chen B, Li XJ, Liao HL, Huang HM. et al. Detection of Aβ oligomers based on magnetic-field-assisted separation of aptamer-functionalized Fe3O4 magnetic nanoparticles and BaYF5:Yb,Er nanoparticles as upconversion fluorescence labels. Talanta. 2017;170:350-7
123. Nam E, Derrick JS, Lee S, Kang J, Han J, Lee SJC. et al. Regulatory activities of dopamine and its derivatives toward metal-free and metal-induced amyloid-beta aggregation, oxidative stress, and inflammation in Alzheimer's disease. ACS Chem Neurosci. 2018;9:2655-66
124. Liu L, Chang Y, Yu J, Jiang MC, Xia N. Two-in-one polydopamine nanospheres for fluorescent determination of beta-amyloid oligomers and inhibition of beta-amyloid aggregation. Sensor Actuators B Chem. 2017;251:359-65
125. Kong LN, Zhou XG, Shi GY, Yu Y. Molybdenum disulfide nanosheets-based fluorescent “off to-on” probe for targeted monitoring and inhibition of β amyloid oligomers. Analyst. 2020;145:6369-77
126. Yin YM, Chen GF, Gong L, Ge K, Gao F. DNAzyme-powered three-dimensional DNA walker nanoprobe for detection amyloid β peptide oligomer in living cells and in vivo. Anal Chem. 2020;92:9247-56
127. Zhang YY, Zhao JW, Yang GM, He Y, Chen SH, Yuan R. Ultrasensitive detection of amyloid β oligomers based on the “DD-A” FRET binary probes and quadrivalent cruciform DNA nanostructure-mediated cascaded amplifier. ACS Appl Mater Interfaces. 2021;13:32013-21
128. Farrar CT, William CM, Eloise H, Tadafumi H, Hyman BT, Ken A. RNA aptamer probes as optical imaging agents for the detection of amyloid plaques. PloS One. 2014;9:e89901
129. Fu HL, Tu PY, Zhao L, Dai JP, Liu BL, Cui MC. Amyloid-β deposits target efficient near-infrared fluorescent probes: synthesis, in vitro evaluation, and in vivo imaging. Anal Chem. 2016;88:1944-50
130. Li Y, Xu D, Chan HN, Poon CY, Ho SL, Li HW. et al. Dual-modal NIR-fluorophore conjugated magnetic nanoparticle for imaging amyloid-β species in vivo. Small. 2018;14:1800901-7
131. Rajeev G, Xifre-Perez E, Prieto BS, Cowin AJ, Marsal LF, Voelcker NH. A label-free optical biosensor based on nanoporous anodic alumina for tumour necrosis factor-alpha detection in chronic wounds. Sensor Actuator B Chem. 2018;257:116-23
132. Tabrizi MA, Ferré-Borrull J, Marsal LF. Highly sensitive aptasensor based on interferometric reflectance spectroscopy for the determination of amyloid β as an Alzheimer's disease biomarkers using nanoporous anodic alumina. Biosens Bioelectron. 2019;137:137279-86
133. Zhu X, Zhang NN, Zhang YT, Liu B, Xu M. A sensitive gold nanoparticle-based aptasensor for colorimetric detection of Aβ1-40 oligomers. Anal Methods. 2018;10:641-4
134. Deng CY, Liu H, Zhang MM, Deng HH, Lei CY, Shen L. et al. A light-up non-thiolated aptasensor for low-mass soluble amyloid-β40 oligomers at high salt concentrations. Anal Chem. 2018;90:1710-7
135. Zhao J, Chang WL, Liu L, Xing XM, Zhang C, Meng HH, Gopinath SCB. et al. Graphene oxide-gold nanoparticle-aptamer complexed probe for detecting amyloid beta oligomer by ELISA-based immunoassay. J Immunol Methods. 2020;489:112942-8
136. Qin SY, Chen ND, Yang XH, Wang Q, Wang KM, Huang J. et al. Development of dual-aptamers for constructing sandwich-type pancreatic polypeptide assay. ACS Sens. 2017;2:308-15
137. Zheng Y, Geng XH, Yang XH, Li SY, Liu YQ, Liu XF. et al. Exploring interactions of aptamers with Aβ40 amyloid aggregates and its application: detection of amyloid aggregates. Anal Chem. 2020;92:2853-8
138. Zhang X, Liu S, Song XL, Wang HW, Wang JF, Wang Y. et al. Robust and universal SERS sensing platform for multiplexed detection of Alzheimer's disease core biomarkers using PAapt-AuNPs conjugates. ACS Sens. 2019;4:2140-9
139. Chan HN, Xu D, Ho SL, He DG, Man SW, Li HW. Highly sensitive quantification of Alzheimer's disease biomarkers by aptamer-assisted amplification. Theranostics. 2019;9:2939-49
140. Shaw LM, Korecka M, Clark CM, Lee VMY, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6:295-303
141. Grimmer T, Riemenschneider M, Forstl H, Henriksen G, Klunk WE, Mathis CA. et al. Beta amyloid in Alzheimer's disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65:927-34
142. Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature. 2018; 554: 249-54.
143. Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131-44
144. Cheignon C, Jones M, Atrian-Blasco E, Kieffer I, Faller P, Collin F. et al. Identification of key structural features of the elusive Cu-Aβ complex that generates ROS in Alzheimer's disease. Chem Sci. 2017;8:5107-18
145. Atrian-Blasco E, Santoro A, Pountney DL, Meloni G, Hureau C, Faller P. Chemistry of mammalian metallothioneins and their interaction with amyloidogenic peptides and proteins. Chem Soc Rev. 2017;46:7683-93
146. Obata Y, Murakami K, Kawase T, Hirose K, Irie K. Detection of amyloid β oligomers with RNA aptamers in App NL-G-F/NL-G-F mice: a model of arctic Alzheimer's disease. ACS Omega. 2020;5:21531-7
147. Lee BI, Lee S, Suh YS, Lee JS, Kim A, Kwon OY. et al. Photoexcited porphyrins as a strong suppressor of β-amyloid aggregation and synaptic toxicity. Angew Chem Int Ed Engl. 2015;54:11472-6
148. Taniguchi A, Shimizu Y, Oisaki K, Sohma Y, Kanai M. Switchable photooxygenation catalysts that sense higher-order amyloid structures. Nat Chem. 2016;8:974-82
149. Zhang XL, Tian YL, Li Z, Tian XY, Sun HB, Hong L. et al. Design and synthesis of curcumin analogues for in vivo fluorescence imaging and inhibiting copper-induced cross-linking of amyloid beta species in Alzheimer's disease. J Am Chem Soc. 2013;135:16397-409
150. Kim K, Lee SH, Choi DS, Park CB. Photoactive bismuth vanadate structure for light-triggered dissociation of Alzheimer's β-amyloid aggregates. Adv Funct Mater. 2018;28:1802813-22
151. Chung YJ, Kim K, Lee BI, Park CB. Carbon nanodot sensitized modulation of Alzheimer's β-amyloid self-assembly, disassembly, and toxicity. Small. 2017;13:1700983-91
152. Chung YJ, Lee BI, Park CB. Multifunctional carbon dots as a therapeutic nanoagent for modulating Cu (II)-mediated β-amyloid aggregation. Nanoscale. 2019;11:6297-306
153. You JC, Chang HL, Lim J, Jang J, Kang Y, Chan BP. Photomodulating carbon dots for spatiotemporal suppression of Alzheimer's β-amyloid aggregation. ACS Nano. 2020;14:16973-83
154. Rinaldi M, Chiosi F, dell'Omo R, Romano MR, Parmeggiani F, Semeraro F. et al. Intravitreal pegaptanib sodium (macugen®) for treatment of diabetic macular oedema: a morphologic and functional study. Br J Clin Pharmacol. 2012;74:940-6
155. Rosenberg JE, Bambury RM, Allen EMV, Drabkin HA, Lara Jr PN, Harzstark AL. et al. A phase ii trial of AS1411 (a novel nucleolin-targeted dna aptamer) in metastatic renal cell carcinoma. Invest New Drugs. 2014;32:178-87
156. Wang RW, Jin C, Zhu XY, Zhou LY, Xuan WJ, Liu Y. et al. Artificial base zT as functional “element” for constructing photoresponsive DNA nanomolecules. J Am Chem Soc. 2017;139:9104-7
157. Xu WT, He WC, Du ZH, Zhu LY, Huang KL, Lu Y. et al. Functional nucleic acid nanomaterials: development, properties, and applications. Angew Chem Int Ed Engl. 2021;60:6890-918
158. Hu YQ, Wang Y, Yan JH, Wen NC, Xiong HJ, Cai SD. et al. Dynamic DNA assemblies in biomedical applications. Adv Sci. 2020;7:2000557-85
159. Wang LY, Song KY, Qu Y, Chang YY, Li ZP, Dong C. et al. Engineering micrometer-sized DNA tracks for high-speed DNA synthesis and biosensing. Angew Chem Int Ed Engl. 2020;59:23344-8
160. Polanco JC, Li C, Liviu-Gabriel B, Martinez-Marmol R, Meunier FA, Jürgen G. Amyloid-β and Tau complexity-towards improved biomarkers and targeted therapies. Nat Rev Neurol. 2018;14:22-39
161. Ozturk M, NilsenHamilton M, Ilgu M. Aptamer applications in neuroscience. Pharmaceuticals. 2021;14:1260-81
162. Bruno JG. Predicting the uncertain future of aptamer-based diagnostics and therapeutics. Molecules. 2015;20:6866-87
163. Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2016;16:181-202
164. Spitzberg JD, Zrehen A, Kooten XF, Meller A. Plasmonic-nanopore biosensors for superior single-molecule detection. Adv Mater. 2019;31:1900422-39
165. Xu S, Liu MX, Bai Y, Liu HW. Multi-dimensional organic mass cytometry: simultaneous analysis of proteins and metabolites on single cells. Angew Chem Int Ed Engl. 2020;60:1806-12
166. Pablo JG, Lindley M, Hiramatsu K, Goda K. High-throughput raman flow cytometry and beyond. Acc Chem Res. 2021;54:2132-43
Author contact
![]() Corresponding authors: E-mail: wangwzedu.cn (W.W.); wwqq99edu.cn (Q.W.); zhangxledu.cn (X.Z.).
Corresponding authors: E-mail: wangwzedu.cn (W.W.); wwqq99edu.cn (Q.W.); zhangxledu.cn (X.Z.).
 Global reach, higher impact
Global reach, higher impact