13.3
Impact Factor
Theranostics 2022; 12(5):1999-2014. doi:10.7150/thno.69119 This issue Cite
Research Paper
Anti-PD-L1 peptide-conjugated prodrug nanoparticles for targeted cancer immunotherapy combining PD-L1 blockade with immunogenic cell death
1. Biomedical Research Institute, Korea Institute of Science and Technology (KIST), Seoul, 02792, Republic of Korea.
2. Department of Bioengineering, Korea University, Seoul, 02841, Republic of Korea.
3. KU-KIST Graduate School of Converging Science and Technology, Korea University, Seoul, 02841, Republic of Korea.
4. Department of Materials Science and Engineering, Seoul National University, Seoul, 08826, Republic of Korea.
#These authors contributed equally to this work.
Abstract
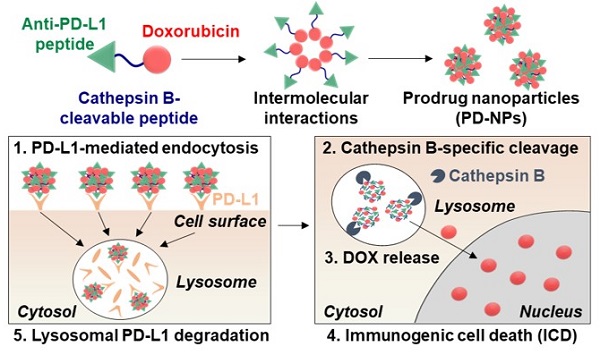
Rationale: Cancer immunotherapy combining immune checkpoint blockade (ICB) with chemotherapeutic drugs has provided significant clinical advances. However, such combination therapeutic regimen has suffered from severe toxicity of both drugs and low response rate of patients. In this study, we propose anti-PD-L1 peptide-conjugated prodrug nanoparticles (PD-NPs) to overcome these obstacles of current cancer immunotherapy.
Methods: The functional peptide, consisted of anti-PD-L1 peptide and cathepsin B-specific cleavable peptide, is conjugated to a doxorubicin (DOX), resulting in prodrug nanoparticles of PD-NPs via intermolecular interactions. The antitumor efficacy and immune responses with minimal side effects by PD-NPs combining PD-L1 blockade and ICD are evaluated in breast tumor models.
Results: The PD-NPs are taken up by PD-L1 receptor-mediated endocytosis and then induce ICD in cancer cells by DOX release. Concurrently, PD-L1 blockade by PD-NPs disrupt the immune-suppressing pathway of cancer cells, resulting in proliferation and reinvigoration of T lymphocytes. In tumor models, PD-NPs accumulate within tumor tissues via enhanced permeability and retention (EPR) effect and induce immune-responsive tumors by recruiting a large amount of immune cells.
Conclusions: Collectively, targeted tumor delivery of anti-PD-L1 peptide and DOX via PD-NPs efficiently inhibit tumor progression with minimal side effects.
Keywords: cancer immunotherapy, immune checkpoint blockade, prodrug nanoparticle, drug delivery, antitumor immune response
 Global reach, higher impact
Global reach, higher impact