13.3
Impact Factor
Theranostics 2022; 12(2):944-962. doi:10.7150/thno.67572 This issue Cite
Research Paper
Tumor-specific activatable biopolymer nanoparticles stabilized by hydroxyethyl starch prodrug for self-amplified cooperative cancer therapy
1. National Engineering Research Center for Nanomedicine, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, 430074, P. R. China
2. Key Laboratory of Biomedical Photonics (HUST), Ministry of Education, Huazhong University of Science and Technology, Wuhan 430074, P. R. China
3. Key Laboratory of Molecular Biophysics of Ministry of Education, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, 430074, P. R. China
4. Hubei Key Laboratory of Bioinorganic Chemistry and Materia Medical, Huazhong University of Science and Technology, Wuhan, 430074, P. R. China
5. Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, Huazhong University of Science and Technology, Wuhan, 430074, P. R. China
6. GBA Research Innovation Institute for Nanotechnology, Guangdong, 510530, P. R. China
7. Wuhan Institute of Biotechnology, High Tech Road 666, East Lake high tech Zone, Wuhan, 430040, P. R. China
# These authors contribute equally.
Abstract
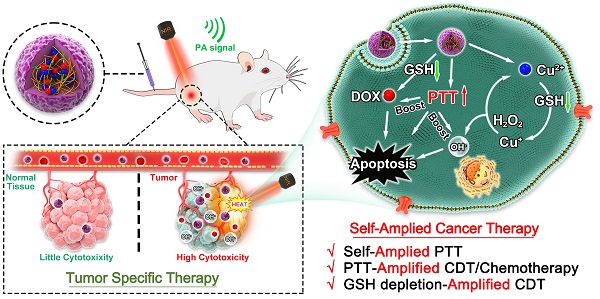
Rationale: Chemodynamic therapy (CDT) is an emerging tumor-specific therapeutic strategy. However, the anticancer activity of CDT is impeded by the insufficient Fenton catalytic efficiency and the high concentration of glutathione (GSH) in the tumor cells. Also, it is challenging to eliminate tumors with CDT alone. Thus, simple strategies aimed at constructing well-designed nanomedicines that can improve therapeutic efficiency of CDT and simultaneously incorporate extra therapeutic modes as helper are meaningful and highly required.
Method: Tailored to specific features of tumor microenvironment (TME), in this study, we developed a biosafe, stable and TME-activated theranostic nanoplatform (P(HSD-Cu-DA)) for photoacoustic imaging (PAI) and self-amplified cooperative therapy. This intelligent nanoplatform was fabricated following a simple one-pot coordination and polymerization strategy by using dopamine and Cu2+ as precursors and redox-responsive hydroxyethyl starch prodrugs (HES-SS-DOX) as stabilizer.
Results: Interestingly, the pre-doped Cu2+ in polydopamine (PDA) framework can endow P(HSD-Cu-DA) NPs with tumor-specific CDT ability and remarkably enhance NIR absorption of PDA. PAI and biodistribution tests proved such nanoplatform can effectively accumulate in tumor tissues. Following enrichment, massive amounts of toxic hydroxyl radicals (·OH, for CDT) and free DOX (for chemotherapy) were generated by the stimulation of TME, which was further boosted by local hyperthermia. Concomitantly, in the process of activating these therapeutic functions, GSH depletion triggered by disulfide bond (-SS-) breakage and Cu2+ reduction within tumor cells occurred, further amplifying intratumoral oxidative stress. Importantly, the framework structure dominated by bioinspired polydopamine and clinical-used HES guaranteed the long-term biosafety of in vivo treatment. As a result, the mutual promotion among different components yields a potent tumor suppression outcome and minimized systemic toxicity, with one dosage of drug administration and laser irradiation, respectively.
Conclusion: This work provides novel insights into designing efficient and tumor-specific activatable nanotherapeutics with significant clinical translational potential for cancer therapy.
Keywords: chemodynamic therapy, one-pot strategy, tumor-specific, GSH depletion, self-amplified therapy
 Global reach, higher impact
Global reach, higher impact