13.3
Impact Factor
Theranostics 2022; 12(2):817-841. doi:10.7150/thno.67932 This issue Cite
Review
Targeting regulated cell death in tumor nanomedicines
1. Department of Ultrasound, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, China.
2. Department of Obstetrics and Gynecology, National Clinical Research Center for Obstetrical and Gynecological Diseases, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, 430030, Wuhan, China.
3. Department of Nuclear Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, China.
4. Department of Ultrasound, Hainan General Hospital, Haikou, 570311, China.
5. Cancer Centre and Center of Reproduction, Development and Aging, Faculty of Health Sciences, University of Macau, Macau, SAR 999078, China.
6. Sino-German Tongji-Caritas Research Center of Ultrasound in Medicine, Department of Medical Ultrasound, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, China.
*These authors contributed equally to this work.
Abstract
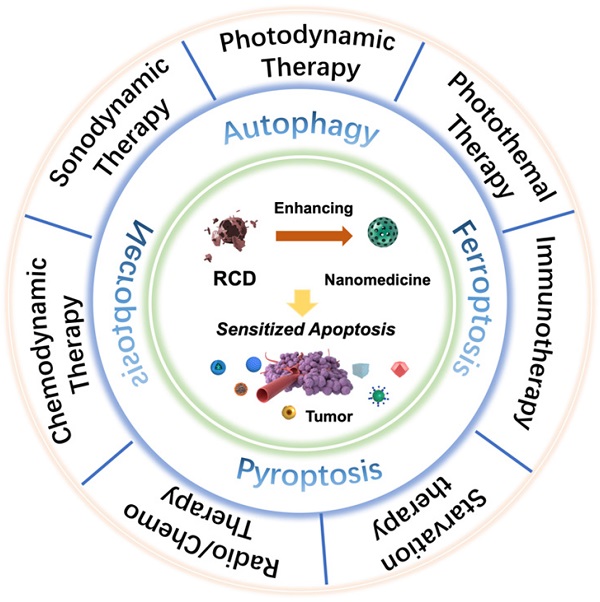
Nanomedicines hold great potential in anticancer therapy by modulating the biodistribution of nanomaterials and initiating targeted oxidative stress damage, but they are also limited by the inherent self-protection mechanism and the evolutionary treatment resistance of cancer cells. New emerging explorations of regulated cell death (RCD), including processes related to autophagy, ferroptosis, pyroptosis, and necroptosis, substantially contribute to the augmented therapeutic efficiency of tumors by increasing the sensitivity of cancer cells to apoptosis. Herein, paradigmatic studies of RCD-mediated synergistic tumor nanotherapeutics are introduced, such as regulating autophagy-enhanced photodynamic therapy (PDT), targeting ferroptosis-sensitized sonodynamic therapy (SDT), inducing necroptosis-augmented photothermal therapy (PTT), and initiating pyroptosis-collaborative chemodynamic therapy (CDT), and the coordination mechanisms are discussed in detail. Multiangle analyses addressing the present challenges and upcoming prospects of RCD-based nanomedicines have also been highlighted and prospected for their further strengthening and the broadening of their application scope. It is believed that up-and-coming coadjutant therapeutic methodologies based on RCDs will considerably impact precision nanomedicine for cancer.
Keywords: Regulated cell death, Tumor therapy, Nanomedicine, Sensitized apoptosis, Nanomaterials
 Global reach, higher impact
Global reach, higher impact