13.3
Impact Factor
Theranostics 2022; 12(2):796-816. doi:10.7150/thno.67375 This issue Cite
Review
Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle
1. Department of Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
2. Biofilm Research Labs, Levy Center for Oral Health, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA, USA
3. Department of Preventive & Restorative Sciences, School of Dental Medicine, University of Pennsylvania, Philadelphia, PA, USA
4. Department of Bioengineering, School of Engineering and Applied Sciences, University of Pennsylvania, Philadelphia, PA, USA
Abstract
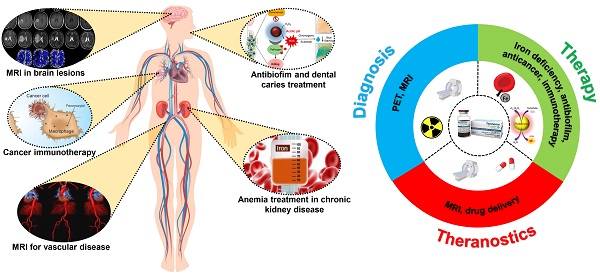
Ferumoxytol is an intravenous iron oxide nanoparticle formulation that has been approved by the U.S. Food and Drug Administration (FDA) for treating anemia in patients with chronic kidney disease. In recent years, ferumoxytol has also been demonstrated to have potential for many additional biomedical applications due to its excellent inherent physical properties, such as superparamagnetism, biocatalytic activity, and immunomodulatory behavior. With good safety and clearance profiles, ferumoxytol has been extensively utilized in both preclinical and clinical studies. Here, we first introduce the medical needs and the value of current iron oxide nanoparticle formulations in the market. We then focus on ferumoxytol nanoparticles and their physicochemical, diagnostic, and therapeutic properties. We include examples describing their use in various biomedical applications, including magnetic resonance imaging (MRI), multimodality imaging, iron deficiency treatment, immunotherapy, microbial biofilm treatment and drug delivery. Finally, we provide a brief conclusion and offer our perspectives on the current limitations and emerging applications of ferumoxytol in biomedicine. Overall, this review provides a comprehensive summary of the developments of ferumoxytol as an agent with diagnostic, therapeutic, and theranostic functionalities.
Keywords: ferumoxytol, magnetic resonance imaging (MRI), iron deficiency, nanozyme, drug delivery
 Global reach, higher impact
Global reach, higher impact