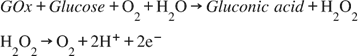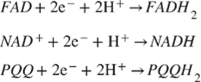13.3
Impact Factor
Theranostics 2022; 12(2):493-511. doi:10.7150/thno.64035 This issue Cite
Review
Glucose biosensors in clinical practice: principles, limits and perspectives of currently used devices
1. Department of Health Sciences, Magna Græcia University of Catanzaro, 88100, Catanzaro, Italy.
2. Department of Experimental and Clinical Medicine, Magna Græcia University of Catanzaro, 88100, Catanzaro, Italy.
Received 2021-6-18; Accepted 2021-10-31; Published 2022-1-1
Abstract
The demand of glucose monitoring devices and even of updated guidelines for the management of diabetic patients is dramatically increasing due to the progressive rise in the prevalence of diabetes mellitus and the need to prevent its complications. Even though the introduction of the first glucose sensor occurred decades ago, important advances both from the technological and clinical point of view have contributed to a substantial improvement in quality healthcare. This review aims to bring together purely technological and clinical aspects of interest in the field of glucose devices by proposing a roadmap in glucose monitoring and management of patients with diabetes. Also, it prospects other biological fluids to be examined as further options in diabetes care, and suggests, throughout the technology innovation process, future directions to improve the follow-up, treatment, and clinical outcomes of patients.
Keywords: assessment of glycemic control, glucose sensors, biological fluids, diabetes technology, point-of-care testing.
Introduction
Glycemic monitoring is currently a routinary and simple operation that is performed intensively worldwide [1,2]. Behind that, there is a long history of socio-demographic, technological and clinical practice [3-5]. The development of glucose sensors has accompanied the evolution of monitoring and treatment of diseases, in particular of diabetes mellitus, substantially improving glycemic control and preventing the rise and progression of diabetes-related complications [6]. Although enzyme-based glucose sensors have dominated the scientific research and the market, these systems inherently suffer from low thermal and chemical stability. Further, one time enzyme-based strips are costly, which discourages and limits frequent testing [7,8]. To account for these limitations, non-enzymatic sensors, and methodology for glucose monitoring in alternative biofluids, rather than blood, have been developed [9]. In recent years, novel materials have also been introduced, but, even with the advent of nanotechnology, the analytic performance of sensing devices is only possible to a certain extent [10]. Referring to glucose monitoring, there is still a demand for continuous and non-invasive detection with more reliable and sensitive devices. A debate on the correlation of glucose levels in alternative biofluids (e.g., tears, saliva, interstitial fluid (ISF), sweat, and urine), with respect to blood is still open and actual [11]. The skin has become very popular in recent years, so that new approaches/devices have been developed to minimize the invasiveness (e.g., short-term subcutaneous implantable sensors) [12]. Non-invasive systems can meet both the patient's needs and the clinical reliability of glucose detection. Studies on the improvement of new non-invasive monitoring systems are likely to continue to grow [4,10,13,14]. Furthermore, to achieve a wide use of these new technologies, the final detection device has to be developed at a very low cost to compete with the currently available blood glucose meters [15]. Consequently, efforts and new approaches to establish glucose monitoring based on alternative biofluids, whose reliability is comparable to that reached on blood, represent a promise for glucose monitoring in daily routine and an important goal for diabetic health care in the future [16-18]. In parallel, the daily clinical research and practice is an incentive for the development of international organizations that deal with the standardization in the use of these devices, whose accuracy is affected by manifold factors (e.g., pre-analytical sampling, sample characteristics and environmental parameters) [19]. The rationale behind this review is not simply to cover technological or clinical advances on glucose monitoring in the last years, but also to provide a global view on the topic using a transversal approach, and a combined vision on the evolution of glycemic control. To this end, we provide an overview of the technologies and methodologies for the evaluation and monitoring of glucose in blood and other less explored biological fluids. Furthermore, we discuss advantages and limits of the glucose biosensors currently used in clinical practice, and future directions that may implement glycemic control and clinical outcomes in the light of currently adopted treatments in diabetes care.
Assessment of glycemic control in diabetes mellitus
Diabetes mellitus (DM) is a widespread, clinically heterogeneous disease characterized by a chronic increase in blood glucose levels due to impaired insulin secretion and/or peripheral insulin resistance [20-23]. Insulin-dependent type 1 diabetes accounts for about 5-10 % of all cases of diabetes, and is characterized by a failure in insulin production for an autoimmune attack on pancreatic beta cells [24,25]. Type 2 diabetes, also known as “non-insulin-dependent” diabetes, is the most prevalent form of diabetes and is caused by a combination of genetic and environmental factors. In this latter form, the relatively low insulin production, due to a progressive beta cell dysfunction, frequently combines with a background of insulin resistance at the level of skeletal muscle, liver, and adipose tissue [26,27]. The most recent classification from the American Diabetes Association (ADA) includes other less common forms of diabetes, in addition to gestational diabetes mellitus, which can develop during pregnancy and usually resolves after delivery [28]. Regardless of the type of diabetes mellitus, many affected people develop a number of acute (diabetic ketoacidosis, hyperosmolar hyperglycemic nonketotic coma, severe hypoglycemia) and chronic microvascular (diabetic retinopathy, nephropathy and neuropathy), and macrovascular (coronary heart disease, cerebrovascular disease, and peripheral vascular disease) complications that are the leading cause of morbidity and mortality among these patients [29-31]. Hypertension, dyslipidemia and heart failure, which are primary predictors of cardiovascular mortality, are also significantly more common in diabetic than in non-diabetic individuals [31,32].
Glycemic control is crucial to prevent the rise and progression of diabetic complications [31,33], so that glycemic targets have been proposed in various specific settings [34]. Currently, the assessment of glycemic status can be carried out by the measurement of glycated hemoglobin (HbA1c), the assessment of self-monitoring blood glucose (SMBG), and the use of continuous glucose monitoring (CGM). HbA1c estimates the average blood glucose levels over approximately 3 months and is recommended 2-4 times a year, depending on patient's treatment goal. SMBG is indicated for self-management and pharmacological adjustments, especially in patients under insulin treatment, while CGM plays an important role in both prevention of hypoglycemia and therapy's effectiveness and safety in patients with type 1 diabetes or in selected cases of insulin-treated type 2 diabetes [35].
Even though a variety of predictive, diagnostic, and prognostic biomarkers (and related technologies) are continuously proposed, blood glucose concentration and HbA1c are still the major biomarkers for both diagnosis and patient monitoring [36,37]. The direct costs related to the management (diagnosis, monitoring, treatment, etc.) of diabetes accounts for billion dollars annually, besides the indirect costs from missed work or decreased productivity [38]. Since the invention of the first enzymatic electrode in 1962 [39], many efforts have been made to improve the performance of glucose sensors technology. Today, the development of devices for glucose monitoring with high reliability need new technologies and strategies to make those sensors more affordable, non-invasive, and suitable for continuous monitoring of glycemic status [40-42].
Evolution of glycemic monitoring
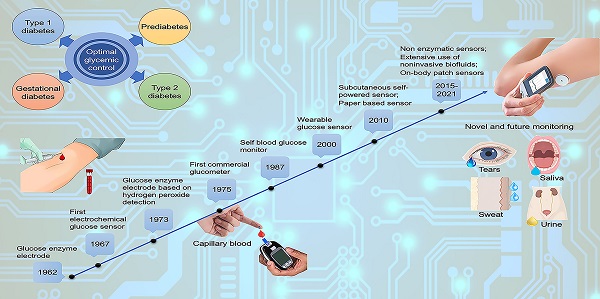
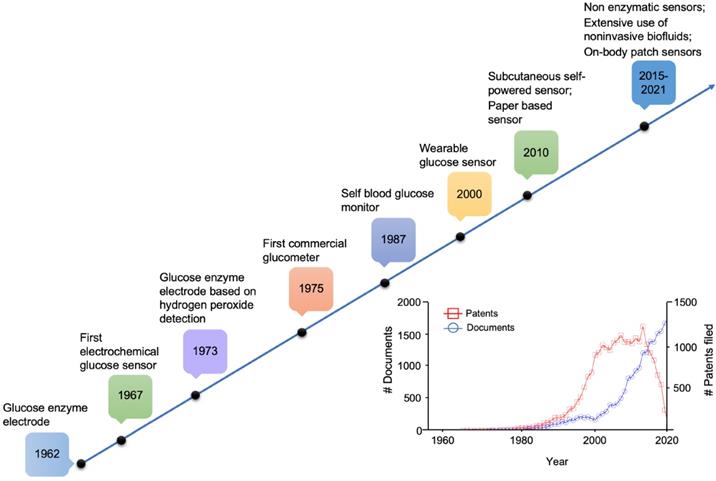
The concept of a glucose sensor was firstly proposed in 1962 by Clark and Lyons, who described an amperometric electrode for the determination of blood glucose through an enzymatic method, using glucose oxidase (GOx or GOD) [39]. This system represented an evolution of a previous electrode proposed by Clark for the determination of oxygen, intended to be mounted on an intravascular catheter [43]. The GOx enzyme catalyzes the oxidation of glucose, leading to the formation of hydrogen peroxide, so that a decrease in the oxygen concentration is proportional to glucose concentration [44]. However, the overall mechanism is strongly influenced by the background oxygen level, that adversely affects sensor's accuracy. In 1967, an electrochemical biosensor was proposed by Updike and Hicks using enzymes immobilized in a polyacrylamide gel on the surface of an oxygen electrode working in single or dual mode (single or dual cathode) [45]. In a dual cathode configuration, one electrode is coated with the active enzyme and the other with the same enzyme, but inactive (i.e., unresponsive electrode), to reduce the above-mentioned limitation of oxygen background concentration [45]. In 1973, Guilbault et al. described a glucose biosensor based on the amperometric monitoring of hydrogen peroxide originated from enzymatic catalysis [46]. Only in 1975, a first sensor for the direct glucose measurement was proposed. In fact, the technology developed by Clark and Lyons was transferred to Yellow Spring Instrument Company (YSI), which presented a whole blood analyzer, Model 23 (a platinum electrode mainly for clinical use due to its cost). As shown in the timeline in Figure 1, starting from the 1960s onwards, the development and application of glucose sensor in the medical sector has aroused considerable interest in both the academic and industrial fields.
Milestones in the development of actual glucose sensor systems technology. In the inset, available literature and estimated patents filed involving glucose sensors (1955-2020). Sourced from Scopus (blue) and Google (red).
The above-mentioned sensors belong to the “first generation” of glucose sensors, which exploit an oxygen electrode, acting as a substrate, and the production of hydrogen peroxide. Intense efforts during the last decades have led to the development of the so-called mediator-based “second generation” of glucose sensors [47,48], the introduction of commercial strips for SMBG [49,50], and the use of further modified electrodes for enhancing the performance [51]. Advancements were obtained by replacing oxygen with a non-physiological (synthetic) electron acceptor (mediator) capable of transporting electrons from the center of the enzyme to the electrode surface. The transfer of electrons between the enzyme active site and the electrode surface is the limiting factor in the functioning of the amperometric glucose sensors [47]. These mediator-based sensors increase the rate of electron transfer between the electrode and the enzyme. Further studies were focused on the development of a technology that did not need any mediator to obtain a reagent-free glucose sensor. The “third generation” of such sensors is based on reagentless devices, and therefore on the direct electron transfer between the enzyme and the electrode without the use of mediators, which are usually toxic. The main advantage is the high selectivity since the working potential is identical to that of the enzyme and, therefore, less prone to any interference [47,48]. More recently, devices based on the direct electro-oxidation of glucose are being proposed as a possible “fourth generation” of sensors, which mainly use noble metals as catalyst to overcome the limits of enzymatic sensors [49]. Despite the target is the glucose detection in complex sample matrix (tears, saliva, ISF, sweat and urine), in most cases, equivalent samples were investigated for glucose performances. Hereafter, literature review is mainly limited to biological samples, which can provide reliable clinical outcomes.
Sensor reliability
Whether they are in development or already in clinical practice/home environment, concerns about the reliability of sensors are still cause of discussion. The gradual evolution of the glycemic monitoring provides results in a few seconds from only 0.3-1 μL of blood (e.g., SMBG) [50-52]. Therefore, the accuracy of glucose sensor (often referred to glucometer) represents still today a debated issue. In fact, glucose levels in the same sample should ideally be compared to a reference or comparative method. Unfortunately, this is technically difficult due to the small volume of capillary blood that can be obtained. This process is also cumbersome because the glucometer measures a whole blood sample, in which glucose levels are unstable due to glycolysis. In fact, blood cells, and in particular erythrocytes, metabolize glucose and may progressively reduce its concentration at a rate of 5-7 %/h as long as plasma remains in contact with the erythrocytes [53]. Therefore, the use of whole blood samples for accuracy comparisons requires consideration of the effects of glycolysis and separation of plasma from the cells for laboratory analysis within a reasonable period (generally within 30 min). However, accuracy comparisons are often conducted on a capillary sample analyzed by a glucose meter versus a venous plasma sample collected at the same time and analyzed with a laboratory method (e.g., venous samples collected in lithium heparin containing tubes) [54,55].
Error grid analysis proposed by Clarke et al., for clinical accuracy (A), and further modified by Parkes et al., for type 1 (B) and type 2 (C) diabetes. System bias plot (D), dashed black lines indicate the predetermined accuracy limits. FS represents the full-scale level for glucose concentration of the tested and reference sensor. Classically, it is set at 400 mg/dL (blood-based sensors).
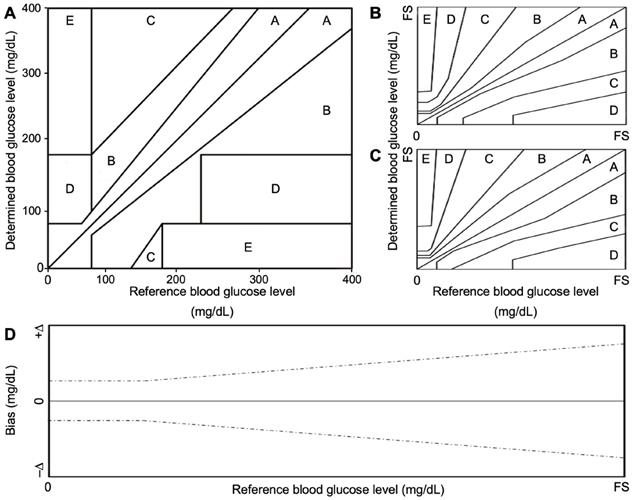
In 1987 Clarke et al. attempted to address clinical agreement by developing an error grid that evaluated the clinical significance of the determined glucose level of a device under test, versus a gold-standard method (Figure 2A) [56]. Clarke error grid is divided into 5 precision zones. A slight deviation (< 20%), or results in the hypoglycemic range (< 70 mg/dL) between the two methods fall within zone A. B zone is characterized by a higher deviation (> 20%) and would not lead to changes in the clinical decision. Conversely, significant differences from the gold-standard method fall in zones C, D or E. The first is characterized by overcorrected acceptable results, while zone D and E represent “potentially dangerous errors to detect and treat” and “erroneous treatment”, respectively [56]. In 2000, this grid was further modified by Parkes et al., for type 1 and type 2 diabetes (Figure 2 B, C, respectively), to avoid discontinuity between risk areas, in which small changes in glucose levels could lead to significant changes of clinical impact (Parkes error grid or consensus error grid) [57]. The above-mentioned analysis was proposed for “clinical accuracy” purpose, to quantify the probability of making a correct therapeutic decision based on the obtained result with a glucometer. Analytical accuracy, instead, is evaluated by comparing the bias between the result provided by the device under test and a gold standard method (specified by the manufacturer) (Figure 2D) [57,58].
Standard organizations and scientific societies differ on the acceptability criteria for accuracy, so there is no single standard for evaluating the accuracy of a blood glucometer. Regulatory organisms worldwide provide and continuously update standards describing the requirements for blood glucose monitoring systems for health care professionals and lay users [59-65]. Table 1 refers to the main standards developed by international organisms for standardization [60-64].
Representative international standards for assessment of acceptable performance
| Regulatory Organism | Country | Device | Standard | Year | Ref. |
|---|---|---|---|---|---|
| Food and Drug Administration | USA | POCTs | FDA-2013-D-1445 | 2020 | [60] |
| Food and Drug Administration | USA | OTC BGMS | FDA-2013-D-1446 | 2020 | [61] |
| International Standard Organization | 165 countries | BGMS | ISO 15197 | 2015 | [62-64] |
| Clinical and Laboratory Standards Institute | >50 countries | POCTs | POCT12-A3 | 2018 | [62] |
POCT, point-of-care test; OCT, over-the-counter; BGMS, blood glucose monitoring test systems; FDA, food and drug administration; ISO, international standard organization.
The analytical performance of the instrument also depends on what purpose the information obtained is being used for: screening, diagnosis, or management. Upon proper comparison, it may be discovered that glucometers should not be used to diagnose diabetes but may instead be suitable for patient monitoring and insulin management [66]. There is open debate on how international standards should be applied and improved when comparing glucometers for automonitoring and laboratory devices [67-73]. Sensors must have certain characteristics to be efficient and of quality. In 1994, the ADA made the first recommendations for the analytical performance of glucose biosensors available, suggesting a threshold <10 % of maximum permissible bias from reference methods for glucose concentrations between 30 and 396 mg/dL. This analytical target was further reduced to <5% in 1996 [52]. According to the Food and Drug Administration (FDA) recommendations, glucose sensors must have an error <20% for glucose concentrations between 30 and 396 mg/dL compared to reference laboratory measurements. The intermediate accuracy of a sensor is defined as accuracy under conditions where test results are obtained by the same method on the same type of sample, in the same position, but where other variables such as operators, equipment, calibration, environmental conditions and/or time intervals differ. Repeatability is evaluated at five diffuse glucose concentrations in the measurement range (30-50 mg/dL, 51-110 mg/dL, 111-150 mg/dL, 151-250 mg/dL, and 251-400 mg/dL) and should be measured over a short period of time, with the same group of users, meters, and reagents. The preferred sample is venous blood. The evaluation of all these, and other parameters allow the evaluation of the technical precision of the device under examination; in the case of biosensors, it is then necessary to complete the study of the functionality of the instrument alongside the evaluation of the clinical precision of the sensor, which consists of comparing the results obtained on a sample by using the sensor in question with a standard laboratory method. The data can be entered into the Clarke or Parkes error grid to allow clinicians to make the right decision without compromising patient's health.
Classification of glucose sensors
Electrochemical glucose sensors can be divided into potentiometric (employed to detect variations of surface charge onto a counter electrode), amperometric (charge flow between the counter electrode and the bio-system), or conductometric (variations in ionic conductance between electrodes) [74,75]. As stated above, over the years, enzymatic amperometric glucose sensors were the first and widespread glucose sensors available [76]. They are generally fabricated by using two families of enzymes, the glucose oxidase and the glucose dehydrogenase (GDH). The reaction, catalyzed by the GOx, is as follows:
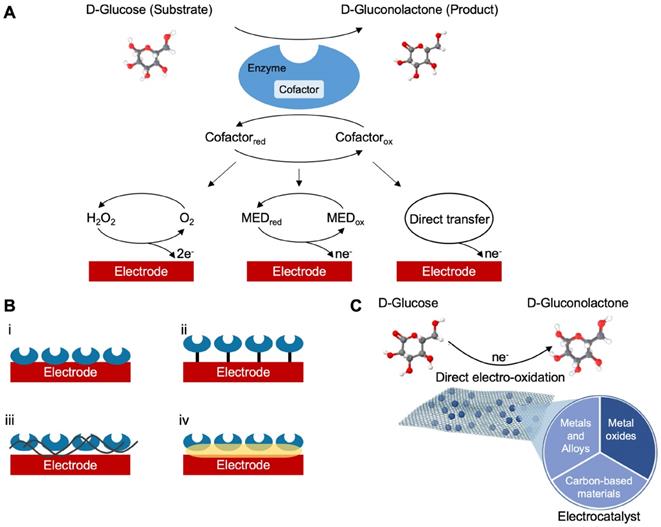
In many complex sample matrices such as blood, the dextrorotatory enantiomer of glucose (D-glucose) equilibrates among α-D-glucose, β-D-glucose structures (> 99.9%), and aldehyde form. Since GOx is inherently highly selective for β-D-glucose, preparations require the interconversion among these forms (enzyme mutarotase, phosphate ions etc.). Since GDH is also selective for β-D-glucose, the same reaction can be catalyzed by replacing GOx. The concept behind a glucose biosensor is based on the fact that the immobilized GOx catalyzes the oxidation of D-glucose by molecular oxygen producing gluconic acid and hydrogen peroxide (Figure 3A). To function as a catalyst, GOx requires a flavin adenine dinucleotide (FAD) redox cofactor. FAD functions as an initial electron acceptor and is reduced to FADH2 [76]. This enzymatic electrode evaluates the glucose level by the amperometric tracking of the released hydrogen peroxide [76]. Glucose dehydrogenases are instead defined as oxidoreductases which are unable to use oxygen as an electron acceptor and therefore transfer electrons to other natural and artificial acceptors. GDHs also need a cofactor. These are mainly nicotine adenine dinucleotide (NAD+ or NADH depending on the oxidation state) or pyrroloquinoline quinone (PQQ) [45]. FAD, NAD+ and PQQ remove hydrogen, H+ and e-, from glucose according to the following:
Natural acceptors can be replaced by artificial electron acceptors such as ferrocene and its derivatives, phenazine methosulfate (PMS), or phenazine ethosulfate. The pure carbon surface is also an improper material used as an electron acceptor from the active center of the enzyme [77]. GDH-PQQ is a particularly efficient enzyme system, with a fast electron transfer rate, but it is relatively expensive. GDH with NAD+ as a cofactor produces NADH rather than H2O2. Nicotine adenine dinucleotide is an important electron acceptor in glucose oxidation, during which NAD's nicotinamide ring accepts one hydrogen ion and two electrons, equivalent to one hydride ion. In this reaction, the generated reduced form of this cofactor is NADH, which can be electrochemically oxidized. As previously introduced, based on the use of specific enzymes and co-factors, glucose sensors can be classified as reported in Table 2.
(A) Schematic classification of the glucose biosensors evolution distinguished into generations according to the sensing mechanism. (B) Representative enzyme immobilization techniques: i, adsorption; ii, covalent bonding; iii, cross-linking; iv, entrapment. (C) Schematic representation of a characteristic direct electro-oxidation of glucose in non-enzymatic glucose sensors and the most investigated materials used as catalyst.
Classification of enzymatic glucose sensors
| Classification | Characteristics |
|---|---|
| 1st Generation | Based on the sensor designed by Clark and Lyons Formation of hydrogen peroxide Oxygen as an electron acceptor Errors due to interference from other electroactive species |
| 2nd Generation | Replacement of oxygen as an electron acceptor Introduction of the non-physiological mediator Limitations in the transfer from the enzymatic active site to the electrode |
| 3rd Generation | Absence of mediator Direct transfer between enzyme and electrode Low operating potential, higher selectivity, less interference |
An electrochemical biosensor is composed by working electrodes (on which the reaction of interest, responsible for the measurement, takes place), reference electrodes and auxiliary electrodes (to ensure that the current does not circulate through the electrode). Glucose concentration is mostly evaluated using the amperometric method, that monitors the current flowing between the working electrode and the reference electrode. The latter involves the application of a potential, that in turn results in the contribution of other electroactive species (e.g., ascorbic acid, uric acid) reducing the selectivity of the electrode. Another limiting aspect of the enzymatic glucose sensor (especially those of the first generation), is due to the oxygen deficit inside the sample. To this end, the use of additional membranes, mediators, and electrocatalysts were investigated [78]. The role of mediators is crucial in glucose electro-oxidation. For example, FAD-GOx provides a low-rate of oxidation and thus the use of mediators allows a rapid glucose oxidation, giving reliable results. Different families of materials were used as redox mediators for FAD-GOx, PQQ-GDH, and NAD-GDH electrodes, such as those of ferrocene derivatives (e.g., ferrocenecarboxylic acid, ferrocenemethanol), osmium complexes (bis-(2,2'-bipyridine) osmium(II), bis-(4,4'-dimethyl-2,2'-bi-pyridine) osmium(II)), ruthenium (ruthenium hexamine), and organic mediators (quinone derivatives) [79,80].
The optimal performance of the electrodes within a sensor requires the choice of suitable materials in relation to the kind of enzymatic reaction taking place. In addition, the selectivity and sensitivity of the reaction strongly depend on the characteristics of the working electrode. In general, sensitivity can be modulated by adjusting the surface area of the working electrode [81]. Porous electrodes, for example, lead to higher sensitivity than planar electrodes because porous electrodes have a larger surface area to accommodate the chemical reaction. An inherent sensitivity to environmental conditions (pH, temperature, humidity and chemical condition of the sample) is one of the bottlenecks of the enzyme-based technology, which reduce the stability of the sensors, especially in those applications in which environmental conditions are not controllable (continuous glucose monitoring, wearable devices). Another factor that determines the quality of the electrode in glucose measurement is the immobilization of the enzyme on the electrode: the permanent immobilization leads to reliable and long-term performance. For this to happen, the enzyme is cross-linked with hydrogel (e.g., chitosan and gelatin), nanomaterials (e.g., carbon nanotubes and graphene) and other stabilizers (e.g., bovine serum albumin) by chemical and physical bonds. Table 3 shows a non-exhaustive list of materials used for the fabrication of working electrodes, the reference enzyme (GOx) to catalyze the reaction and the immobilization technique (Figure 3B) [7,76,77].
Representative substrate enzyme immobilization method
| Substrate | Enzyme | Immobilization |
|---|---|---|
| Au/Ag-NCs | GOx | Trapping |
| ITO/ Chitosan-Polypyrrole Au-NPs | GOx | Trapping |
| Ag/CNT/Chitosan | GOx | Layer technique |
| BDD/Graphene/ Pt-NPs | GOx | Absorption |
| Si/ VACNF | GOx | Absorption |
| Graphite NPs | GOx | Covalent bond |
NC, nano cube; ITO, indium tin oxide; NP, nano particle; CNT, carbon nano tube;
BDD, boron-doped diamond; VACNF, vertically aligned carbon nanofiber.
Apart from the above reported amperometric biosensors, reference method for the clinical determination of glucose using spectrophotometry in many laboratories is based on hexokinase (HK) assay. In this case HK catalyses the phosphorylation of glucose (glucose-6-phosphate) using adenosine triphosphate (ATP), becoming adenosine diphosphate (ADP). Bacterial glucose-6-phosphate dehydrogenase (G-6-PDH), in the presence of Nicotinamide adenine dinucleotide phosphate (NADP), causes the oxidation of glucose-6-phosphate to gluconate-6-phosphate. The production rate of NADPH is directly proportional to glucose concentration (and is measured photometrically at 340 nm), according to the following reaction:
This test is a reference method for the quantitative determination of glucose in serum, in human plasma, urine and cerebrospinal fluid [52].
The novel concept of nonenzymatic sensors is being proposed for their foreseen performances in terms of stability (some evidence report sufficient stability up to 30 days in complex samples), easier fabrication process (Figure 3C), besides the very high sensitivity and the variety of sensor materials that are currently investigated [49]. Obviously, the design of glucose sensors should consider parameters such as sensitivity and limit of detection (LOD), especially in relation to the biofluid used to identify the best suited technology. Non enzymatic sensors based on metal, metal-oxide, or based on carbon (carbon nanotubes, graphene) are characterized by a higher sensitivity with respect to enzymatic counterparts [82-86]. For example, non-enzymatic sensors based on carbon nanotubes decorated with Nickel evidenced a sensitivity of 70 mA mM-1cm-2, which is outstanding if compared with GOx based sensors with a sensitivity that, in most cases, is different order of magnitude lower [87]. A similar conclusion can be observed considering LOD, even though, it evidenced a higher degree of variability, non-enzymatic sensor reaches more often LOD on the nM range. Despite a vast scientific literature on non-enzymatic glucose sensors has been produced in the last years, the technology has not reached the commercial phase and thus clinical experience is still limited. Sensor stability represents a major concern in CGM devices, which are expected to provide reliable data for a sufficient timeframe (the devices currently on the market are indicated for 7 days of use requiring multiple calibrations per day). However, even though invasive, extended time CGM based on a subcutaneous approach is able to provide accurate measurement for up to 1 year. The latter, generally requires 2-3 weeks stabilization period after surgery and are larger than the subcutaneous counterparts [88]. Despite recent data from nonenzymatic sensors evidenced a higher sensitivity with respect to enzymatic counterparts, they still suffer of a poor stability (i.e., surface fouling of the electrode) and lacks glucose selectivity [49]. It is widely known that enzymatic sensors such as those based on glucose oxidase are more stable than other enzymes, easy to obtain, and cheap; on the other hand, it quickly loses its activity at pH<2 or pH>8 and can be irrevocably damaged at temperatures above 40 °C [76]. Also, it is affected by environmental unstable humidity before its use [77]. Despite the chief motive of this review is to provide a multidisciplinary approach for a wide audience in the technical/clinical field, being a highly attractive area, a vast literature is witnessed by systematic readings that can be reached to deepen technological advancements on enzymatic, nonenzymatic, and optical based glucose sensors [4,9,49,50,51,75,76,78,79,88].
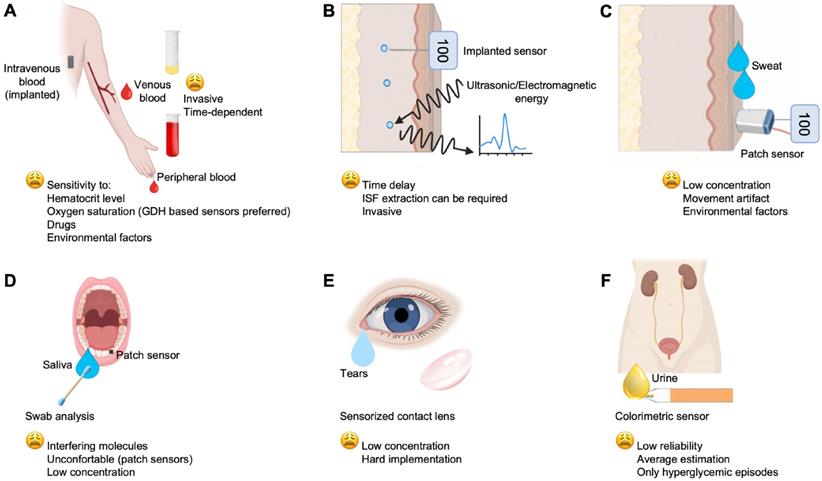
Main biofluids and technologies investigated for glucose monitoring, which include: (A) the gold standard venous blood and the widespread used peripheral blood for auto-monitoring; (B) ISF; (C) sweat; (D), saliva; (E) tears; (F) urine.
Representative commercial and non-commercial glucose sensor for laboratory settings and SMBG
| Manufacturer | Sample | Type | Sensor material | Method | Sensitivity | Linear Range (mg/dL) | LOD (mg/dL) |
|---|---|---|---|---|---|---|---|
| Commercial | |||||||
| Roche Cobas [92] (laboratory) | S, P, U, CSF | Enzymatic (G6PD/NADP) | - | Photometric | 1.003§ | 2-750 | 2 |
| Roche Accu-check [93,94] (SMBG) | PB | Enzymatic (GDH/FAD) | Palladium | Electrochemical | 0.127 μA/mM | 10-600 | 10 |
| Non-Commercial | |||||||
| Yang et al. [95] | Glc/NaOH* | Non-enzymatic | PDDA-graphene/CuO | Amperometric | 4982.2 μA mM-1cm-2 | 0.072-72 | 0.004 |
| Zang et al. [96] | WB | GOx/HRP | TMB/GOx/HRP bi-enzymatic | Photometric | 1.1 (a.u.)/(mg/dL) | 49-284 | 5 |
| Màrquez et al. [97] | WB | Enzymatic (GOx/HRP) | Calcium alginate hydrogel | Amperometric | 0.27 μA mM-1cm-2 | 36-218 | 0.007 |
S, serum; P, plasma; U, urine; CSF, cerebrospinal fluid; PB, peripheral blood; WB, whole blood; LOD, limit of detection; PDDA, poly-dimethyl diallyl ammonium chloride; TMB, 3,3', 5,5' tetramethylbenzidine dihydrochloride; HRP, horseradish peroxidase. §angular coefficient provided by the linear regression (calibration curve y = 18.07·x-0.11 mg/dL). *Glucose in alkaline solution.
Another way to disentangle the variety of devices developed/commercialized can be changing the sample type used. Venous and peripheral blood glucose concentration (Figure 4A) is the main basis for the diagnosis, monitoring and treatment of diabetes, but for auto-monitoring, other biological fluids can be also used for glucose determination; for example, ISF, sweat, tears, saliva and urine. Currently, the World Health Organization (WHO), has established the expected values for fasting blood glucose (FBG) of normal, euglycemic people, at 70-100 mg/dL, and the blood glucose after 2 h from oral glucose tolerance test (OGTT) at < 140 mg/dL (https://www.who.int). Indeed, it is remarkable that glucose levels in biofluids, such as ISF (Figure 4B), sweat (Figure 4C), saliva, tears, and urine (Figure 4 D, E, F, respectively) are correlated with those in the blood [76]. Therefore, many studies have focused on these biofluids to develop noninvasive sensors and methods for glucose monitoring. Most importantly, glucose level in these fluids is lower than that in the bloodstream. Its concentration range varies between 36-720 mg/dL in blood, 36-400 mg/dL in ISF, 0.0001-32 mg/dL in saliva, 0.00018-20 mg/dL in sweat and 0.0009-90 mg/dL in tears [11]. Hereafter, glucose monitoring is described according to the site of measurement and the applied technologies.
Blood glucose monitoring
Most of the gold standard tests for clinical diagnostics exploits the use of blood, and glucose is no exception [89]. However, blood sampling is invasive and expensive for a large number of tests per day. Alternatively, implantable sensors can be used for continuous glucose monitoring. SMBG mainly refers to the monitoring of peripheral blood glucose concentration in a specific time of sampling [90]. Blood sampling at capillary level results in higher glucose concentration than in venous blood and is affected by the metabolic state [91]. Currently, there are different commercial SMBG (e.g., Roche, Sano, Omron, Johnson and Johnson, Bayer, Abbott, Echeng, Ecco, etc.) (Table 4) [92-97] Apart from the invasive blood sampling, the portability, and the relative simplicity of operation, together with their relative high accuracy, have allowed a wide diffusion of these devices. However, being used multiple times a day, their relative cheapness is apparent only.
Role of the pre-analytical phase
Factors affecting accuracy of results may occur in any step of the diagnostic process. In this context, pre-analytical aspects are of crucial importance [98]. Operators should standardize the use of SMGB by reactivating circulation in the finger chosen for the puncture by massaging the hand from the palm to the fingertips; hands need to be preferentially washed with water and soap and dried; an appropriate lancing device and puncture depth should be set. The first blood drop is generally considered the best biological matrix to be used [99]. Milking the finger, instead of massaging, should be avoided. Opened or expired test strips can also represent a source of SMBG inaccuracy and should not be used.
Glucose sensor inaccuracy in specific clinical settings
Many variables, including hematocrit, hypoxemia, hypotension, temperature, altitude, and humidity may influence the reliability of glucose measurements [52,100]. In strip-based glucose assays, high hematocrit values, due to increased blood viscosity, can reduce the diffusion of plasma and determine underestimated results, whereas low hematocrit levels, like in anemia, are associated with overestimated readings [101,102]. Most glucose biosensors are, therefore, reliable only if hematocrit value is not far from the normal range.
Glucose oxidase-based devices are sensitive to oxygen and should be only used with capillary blood from patients with normal oxygen saturation. In case of higher oxygen tension (oxygen therapy or arterial blood), glucose readings are falsely low, while in case of lower oxygen tension (hypoxia, high altitude, venous blood), test results are falsely high [100,103]. Therefore, for patients in critical care, Point-of-Care Test (POCT) should prefer the use of glucose dehydrogenase-based monitors since they are not sensitive to oxygen. These latter devices are not recommended in patients on peritoneal dialysis, in which the osmotic agent icodextrin, a widely used glucose polymer, may cause falsely elevated glucose readings and improper insulin administration in patients with diabetes [104,105]. In the case of glucose oxidase-based monitors, physiological and pharmacological interfering substances for glucose readings include uric acid, galactose, xylose, L-DOPA, acetaminophen, and ascorbic acid [100,106,107].
Glucose monitoring in interstitial fluid
Glucose levels can be monitored in the extracellular fluid that surrounds tissue cells, i.e., ISF (Figure 4B). In tissue, the cells are not directly in contact with the capillaries, while exchanges between blood and cells are mediated by ISF, thus allowing the passage of electrolytes, nutrients, and waste, as well as hormones [98]. Glucose measured in the ISF represents a good indicator of blood glucose level due to the continuous supply of this nutrient from vessels to the interstitial area. The diffusion of glucose from the capillary to the ISF occurs, however, with a short delay of 5-10 min, thus limiting the reliability of results in hypoglycemic emergencies [99].
Iontophoresis or reverse iontophoresis is based on the injection of current through the measurement site, which causes migration of ions and glucose from the ISF to the surface and electrodes [100]. Ion migration generates the charge flow, while the glucose (uncharged) is transported exploiting the current, by convective flow [101]. Thus, it is collected in the passage through the skin, at the cathode where a glucose biosensor is placed. Glucose extraction is approximately in linear relation with the density and duration of iontophoretic current (<0.5 mA/cm2) [102]. The main drawback of this technique is that it may cause skin irritation, mainly in case of longer exposures to iontophoretic treatment. On the other hand, a minimum duration is required to get enough glucose for measurement [103,104]. Sweat represents an interference factor. Also, concerns are raised to whether this technology can reflect rapid changes in blood glucose (common to all the non-blood glucose sensors). Sonophoresis involves low frequency ultrasonic wave in order to increase skin permeability and have access to ISF [105]. Extracted ISF can be easily analyzed externally by optical/electrochemical glucose biosensor [106]. In-vivo microdialysis is an important technique in CGM devices; glucose is sampled from the body by a subcutaneous probe consisting of a semipermeable hollow fiber. The membrane surface of the probe is biocompatible and safe for the patient. The sensing electrodes, located in an external unit coupled to the probe, are exposed to a relatively clean sample solution, which makes them less prone to biofouling and more accurate than implantable needle biosensors [107]. Although results provided by microdialysis are sufficiently precise, this technique is not yet commonly used due to the high costs of the instrumentation. Furthermore, the instruments are bulky and cannot be worn during daily activities [108]. Dexcom G6 (Dexcom), Guardian REAL-time (Medtronic) and FreeStyle Navigator (Abbott) are examples of few minimally invasive devices, which are currently available in the market. These devices use subcutaneous sensors to determine glucose concentration in ISF. In optical devices, glucose concentration is evaluated through the interaction with light (reflection, absorption, scattering) and represents a viable and investigated approach. Near infrared (NIR) spectroscopy is a method that uses a light beam with a wavelength in the near-infrared range of the electromagnetic spectrum (750-2500 nm). When infrared radiation hits a molecule in patient's skin, it releases energy and the molecule vibrates (stretching or bending vibration), producing an oscillating magnetic field due to changes in its dipole moment. NIR spectroscopy allows the measurement of glucose in tissues with a depth of 1-100 mm. It uses three basic measurement modes: transmittance, reflectance (including diffuse reflectance), and interactance. The light beam is partially absorbed and then diffused due to the interaction between light and chemical components present in the tissues. Glucose concentration is estimated as variations in the intensity of the light transmitted and reflected in a tissue [103]. The major problem with non‐invasive optical sensors is the specific measurement of glucose with sufficient analytical quality [109].
A complimentary alternative to NIR is represented by Raman spectroscopy, which was recently proposed as a non-invasive glucose sensing technique, even though the commercial application is still to be demonstrated [110]. The LOD in this case is in the mM range, which together with the low sensitivity, has required the need for a robust chemometric analysis [110,111]. It was investigated as non-invasive tool in ISF and in different other tissues and, in particular cases, Raman spectroscopy was applied to anterior aqueous humor since it is correlated with blood glucose, providing at the same time few optically active (interfering) molecules [110-114]
Optical coherence tomography (OCT) scans the biotissues with low coherent light source. The refractive index mismatch (Δn) between cells and ISF and the scattering properties in the dermis, gives information about glucose concentrations according to the principles of low coherency interferometry. OCT can typically produce an image at a tissue depth of up to several mm with a very high resolution (<10-15 μm). The dispersion properties of the tissue are highly dependent on the ratio between the refractive index of the diffusion centers (cellular components, proteins, etc.) and that of the ISF. An increase in the concentration of glucose in the ISF causes an increase in its refractive index, thus determining a decrease in the dispersion coefficient. Therefore, starting from the OCT data, generated by the backscattered light, it is possible to obtain an estimate of glucose concentration in the ISF. However, OCT is sensitive to motion artifact due to the inhomogeneity of tissue [115,116].
Photo-acoustic spectroscopy combines a light emitted from a laser with the acoustic response produced. The ultrasonic transmitter measures the peak-to peak variation of pressure waves due to absorption. First in-vivo scientific evidence highlighted greater sensitivity in the determination of glucose than other more traditional spectroscopic techniques and this is due to the relatively poor photoacoustic response of water, which facilitates the determination of some compounds, such as glucose and hydrocarbons [117,118].
Fluorescence is based on the generation of fluorescence by glucose molecules in blood when excited by lights at specific frequencies (308 nm). Fluorescence intensity is dependent upon glucose concentration, and glucose levels in tears reflect concentrations similar to those in blood [119]. There are few fluorescence-based glucose detection methods that have reached the stage of testing in vivo, and none has been approved for clinical practice in diabetes management. Spectroscopic techniques are inherently characterized by a higher stability (>30 days), paid by a lower sensitivity and LOD. However, when the analysis is mediated by the presence of an external substrate (e.g., Surface enhanced Raman spectroscopy, fluorescent nanomaterials etc.), the stability can be negatively influenced [120-122]. Although a large literature is present on non-invasive glucose monitoring, the research of novel non-invasive techniques is still in progress. Table 5 provides a summarization about the reported technologies [103,104,110-114,117-119,123-145].
Glucose monitoring in sweat
Sweat-based glucose sensors offer a further theoretical alternative to non-invasive methods for glucose measurement (Figure 4C). However, attention must be paid on the complex, and variable chemical composition of sweat. For example, sweat collection and detection vary, depending on the environmental conditions. In addition to the difficulty of measuring glucose levels in sweat due to its much lower concentration than in blood, lactic acid content in sweat, changes in environmental temperature and various medications can induce errors in enzyme-based glucose detection. Mechanical friction and deformation of the devices on soft human skin might delaminate the enzymes from the sensor, causing mechanical fractures that could affect the glucose estimation [146]. An innovative approach to sweat-based glucose measurement was investigated by Saraoglu et al., based on the combined use of humidity sensor and artificial neural networks (ANN). A comparison of glucose measurements obtained in sweat and in blood showed a relative error ranging between 2.90%-15.81% and 5.13%-16.25%. These relative errors were established for blood glucose measurements from human palm perspiration [147]. Gao et al. pioneered another example of a fully integrated flexible sensor array platform (FISA) for in situ sweat analysis, able to measure multiple sweat metabolites (glucose and lactate) and electrolytes (sodium and potassium), as well as skin temperature in a wearable patch-type platform (patch) [148]. It was proposed that the increase in sweat rate and duration led to the dilution of sweat glucose over time, which corresponded to an observed increase in skin temperature. However, different parts of the body showed different rates of sweat, which led to varying concentrations of analytes at a given time due to the dilution effect. Therefore, the study indicated that careful assessment of sweat composition and environmental parameters is required for accurate blood glucose monitoring [149]. Wang et al. developed a non-invasive, wearable glucose monitoring platform in the form of an amperometric glucose sensing tattoo on a flexible substrate comprising iontophoretic and glucose sensing electrodes [149]. Although the tattoo-based device was designed for single-use, such a sensor offers considerable promise for continuous blood glucose monitoring in the non-invasive ISF by offering a body-compatible, flexible, and cost-effective platform [150]. Similarly, there are also a series of patch sensors that are used to assess blood glucose via sweat (or via ISF, but with a minimal invasiveness caused by microneedles) for a day. However, the requirement to sweat whenever a measurement is to be made may be inconvenient or impractical for many potential users [151]. Table 6 provides a non-exhaustive summarization above the reported technologies [18,147,148,152].
Glucose monitoring in saliva
Saliva can be collected in a non-invasive way, without the need for specific equipment or trained personnel. Since saliva collection requires fewer skills than blood collection, its analysis is more valuable for children and the elderly. Saliva analysis can also provide a cost-effective approach for screening large populations [153]. However, various impurities in saliva from ingested food and digested metabolites can hinder accurate measurement of glucose concentration (Figure 4D). In general, glucose in saliva can be measured after filtering large biomolecules mixed in saliva [154]. Glucose moves easily from plasma through the membranes of the blood vessels to the gum fluid, through the gingival sulcus, reaching saliva. Therefore, hyperglycemia in diabetic patients could lead to higher salivary glucose, whose concentration at this level is typically in the range of 0.5-1 mg/dL, thus much lower than glucose in the blood [155-158]. Recent developments in highly sensitive materials are paving the way for generating easy and low-cost methods for acquiring salivary glucose (see Table 7) [155-158]. For example, Macaya et al. developed electrochemical sensors using a transistor with a channel consisting of poly (3,4-ethylenedioxyitophene): poly (styrene sulfonate) (PEDOT: PSS) and a Pt electrode with a minimum detection limit of 1 μM (≃ 18•10-3 mg/dL) [159]. In addition to being a valid alternative for glucose measurement, saliva can be used as a versatile biofluid for evaluating other clinically significant analytes (e.g., lactate and cholesterol). However, there are limitations in mouthguard devices as they are uncomfortable for long-term use, can lead to adverse effects related to dental conditions, are strictly site-specific, and the amount of these biofluids is limited [159-161].
Main technologies and characteristics of the glucose evaluation in ISF
| Technologies | Type | Advantages | Disadvantages | Measurement site | Performance | Ref. |
|---|---|---|---|---|---|---|
| Electrical | Impedance Spectroscopy | It can measure glucose levels in the vascular compartment, so no time lag in sensor response Low-cost instrument | Temperature and diseases affecting skin may affect measurements Changes in blood dielectric properties may cause error in measurement | Skin, wrist | Sens: 0.02-0.05 Ω/(mg/dL) | [103, 104] |
| Optical | Raman | Non-invasive sharp spectral features Broad SERS substrate enhancement | Weak Raman signal Background noise | Wrist, finger, aqueous humor | [110-114] | |
| Optical | OCT | Real time monitoring High resolution High signal to noise ratio High penetration depth Robust to blood pressure, heart rate, and haematocrit variability. | sensitive to motion artifacts sensitive to large changes in temperature | Forearm | Sens. 0.015-0.045 a.u./(mg/dL) | [117,118,123] |
| Optical/Mechanical | Photoacoustic spectroscopy | It is not affected by ionic strength. Higher sensitivity | Problem of scattering in the tissues Sensitive to environmental parameters, chemical interferences from other metabolites, physical interference from temperature and pressure changes. | Aqueous humor, finger and forearm | Sens. 0.035-0.098 /100(mg/dL) | [118,124] |
| Optical | Fluorescence | No damage to the body Information about the structure and micro-environment of molecules | Scattering phenomena Fluorescence can depend on skin pigmentation, redness, epidermal thickness | Skin | Range: up to 454 mg/dL | [119] |
| Optical | NIR | Skin penetration up to 1-100 mm High sensitivity Low cost and widespread | Poor signal to noise ratio Calibration issues Baseline drift Thermal noise Physiological factors Environmental factors | Tongue, oral mucosa, lip, ear lobe, finger, forearm, cheek. | Range:30-300 mg/dL and up to 600 mg/dL | [125-143] |
| Electrochemical | Iontophoresis | No mechanical hardware Simpler concept | Filtered ISF and thus more similar to sweat or saliva Skin irritation over long term Onset sweating | Skin, wrist, forearm | [144] | |
| Microwave | No-ISF extraction is required Flexible substrate | Lower precision | Skin | Range: 36-454 mg/dL | [145] |
Representative characteristics of non-commercial sweat-based glucose sensors
| Manufacturer | Type | Sensor material | Configuration | Sensitivity | Linear range (mg/dL) | LOD (mg/dL) | |
|---|---|---|---|---|---|---|---|
| Lee et al. [18] | GOx | Au/Nafion/Glutaraldehyde | Amperometric | 28 μA mM-1cm-2 | 0-18 | 0.2 | |
| Gao et al. [148] | GOx | Chitosan/CNT /Prussian blue/Au | Amperometric | 2.35 nA μM-1 | 0-3.6 | - | |
| Saraoğlu et al. [147] | Non-enzymatic (humidity) | Thin film | Capacitive- ANN | - | 83-116.5 | - | |
| Lu et al. [152] | Non-enzymatic | Chitosan/NiCo2O4/Au | Amperometric | 0.5 μA μM-1 | 0.2-4 | 0.2 | |
Representative commercial and non-commercial characteristics of saliva-based glucose sensors
| Manufacturer | Type | Sensor material | Configuration | Sensitivity | Linear Range (mg/dL) | LOD (mg/dL) | |
|---|---|---|---|---|---|---|---|
| Commercial | |||||||
| The IQ Global Group [156] | GOx | Organic Transistor ITO/P3HT/Poly (4-vinylphenol) | Amperometric | - | 3.6-545 | - | |
| Non-commercial | |||||||
| Macaya et al. [155] | GOx | Pt/PEDOT:PSS | Resistive | 0.1 R/R0/(mg/dL) | 0.02-545 | 0.02 | |
| Chakraborty et al. [157] | Non-enzymatic | porous CuO | Amperometric | ∼2299 μAmM-1 cm-2 | 0.09-4 | 0.008 | |
| Liu et al. [158] | GOx/HRP | MWCNT | Amperometric | 67.93 nAmM-1 | 0.9-27 | 0.005 | |
P3HT, poly(3-hexylthiophene); R/R0, relative resistance variation; PEDOT:PSS, poly(3,4-ethylenedioxythiophene) polystyrene sulfonate; MWCNT, multi-walled carbon nanotube.
Representative commercial and non-commercial characteristics of tears-based glucose sensors
| Manufacturer | Type | Sensor material | Configuration | Sensitivity | Linear Range (mg/dL) | LOD (mg/dL) |
|---|---|---|---|---|---|---|
| Commercial | ||||||
| Roche - ACCU-CHEK Aviva Plus [94,163] | PQQ-GDH | nitrosoaniline-derivative (Mediator) | Amperometric | 0.127 μAmM-1 | 0.009-2.67 | 0.016 |
| Non-Commercial | ||||||
| Kownacka et al. [164] | GOx | Pt/Ir | Amperometric | - | 1.8-18 | - |
| Kim et al. [165] | Non-enzymatic | Nanoparticle Embedded Contact Lens | Photometric | 0.089Δr'n/mM | 0-44 | - |
| Romeo et al. [166] | Non-enzymatic | PET/Au/CuO/Nafion | Amperometric | 850 μAmM-1 cm-2 | 0.055-12.6 | 0.05 |
PQQ-GDH, pyrroloquinoline quinone dehydrogenase; PET, polyethylene terephthalate; Δr'n, difference of relative reflectance before and after reaction with glucose.
Glucose monitoring in tears
Several studies have suggested testing glucose in tear fluid as a blood substitute, thereby promoting the development of other techniques [162,163]. If a good correlation between tear glucose and blood glucose can be established, the measurement of tear glucose levels could provide an interesting method of indirect measurement of blood glucose within normal, hyperglycemic, and hypoglycemic limits [164]. To be analytically useful, sensing techniques require a very low detection limit (since glucose is present in tear fluid at levels 50-100 times lower than blood), high analytical sensitivity and selectivity, and the ability to quantitatively measure a very small sample in a short period of time (Figure 4E) [165]. Yao et al. have integrated functional contact lenses consisting of a differential glucose biosensor module, metal interconnections, a readout circuit, an antenna, and a telecommunication circuit to monitor tear glucose levels wirelessly, continuously and non-invasively [165]. The biosensor has a detection limit of 0.18 mg/dL and shows good linearity over the typical range of glucose concentrations in a tear film (0.18-10.5 mg/dL). Contact lenses can be worn for hours without discomfort and, therefore, provide an ideal vehicle for non-invasive and continuous glucose monitoring. However, a persistent problem for these types of devices is the implementation of a suitable power source. Recently, Badugu et al. introduced an optical chemical sensor for glucose detection in the ocular fluid [12]. Scientific data evidenced how commercially available test strips exhibit the required performance to evaluate glucose concentration in low-volume tears [166-168]. Reported data are summarized in Table 8 [94,163-166].
Glucose monitoring in urine
Urine is a noninvasive fluid for inexpensive, easy to use glucose testing, that has historically dominated diabetes monitoring before the blood glucose sensors era (Figure 4F) [169]. However, as circulating glucose (up to modestly high level) is physiologically reabsorbed by the kidney [170], conditions of hypoglycemia, euglycemia and moderate hyperglycemia cannot be either identified and/or differentiated. Glycosuria is normally evidenced when the renal reabsorption threshold is exceeded (>180 mg/dL), even if there is individual variability [171,172]. Thus, it is still of clinical interest for the evaluation of hyperglycemic episodes in poorly controlled patients, although there is no real-time relationship between glycosuria and blood glucose peaks. More recently, urine glucose determination may also help in the assessment of drug efficacy when using sodium-glucose cotransporter-2 (SGLT2) inhibitors, a class of oral anti-diabetic drugs that inhibit glucose reabsorption in the kidney, with elimination of glucose via urine [173]. The evaluation of glycosuria should be performed only on fresh urine samples, since old or high bacterial load samples may alter the levels of glucose in urine. However, the lack of precision and sensitivity of this test limits its use [172], and as underlined by current literature in this field, efforts are in progress to provide more reliable glucose sensors for urine. To date, most of the proposed sensors are based on colorimetric approaches, as glycosuria is generally assessed by colorimetric strip-based devices, with enzymatic methods and chromogenic reagents [174]. A recent, non-commercial alternative to colorimetric strips is represented by Surface-enhanced Raman scattering (SERS). This non-invasive sensing technique requires a very low sample volume (μL range) and no sample pre-processing, while it exhibits a high sensitivity and specificity for glucose [175,176]. Reported data of urine-based glucose sensors are summarized in Table 9 [174-179].
Future directions
Glucose related sensing technologies are not novel, and relative devices have indeed required decades of evolution to become mature and established on the market. Currently, the evaluation of glucose levels in the blood or in other non-invasive fluids has evidenced the limits of the actual technology. Optical evaluation of glucose in ISF is inherently interfered by different molecules, such as lactate and urea, reducing the reliability of results. Also, studies have highlighted that glucose levels in the blood, as well as in ISF (and likely in other biofluids) are influenced by commonly used pharmacological treatments (e.g., acetaminophen, albuterol, lisinopril, atenolol, and atorvastatin). Besides, glucose oxidase and glucose dehydrogenase monitors should be avoided, respectively, in patients with abnormal oxygen saturation, and in peritoneal dialysis using icodextrin [100,180]. Furthermore, even if non-invasive devices (such as those used for CGM) in some instances have replaced traditional glucometers, the detection of hypoglycemic events may be hampered by the time delay of glucose spread from blood to ISF. To overcome these drawbacks, current technology and methods should be improved, while other technological strategies should be searched for, and pursued. Barriers to glucose detection in non-invasive fluid sampling include analytical inaccuracy due to device miniaturization, the need to improve sensors' sensitivity and algorithms to convert sensor signals into glucose levels, in addition to a better understanding, by physicians, of the clinical significance of glucose values in non-blood fluids.
Another major point is to what extent glucose devices can be crucial to improve treatment and clinical outcomes. While devices for both SMBG and CGM have shown to be useful tools in type 1 diabetes with HbA1c and hypoglycemia as primary outcomes [108], devices for SMBG have not consistently shown a significant reduction in HbA1c in noninsulin treated patients with type 2 diabetes. On the other hand, in type 2 diabetes under insulin and/or hypoglycemic oral treatment, the use of a CGM device reduces HbA1c, but not the rates of hypoglycemia [108], while in gestational diabetes these devices help to achieve a better daytime glucose profile, providing an improvement in both maternal and neonatal outcomes [108, 181].
Representative commercial and non-commercial characteristics of urine-based glucose sensors
| Manufacturer | Type | Sensor material | Configuration | Sensitivity | Range (mg/dL) | LOD (mg/dL) |
|---|---|---|---|---|---|---|
| Commercial | ||||||
| Sysmex, UC‐11A test strips [174] | GOx | - | Colorimetric (Semiquantitative) | - | 50-2000 | 50 |
| Non-Commercial | ||||||
| Kong et al. [175,176] | SERS | Metal carbonyl compounds | Raman responses | 1800-2200 cm-1 | 1.8-180 | 1.8 |
| Lee et al. [177] | GOx | Paper/ PAni-NPs/RBCM | Colorimetric | 0.2562 λ/(μg/mL) | 0-1018 | 10 |
| Janyasupab et al. [178] | Non-enzymatic | CoFe/NG | Amperometric | 45.36 μAmM-1 cm-2 | 5-55 | ∼4 |
| Sun et al. [179] | Non-enzymatic | Cu-MOF | Amperometric | 89 μAmM-1 cm-2 | 0.001-90 | 0.2·10-3 |
SERS, surface-enhanced Raman scattering; PAni-NPs, polyaniline-nanoparticles; RBCM, red blood cell membrane; NG, nitrogen-doped graphene; MOF, metal-organic framework. λ, absorbance at 563 nm.
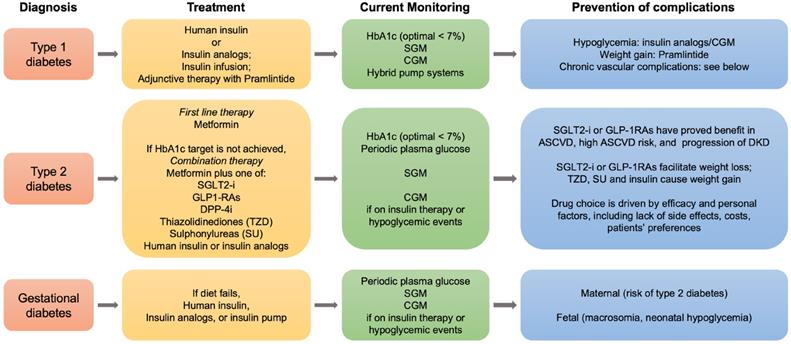
Roadmap for the management of diabetes mellitus. Summarized steps for the treatment and follow-up of type 1, type 2 and gestational diabetes are indicated. SGLT2, sodium glucose cotransporter 2; GLP-1RAs, glucagone-like peptide-1 receptor agonists; DPP-4i, dipeptydil peptidase-4 inhibitors; HbA1c, hemoglobin A1c; SGM, self glucose monitoring; CGM, continuous glucose monitoring; ASCVD, atherosclerotic cardiovascular disease; DKD, diabetic kidney disease.
Despite the apparent success in both treatment and control of diabetes, in the last decade, however, a deterioration of glycemic control in US adults with diabetes has been reported [182], so that major efforts, also in the field of diabetes technology, should be undertaken to reverse these findings.
As patients with diabetes experience the reduction of about a decade in their lifespan due to cardiovascular complications and renal failure, new therapeutic strategies have been developed and are in progress to ameliorate this outcome. In this context, the current roadmap for diabetes treatment, in particular for type 2 diabetes, has recently been revised by the ADA and the European Association for the Study of Diabetes, in light of the potential benefits of recently developed anti-diabetic drugs in preventing the vascular chronic complications linked to diabetes (Figure 5).
Besides improving glycemic control and reducing hypoglycemic events, new single or combined pharmacological treatments may offer new tools potentially suitable for the clinical needs of diabetic patients. Adjunctive therapies in type 1 (insulin plus pramlintide, or SGLT2 inhibitors), and combination therapies in type 2 diabetes (metformin plus SGLT2 inhibitors or glucagon-like peptide analogs) may be compared with traditional treatments thanks to the use of conventional glucose devices and the introduction of new devices for CGM [183, 184]. In this sense, technology well supports pharmacological research in diabetes and helps disentangling the pros and cons of each possible treatment. With the evolution of diabetes technology, non-invasive glucose monitoring has the potential to change the future of diabetes management [185], and to become soon, over the finger-pricking approaches, the preferred choice in young patients with type 1 diabetes, and in adults with prediabetes and overt type 2 diabetes (Figure 6).
Literature has evidenced novel and affordable biomarkers for the theranostics of diabetes [186-188], but current sensing technologies are poorly focused on such molecules, thus providing a bottleneck to the use of such biomarkers. Whether the development of lab-on-a chips may be useful to improve diagnostics, treatment, and clinical outcomes in diabetes is a big challenge that deserves further research.
Conclusions
Overall, strategies to evolve the world of biosensors have been already undertaken by many researchers, and the results obtained bode well for future improvements both in device technology and in patients' lives. Even though blood-based glucose monitoring is still a gold standard technology, the exploitation of different biofluids for continuous glucose monitoring with no invasiveness may represent an attractive and promising option for future directions. Fundamental, in this case, is the development of specific biosensors. On the other hand, the important benefit of minimal invasiveness and continuous glucose monitoring of new medical devices may improve patient comfort and awareness of the glycemic status, with foreseeable improvements in clinical outcomes and, in general, with a positive impact in the healthcare system.
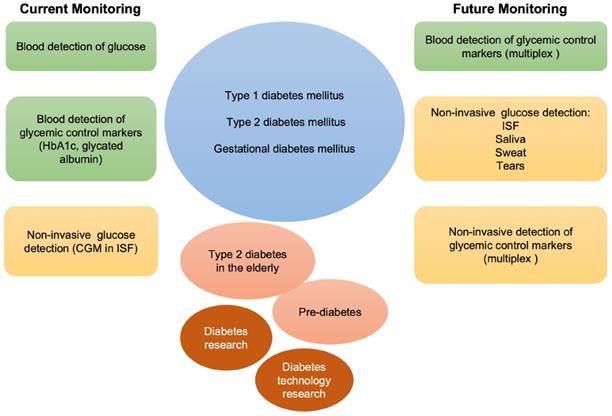
Schematic representation of current and future monitoring of glycemic control in type 1, type 2 and gestational diabetes. In green, blood is used for the detection of glucose and other markers of glycemic control. In orange, non-invasive biological fluids for glucose detection. Currently, the only approved FDA non-invasive methods for glucose detection employ ISF. Multiplex invasive or non-invasive assays may be foreseen in the future to integrate glucose measurement in the follow-up of patients with diabetes, elderly type 2 diabetes, and prediabetes, as well as to support diabetes related research.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Battelino T, Bergenstal RM. Continuous glucose monitoring-derived data report-simply a better management tool. Diabetes Care. 2020;43:2327-9
2. Fang M. Trends in diabetes management among US adults: 1999-2016. J Gen Intern Med. 2020;35:1427-34
3. Caruso R, Rebora P, Dellafiore F, Fabrizi D, Riegel B, Ausili D. et al. Clinical and socio-demographic determinants of inadequate self-care in adults with type 1 diabetes mellitus: the leading role of self-care confidence. Acta Diabetol. 2019;56:151-61
4. Adeel M, Rahman MM, Caligiuri I, Canzonieri V, Rizzolio F, Daniele S. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens Bioelectron. 2020;165:112331
5. Islam MS. Diabetes: from research to clinical practice. Adv Exp Med Biol. 2021;1307:1-5
6. Bailey TS. Clinical implications of accuracy measurements of continuous glucose sensors. Diabetes Technol Ther. 2017;19:S51-4
7. Fahmy Taha MH, Ashraf H, Caesarendra W. A Brief description of cyclic voltammetry transducer-based non-enzymatic glucose biosensor using synthesized graphene electrodes. Appl Syst Innov. 2020;3:32
8. Yadav J, Rani A, Singh V, Murari BM. Prospects and limitations of non-invasive blood glucose monitoring using near-infrared spectroscopy. Biomed Signal Process Control. 2015;18:214-27
9. Hwang D-W, Lee S, Seo M, Chung TD. Recent advances in electrochemical non-enzymatic glucose sensors - A review. Anal Chim Acta. 2018;1033:1-34
10. Sharma S, Shekhar S, Gautam S, Sharma B, Kumar A, Jain P. Carbon-based nanomaterials as novel nanosensors. In: Kaushik P, Gomes F, eds. Nanofabrication for smart nanosensor applications. 2020:323-47
11. Lee H, Hong YJ, Baik S, Hyeon T, Kim D-H. Enzyme-based glucose sensor: from invasive to wearable device. Adv Healthc Mater. 2018;7:1701150
12. Bruen D, Delaney C, Florea L, Diamond D. Glucose sensing for diabetes monitoring: recent developments. Sensors (Basel). 2017;17:1866
13. Rassel S, Xu C, Zhang S, Ban D. Noninvasive blood glucose detection by quantum cascade laser. Analyst. 2020;145:2441-56
14. Zhang G, Mei Z, Zhang Y, Ma X, Lo B, Chen D. et al. A non-invasive blood glucose monitoring system based on smartphone PPG signal processing and machine learning. IEEE Trans Industr Inform. 2020;16:7209-7218
15. Stone JY, Bailey TS. Benefits and limitations of continuous glucose monitoring in type 1 diabetes. Expert Rev Endocrinol Metab. 2020;15:41-9
16. Kim J, Kim M, Lee MS, Kim K, Ji S, Kim Y-T. et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat Commun. 2017;8:14997
17. Arakawa T, Kuroki Y, Nitta H, Chouhan P, Toma K, Sawada S. et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens Bioelectron. 2016;84:106-11
18. Lee H, Song C, Hong YS, Kim MS, Cho HR, Kang T. et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci Adv. 2017;3:e1601314
19. Freckmann G, Nichols JH, Hinzmann R, Klonoff DC, Ju Y, Diem P. et al. Standardization process of continuous glucose monitoring: traceability and performance. Clin Chim Acta. 2021;515:5-12
20. American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl. 1):S15-33
21. Skyler JS, Bakris GL, Bonifacio E, Darsow T, Eckel RH, Groop L. et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66:241-55
22. Gale EA. Declassifying diabetes. Diabetologia. 2006;49:1989-95
23. Esser N, Utzschneider KM, Kahn SE. Early beta cell dysfunction vs insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia. 2020;63:2007-21
24. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69-82
25. Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A. et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473-9
26. Brunetti A, Chiefari E, Foti D. Recent advances in the molecular genetics of type 2 diabetes mellitus. World J Diabetes. 2014;5:128-40
27. Soumaya K. Molecular mechanisms of insulin resistance in diabetes. Diabetes. 2013;771:240-51
28. Chiefari E, Arcidiacono B, Foti D, Brunetti A. Gestational diabetes mellitus: an updated review. J Endocrinol Invest. 2017;40:899-909
29. Rangel ÉB, Rodrigues CO, De Sá JR. Micro- and macrovascular complications in diabetes mellitus: preclinical and clinical Studies. J Diabetes Res. 2019: 2161085.
30. Greco M, Chiefari E, Accattato F, Corigliano DM, Arcidiacono B, Mirabelli M. et al. MicroRNA-1281 as a novel circulating biomarker in patients with diabetic retinopathy. Front Endocrinol. 2020;11:528
31. American Diabetes Association. Cardiovascular disease and risk management: Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl. 1):S125-50
32. De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. Front Endocrinol. 2018;9:2
33. Palella E, Cimino R, Pullano SA, Fiorillo AS, Gulletta E, Brunetti A. et al. Laboratory parameters of hemostasis, adhesion molecules and inflammation in type 2 diabetes mellitus: correlation with glycemic control. Int J Environ Res Public Health. 2020;17:300
34. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T. et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42:1593-603
35. Silva DD, Bosco A. An educational program for insulin self-adjustment associated with structured self-monitoring of blood glucose significantly improves glycemic control in patients with type 2 diabetes mellitus after 12 weeks: a randomized, controlled pilot study. Diabetol Metab Syndr. 2015;7:2
36. Salek-Maghsoudi A, Vakhshiteh F, Torabi R, Hassani S, Ganjali MR, Norouzi P. et al. Recent advances in biosensor technology in assessment of early diabetes biomarkers. Biosens Bioelectron. 2018;99:122-35
37. Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK. Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark Insights. 2016;11:95-104
38. Nichols GA, Bell K, Kimes TM, O'Keeffe-Rosetti M. Medical care costs associated with long-term weight maintenance versus weight gain among patients with type 2 diabetes. Diabetes Care. 2016;39:1981-6
39. Clark LC, Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann NY Acad Sci. 1962;102:29-45
40. Mazze RS, Shamoon H, Pasmantier R, Lucido D, Murphy J, Hartmann K. et al. Reliability of blood glucose monitoring by patients with diabetes mellitus. Am J Med. 1984;77:211-7
41. Pasqualetti S, Braga F, Panteghini M. Pre-analytical and analytical aspects affecting clinical reliability of plasma glucose results. Clin Biochem. 2017;50:587-94
42. Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res Clin Pract. 2017;133:178-92
43. Clark LC, Wolf R, Granger D, Taylor Z. Continuous recording of blood oxygen tensions by polarography. J Appl Physiol. 1953;6:189-93
44. Tao Z, Raffel RA, Souid A-K, Goodisman J. Kinetic studies on enzyme-catalyzed reactions: oxidation of gucose, decomposition of hydrogen peroxide and their combination. Biophys J. 2009;96:2977-88
45. Updike SJ, Hicks GP. The enzyme electrode. Nature. 1967;214:986-8
46. Guilbault GG, Lubrano GJ. An enzyme electrode for the amperometric determination of glucose. Anal Chim Acta. 1973;64:439-55
47. Wang J. Glucose biosensors: 40 years of advances and challenges. Electroanalysis. 2001;13:983-8
48. Rahman MM, Ahammad AJS, Jin J-H, Ahn SJ, Lee J-J. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors (Basel). 2010;10:4855-86
49. Niu XH, Shi LB, Zhao HL, Lan MB. Advanced strategies for improving the analytical performance of Pt-based nonenzymatic electrochemical glucose sensors: a minireview. Anal Methods. 2016;8:1755-64
50. Vashist SK, Zheng D, Al-Rubeaan K, Luong JHT, Sheu F-S. Technology behind commercial devices for blood glucose monitoring in diabetes management: a review. Anal Chim Acta. 2011;703:124-36
51. Sabu C, Henna TK, Raphey VR, Nivitha KP, Pramod K. Advanced biosensors for glucose and insulin. Biosens Bioelectron. 2019;141:111201
52. Yoo E-H, Lee S-Y. Glucose biosensors: an overview of use in clinical practice. Sensors (Basel). 2010;10:4558-76
53. Jung J, Garnett E, Rector K, Jariwala P, Devaraj S. Effect of collection tube type on glucose stability in whole blood. Ann Clin Lab Sci. 2020;50:557-9
54. Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3:971-80
55. Kirk JK, Stegner J. Self-monitoring of blood glucose: practical aspects. J Diabetes Sci Technol. 2010;4:435-39
56. Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10:622-8
57. Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23:1143-48
58. Pfützner A, Klonoff DC, Pardo S, Parkes JL. Technical aspects of the Parkes error grid. J Diabetes Sci Technol. 2013;7:1275-81
59. Canadian Standards Association. Performance specifications for portable whole blood glucose monitor systems for use in diabetes management. 1994. CSA Z316.4-94.
60. US Department of Health, Human Services, Food, Drug Administration. Blood glucose monitoring test systems for prescription point-of-care use: draft guidance for industry and Food and Drug Administration staff. 2014. FDA-2013-D-1445-0002.
61. US Department of Health, Human Services, Food, Drug Administration, Self-monitoring blood glucose test systems for over-the-counter use guidance for industry, Food, Drug Administration staff. FDA-2013-D-1446. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/self-monitoring-blood-glucose-test-systems-over-counter-use-0.
62. International Organization for Standardization. In vitro diagnostic test systems-requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. ISO 15197. 2013
63. International Organization for Standardization. In vitro diagnostic test systems-requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus (ISO15197:2013). EN ISO 15197:2015.
64. International Organization for Standardization. In vitro diagnostic medical devices-measurement of quantities in biological samples-metrological traceability of values assigned to calibrators and control materials. EN ISO 17511. 2003
65. CLSI. Point-of-care blood glucose testing in acute and chronic care facilities; Approved guideline - 3rd ed. CLSI document POCT12-A3. Wayne, PA: Clinical and Laboratory Standards Institute. 2013
66. Balion C, Grey V, Ismaila A, Blatz S, Seidlitz W. Screening for hypoglycemia at the bedside in the neonatal intensive care unit (NICU) with the Abbott PCx glucose meter. BMC Pediatr. 2006;6:28
67. Krouwer JS. Why the new FDA glucose meter POCT guidance is disappointing. J Diabetes Sci Technol. 2017;11:1274
68. Freckmann G, Baumstark A, Pleus S. Do the new FDA guidance documents help improving performance of blood glucose monitoring systems compared with ISO 15197? J Diabetes Sci Technol. 2017;11:1240-6
69. Pleus S, Baumstark A, Jendrike N, Mende J, Link M, Zschornack E. et al. System accuracy evaluation of 18 CE-marked current-generation blood glucose monitoring systems based on EN ISO 15197: 2015. BMJ Open Diabetes Res Care. 2020;8:e001067
70. Breitenbeck N, Brown A. Accuracy assessment of a blood glucose monitoring system for self-testing with three test strip lots following ISO 15197:2013/EN ISO 15197:2015. J Diabetes Sci Technol. 2016;11:854-5
71. Jeanny G, Hope P. Surveillance of the system accuracy of two systems for self-monitoring of blood glucose after market approval. J Diabetes Sci Technol. 2015;10:240-1
72. Krouwer JS, Cembrowski GS. A review of standards and statistics used to describe blood glucose monitor performance. J Diabetes Sci Technol. 2010;4:75-83
73. Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH. et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-40
74. Chaubey A, Malhotra BD. Mediated biosensors. Biosens Bioelectron. 2002;17:441-56
75. Pullano SA, Critello CD, Mahbub I, Tasneem NT, Shamsir S, Islam SK. et al. EGFET-Based sensors for bioanalytical applications: a review. Sensors (Basel). 2018;18:4042
76. Tang L, Chang SJ, Chen C-J, Liu J-T. Non-invasive blood glucose monitoring technology: a review. Sensors (Basel). 2020;20:6925
77. Frew JE, Hill HA. Electrochemical biosensors. Anal Chem. 1987;59:933A-44A
78. Wang J. Electrochemical glucose biosensors. Chem Rev. 2008;108:814-25
79. Heller A, Feldman B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem Rev. 2008;108:2482-505
80. Heller A, Feldman B. Electrochemistry in diabetes management. Acc Chem Res. 2010;43:963-73
81. Turner AP. Biosensors: sense and sensibility. Chem Soc Rev. 2013;42:3184-96
82. Qiao Y, Liu Q, Lu S, Chen G, Gao S, Lu W. et al. High-performance non-enzymatic glucose detection: using conductive Ni-MOF as an electrocatalyst. J Mater Chem B. 2020;8:5411-5
83. Qiao Y, Zhang R, He F, Hu W, Cao X, Jia J. et al. A comparative study of electrocatalytic oxidation of glucose on conductive Ni-MOF nanosheet arrays with different ligands. New J Chem. 2020;44:17849-53
84. Niu Q, Lu W, Bao C, Wei M, Chen Z-A, Jia J. Glucose-sensing abilities of mixed-metal (Ni Co) Prussian blue analogs hollow nanocubes. J Electroanal Chem. 2020;874:114507
85. Wu X, Bao C, Niu Q, Lu W. A novel method to construct 3D FeWO4 microsphere-array electrode as non-enzymatic glucose sensor. Nanotechnology. 2019;30:165501
86. Wang G, He X, Wang L, Gu A, Huang Y, Fang B. et al. Non-enzymatic electrochemical sensing of glucose. Microchim Acta. 2012;180:161-86
87. Başkaya G, Yıldız Y, Savk A, Okyay TO, Eriş S, Sert H. et al. Rapid, sensitive, and reusable detection of glucose by highly monodisperse nickel nanoparticles decorated functionalized multi-walled carbon nanotubes. Biosens Bioelectron. 2017;91:728-33
88. Nichols SP, Koh A, Storm WL, Shin JH, Schoenfisch MH. Biocompatible materials for continuous glucose monitoring devices. Chem Rev. 2013;113:2528-49
89. Boyd R, Leigh B, Stuart S. Capillary versus venous bedside blood glucose estimations. Emerg Med J. 2005;22:177-9
90. Farrer M, Albers CJ, Neil HA, Adams PC, Laker MF, Alberti KG. Assessing the impact of blood sample type on the estimated prevalence of impaired glucose tolerance and diabetes mellitus in epidemiological surveys. Diabet Med. 1995;12:325-9
91. Rebel A, Rice MA, Fahy BG. The accuracy of point-of care glucose measurements. J Diabetes Sci Technol. 2012;6:396-411
92. Manual https://pim-eservices.roche.com/LifeScience/Document/e370d613-6441-eb11-0291-005056a71a5d
93. Manual https://www.rochediabetescaremea.com
94. Cha KH, Qin Y, Meyerhoff ME. Origin of low detection limit and high selectivity of Roche Accu-Chek test strips that enables measurement of tear glucose levels. Electroanalysis. 2015;27:670-6
95. Yang J, Lin Q, Yin W, Jiang T, Zhao D, Jiang L. A novel nonenzymatic glucose sensor based on functionalized PDDA-graphene/CuO nanocomposites. Sens Actuators B Chem. 2017;253:1087-95
96. Zhang H, Chen Z, Dai J, Zhang W, Jiang Y, Zhou A. A low-cost mobile platform for whole blood glucose monitoring using colorimetric method. Microchem J. 2020;162:105814
97. Márquez A, Jiménez-Jorquera C, Domínguez C, Muñoz-Berbel X. Electrodepositable alginate membranes for enzymatic sensors: An amperometric glucose biosensor for whole blood analysis. Biosens Bioelectron. 2017;97:136-42
98. Montagnana M, Caputo M, Giavarina D, Lippi G. Overview on self-monitoring of blood glucose. Clin Chim Acta. 2009;402:7-13
99. Hortensius J, Slingerland RJ, Kleefstra N, Logtenberg SJJ, Groenier SH, Houweling ST. et al. Self-monitoring of blood glucose: the use of the first or the second drop of blood. Diabetes Care. 2011;25:956-60
100. Ginsberg BH. Factors affecting blood glucose monitoring: sources of errors in measurements. J Diabetes Sci Technol. 2009;3:903-13
101. Barreau PB, Buttery JE. Effect of hematocrit concentration on blood glucose value determined on Glucometer II. Diabetes Care. 1988;11:116-8
102. Tang Z, Lee JH, Louie RF, Kost GJ. Effects of different hematocrit levels on glucose measurement with handlheld meters for point-of-care testing. Arch Pathol Lab Med. 2000;124:1135-40
103. Weinzimer SA. Analysis: PENDRA: The once and future noninvasive continuous glucose monitoring device? Diabetes Technol Ther. 2004;6:442-4
104. Caduff A, Dewarrat F, Talary M, Stalder G, Heinemann L, Feldman Y. Non-invasive glucose monitoring in patients with diabetes: A novel system based on impedance spectroscopy. Biosens Bioelectron. 2006;22:598-604
105. Frias JP, Lim CG, Ellison JM, Montandon CM. Review of adverse events associated with false glucose readings measured by GDH-PQQ-based glucose test strips in the presence of interfering sugars. Diabetes Care. 2010;33:728-9
106. Schleis TG. Interference of maltose, icodextrin, galactose, or xylose with some blood monitoring systems. Pharmacotherapy. 2007;27:1313-21
107. Montagnana M, Lippi G. Self-monitoring of blood glucose in diabetic patients. Riv Ital Med Lab. 2013;9:195-204
108. American Diabetes Association. Diabetes technology: Standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl. 1):S85-99
109. Shokrekhodaei M, Quinones S. Review of non-invasive glucose sensing techniques: optical, electrical and breath acetone. Sensors (Basel). 2020;20:1251
110. Pandey R, Paidi SK, Valdez TA, Zhang C, Spegazzini N, Dasari RR. et al. Noninvasive monitoring of blood glucose with Raman spectroscopy. Acc Chem Res. 2017;50:264-72
111. Kang JW, Park YS, Chang H, Lee W, Singh SP, Choi W. et al. Direct observation of glucose fingerprint using in vivo Raman spectroscopy. Sci Adv. 2020;64:eaay5206
112. Yang D, Afroosheh S, Lee JO, Cho H, Kumar S, Siddique RH. et al. Glucose sensing using surface-enhanced Raman-mode constraining. Anal Chem. 2018;90:14269-78
113. Pelletier CC, Lambert JL, Borchert M. Determination of glucose in human aqueous humor using Raman spectroscopy and designed-solution calibration. Appl Spectrosc. 2005;59:1024-31
114. La Via WV, Lambert JL, Pelletier MJ, Morookian JM, Sirk SJ, Mickiene D. et al. Measurement of amphotericin B concentration by resonant Raman spectroscopy - a novel technique that may be useful for non-invasive monitoring. Med Mycol. 2006;44:169-74
115. Lan YT, Kuang YP, Zhou LP, Wu GY, Gu PC, Wei HJ. et al. Noninvasive monitoring of blood glucose concentration in diabetic patients with optical coherence tomography. Laser Physics Letters. 2017;14:035603
116. Heinemann L, Kramer U, Klotzer HM, Hein M, Volz D, Hermann M. et al. Noninvasive glucose measurement by monitoring of scattering coefficient during oral glucose tolerance tests. Diabetes Technol Ther. 2000;2:211-20
117. Kirill VL, Mohsen SE, Massoud M, Rinat OE. Noninvasive blood glucose monitoring with optical coherence tomography. Diabetes Care. 2002;25:2263-7
118. Tanaka Y, Tajima T, Seyama M, Waki K. Differential continuous wave photoacoustic spectroscopy for non-invasive glucose monitoring. IEEE Sens J. 2019;20:4453-8
119. Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJS. Fluorescence-based glucose sensors. Biosens Bioelectron. 2005;20:2555-65
120. Chen L, Hwang E, Zhang J. Fluorescent nanobiosensors for sensing gucose. Sensors (Basel). 2018;18:1440
121. Ju J, Liu W, Perlaki CM, Chen K, Feng C, Liu Q. Sustained and cost effective silver substrate for surface enhanced Raman spectroscopy based biosensing. Sci Rep. 2017;7:6917
122. Lundsgaard-Nielsen SM, Pors A, Banke SO, Henriksen JE, Hepp DK, Weber A. Critical-depth Raman spectroscopy enables home-use non-invasive glucose monitoring. PLoS ONE. 2018;13:e0197134
123. Villena Gonzales W, Mobashsher A, Abbosh A. The progress of glucose monitoring—a review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors (Basel). 2019;19:800
124. Zhang R, Gao F, Feng X, Jin H, Zhang S, Liu S. et al. “Guide star” assisted noninvasive photoacoustic measurement of glucose. ACS Sensors. 2018;3(12):2550-7
125. Robinson MR, Eaton RP, Haaland DM, Keep GW, Thomas EV, Stalled BR. et al. Non-invasive glucose monitoring in diabetic patients: a preliminary evaluation, Clin. Chem. 1992;38:1618-22
126. Heise HM, Marbach R, Koschinsky TH, Gries HM. Non-invasive blood glucose sensors based on near-infrared spectroscopy. Artif Organs. 1994;18:439-47
127. Kay-Uwe J, Christoph F, Klaus D, Ulrich AM, Bernardo M. Application of near-infrared spectroscopy for non-invasive determination of blood/tissue glucose using neural networks. Z Phys Chem. 1995;191:2179-90
128. Muller UA, Mertes B, Fischbacher C, Jageman KU, Danzer K. Non-invasive blood glucose monitoring by means of near infrared spectroscopy: methods for improving the reliability of the calibration models. Int J Artif Organs. 1997;20:285-90
129. Danzer K, Fischbacher CH, Jagemann KU, Reichelt KJ. Near infrared diffuse reflection spectroscopy for non-invasive blood glucose monitoring. LEON Newslett. 1998;12:9-11
130. Stephen FM, Timothy LR, Thomas BB, Suresh NT, Stephen LM. Noninvasive prediction of glucose by near-infrared diffuse reflectance spectroscopy. Clin Chem. 1999;45:1651-8
131. Shu-Jen Y, Charles FH, Omar SK. Monitoring blood glucose changes in cutaneous tissue by tissue by temperature-modulated localized reflectance measurements. Clin Chem. 2003;49:924-34
132. Maruo K, Tsurugi M, Tumura M, Ozaki Y. In vivo non-invasive measurement of blood glucose by near-infrared diffuse-reflectance spectroscopy. Appl Spectrosc. 2003;57:1236-44
133. Maruo K, Tsurugi M, Jakusei C, Tomohiro O, Hidenobu A, Yukio Y. et al. Noninvasive blood glucose assay using a newly developed near-infrared system. IEEE J Sel Top Quant Electron. 2003;9:322-30
134. Araujo-Andrade C, Facundo R, Martinez-Mendoza JR, Terrones H. Infrared and Raman spectra, conformational stability, ab initio calculations of structure, and vibrational assignment of α and β glucose. J Mol Struct. 2005;714:143-6
135. Han G, Chen S, Wang X, Wang J, Wang H, Zhao Z. Noninvasive blood glucose sensing by near-infrared spectroscopy based on PLSR combines SAE deep neural network approach. Infrared Phys Technol. 2021;113:103620
136. Xu K, Qiu Q, Jiang J, Yang X. Non-invasive glucose sensing with near-infrared spectroscopy enhanced by optical measurement conditions reproduction technique. Opt Lasers Eng. 2005;43:1096-106
137. Jain P, Joshi AM. A precise non-invasive blood glucose measurement system using NIR spectroscopy and Huber's regression model. Opt Quantum Electron. 2019;51:51
138. Brown CD, Davis HT, Ediger MN, Fleming CM, Hull EL, Rohrscheib M. Clinical assessment of near-infrared spectroscopy for noninvasive diabetes screening. Diabetes Technol Ther. 2005;7:456-66
139. Tashtoush AM. Non-invasive blood glucose measurement using laser technology (NIBGM). Int J Com Dig Sys. 2021;10:297-307
140. Kamalraj R, Neelakandan S, Ranjith Kumar M, Chandra Shekhar Rao V, Anand R, Singh H. Interpretable filter based convolutional neural network (IF-CNN) for glucose prediction and classification using PD-SS algorithm. Measurement. 2021;183:109804
141. Chuah ZM, Paramesran R, Thambiratnam K, Poh S-C. A two-level partial least squares system for non-invasive blood glucose concentration prediction. Chemometrics and Intelligent Laboratory Systems. 2010;104:347-51
142. Nystrom J, Lindholm-Sethson B, Stenberg L, Ollmar SJ, Eriksson W, Geladi P. Combined near-infrared spectroscopy and multifrequency bio-impedance investigation of skin alternation in diabetes patients based on multivariate analyses. Med Biol Eng Comput. 2003;41:324-9
143. Guevara G. Joint optical-electrical technique for non-invasive glucose monitoring. Mex J Phys. 2010;56:430-4
144. Heikenfeld J, Jajack A, Feldman B, Granger SW, Gaitonde S, Begtrup G. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat Biotechnol. 2019;37:407-19
145. Baghelani M, Abbasi Z, Daneshmand M, Light PE. Non-invasive continuous-time glucose monitoring system using a chipless printable sensor based on split ring microwave resonators. Sci Rep. 2020;10:12980
146. Burmeister JJ, Arnold MA. Evaluation of measurement sites for noninvasive blood glucose sensing with near-infrared transmission spectroscopy. Clin Chem. 1999;45:1621-7
147. Saraoğlu HM, Koçan M. A study on non-invasive detection of blood glucose concentration from human palm perspiration by using artificial neural networks. Expert Syst. 2010;27:156-65
148. Gao W, Emaminejad S, Nyein HYY, Challa S, Chen KV, Peck A. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529:509-14
149. Kim J, Campbell AS, Wang J. Wearable non-invasive epidermal glucose sensors: a review. Talanta. 2018;177:163-70
150. Bandodkar AJ, Wenzhao J, Yardımcı C, Wang X, Ramirez J, Wang J. Tattoo-based noninvasive glucose monitoring: a proof-of-concept study. Anal Chem. 2015;87:394-8
151. Chung M, Fortunato G, Radacsi N. Wearable flexible sweat sensors for healthcare monitoring: a review. J R Soc Interface. 2019;16:20190217
152. Lu Y, Jiang K, Chen D, Shen G. Wearable sweat monitoring system with integrated micro-supercapacitors. Nano Energy. 2019;58:624-32
153. Kaufman E, Lamster IB. The diagnostic applications of saliva—A review. Crit Rev Oral Biol Med. 2002;13:197-212
154. Du Y, Zhang W, Wang ML. An on-chip disposable salivary glucose sensor for diabetes control. J Diabetes Sci Technol. 2016;10:1344-52
155. Macaya DJ, Nikolou M, Takamatsu S, Mabeck JT, Owens RM, Malliaras GG. Simple glucose sensors with micromolar sensitivity based on organic electrochemical transistors. Sens Actuators B Chem. 2007;123:374-8
156. Dastoor P, Belcher W. Organic thin film transistors and the use thereof in sensing applications. US patent US9766199B2. 2017
157. Chakraborty P, Dhar S, Deka N, Debnath K, Mondal SP. Non-enzymatic salivary glucose detection using porous CuO nanostructures. Sens Actuators B Chem. 2020;302:127134
158. Liu J, Sun S, Shang H, Lai J, Zhang L. Electrochemical biosensor based on bienzyme and carbon nanotubes incorporated into an os-complex thin film for continuous glucose detection in human saliva. Electroanalysis. 2016;28:2016-21
159. Panchbhai AS. correlation of salivary glucose level with blood glucose level in diabetes mellitus. J Oral Maxillofac Res. 2012;3:334-9
160. Yamaguchi M, Mitsumori M, Kano Y. Noninvasively measuring blood glucose using saliva. IEEE Eng Med Biol Mag. 1998;17:59-63
161. Heller A. Implanted electrochemical glucose sensors for the management of diabetes. Ann Rev Biomed Eng. 1999;1:153-75
162. Lee SH, Cho YC, Bin Choy Y. Noninvasive self-diagnostic device for tear collection and glucose measurement. Sci Rep. 2019;9:4747
163. Makaram P, Owens D, Aceros J. Trends in nanomaterial-based non-invasive diabetes sensing technologies. Diagnostics (Basel). 2014;4:27-46
164. Yao H, Shum AJ, Cowan M, Lähdesmäki I, Parviz BA. A contact lens with embedded sensor for monitoring tear glucose level. Biosens Bioelectron. 2011;26:3290-6
165. Cha KH, Jensen GC, Balijepalli AS, Cohan BE, Meyerhoff ME. Evaluation of commercial glucometer test strips for potential measurement of glucose in tears. Anal Chem. 2014;86:1902-8
166. Kownacka AE, Vegelyte D, Joosse M, Anton N, Toebes BJ, Lauko J. et al. Clinical evidence for use of a non-invasive biosensor for tear glucose as an alternative to painful finger-prick for diabetes management utilizing biopolymer coating. Biomacromolecules. 2018;19:4504-11
167. Kim S, Jeon H-J, Park S, Lee DY, Chung E. Tear glucose measurement by reflectance spectrum of a nanoparticle embedded contact lens. Sci Rep. 2020;10:8254
168. Romeo A, Moy A, Leung TS, Gabriel G, Villa R, Sánchez S. Inkjet printed flexible non-enzymatic glucose sensor for tear fluid analysis. Appl Mater Today. 2018;10:133-41
169. Clarke SF, Foster JR. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br J Biomed Sci. 2012;69:83-93
170. Hayford JT, Weydert JA, Thompson RG. Validity of urine glucose measurements for estimating plasma glucose concentration. Diabetes Care. 1983;6:40-4
171. Renard E. Monitoring glycemic control: the importance of self-monitoring of blood glucose. Am J Med. 2005;118:12S-9S
172. Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, Peterson CM. et al. Tests of glycemia in diabetes. Diabetes Care. 2012;27:1761-73
173. Fathi A, Vickneson K, Singh JS. SGLT2-inhibitors; more than just glycosuria and diuresis. Heart Fail Rev. 2021;26:623-42
174. Oyaert M, Delanghe JR. Semiquantitative, fully automated urine test strip analysis. J Clin Lab Anal. 2019;33:e22870
175. Kong KV, Lam Z, Lau WKO, Leong WK, Olivo M. A transition metal carbonyl probe for use in a highly specific and sensitive SERS-based assay for glucose. J Am Chem Soc. 2013;135:18028-31
176. Kong KV, Ho CJH, Gong T, Lau WKO, Olivo M. Sensitive SERS glucose sensing in biological media using alkyne functionalized boronic acid on planar substrates. Biosens Bioelectron. 2014;56:186-91
177. Lee T, Kim I, Cheong DY, Roh S, Jung HG, Lee SW. et al. Selective colorimetric urine glucose detection by paper sensor functionalized with polyaniline nanoparticles and cell membrane. Anal Chim Acta. 2021;1158:33838
178. Janyasupab M, Liu C-W, Chanlek N, Chio-Srichan S, Promptmas C, Surareungchai W. A comparative study of non-enzymatic glucose detection in artificial human urine and human urine specimens by using mesoporous bimetallic cobalt-iron supported N-doped graphene biosensor based on differential pulse voltammetry. Sens Actuators B Chem. 2019;286:550-63
179. Sun Y, Li Y, Wang N, Xu QQ, Xu L, Lin M. Copper-based metal-organic framework for non-enzymatic electrochemical detection of glucose. Electroanalysis. 2018;30:474-8
180. Basu A, Slama MQ, Nicholson WT, Langman L, Peyser T, Carter R. et al. Continuous glucose monitor interference with commonly prescribed medications: a pilot study. J Diabetes Sci Technol. 2017;11:936-41
181. Scott EM, Feig DS, Murphy HR, Law GR, CONCEPTT Collaborative Group. Continuous glucose monitoring in pregnancy: importance of analyzing temporal profiles to understand clinical outcomes. Diabetes Care. 2020;43:1178-84
182. Fang M, Wang D, Coresh J, Sevin E. Trends in diabetes treatment and control in U.S. adults, 1999-2018. N Engl J Med. 2021;384:2219-28
183. Garg SK, Brackett S, Reinicke T, Hirsch IB. New medications for the treatment of diabetes. Diabetes Technol Ther. 2020;22:S149-72
184. American Diabetes Association. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl. 1):S111-24
185. Zhao M & Leung PS. Revisiting the use of biological fluids for noninvasive glucose detection. Future Med Chem. 2020;12:645-27
186. Menni C, Fauman E, Erte I, Perry JRB, Kastenmuller G, Shin S-Y. et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. 2013;62:4270-6
187. Wang Y, Yuan Y, Zhang Y, Lei C, Zhou Y, He J. et al. Serum 1,5-anhydroglucitol level as a screening tool for diabetes mellitus in a community-based population at high risk of diabetes. Acta Diabetol. 2016;54:425-31
188. Wu J, Dong M, Rigatto C, Liu Y, Lin F. Lab-on-chip technology for chronic disease diagnosis. NPJ Digit Med. 2018;1:7
Author contact
![]() Corresponding authors: Prof. Antonio Brunetti; e-mail: brunettiit. Prof. Antonino S. Fiorillo; e-mail: ninoit
Corresponding authors: Prof. Antonio Brunetti; e-mail: brunettiit. Prof. Antonino S. Fiorillo; e-mail: ninoit
 Global reach, higher impact
Global reach, higher impact