13.3
Impact Factor
Theranostics 2022; 12(1):422-433. doi:10.7150/thno.68182 This issue Cite
Research Paper
Evans blue-modified radiolabeled fibroblast activation protein inhibitor as long-acting cancer therapeutics
1. State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics & Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, 4221-116 Xiang'An South Rd, Xiamen 361102, China;
2. Institute of Clinical Pharmacy & Pharmacology, Jining First People's Hospital, Jining Medical University, Jining 272000, China;
3. Department of Diagnostic Radiology, Yong Loo Lin School of Medicine, National University of Singapore, 119074, Singapore;
4. Nanomedicine Translational Research Program, NUS Center for Nanomedicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Singapore
5. Clinical Imaging Research Centre, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117599, Singapore
6. Departments of Surgery, Chemical and Biomolecular Engineering, and Biomedical Engineering, Yong Loo Lin School of Medicine and Faculty of Engineering, National University of Singapore, Singapore, 119074, Singapore
# These authors contributed equally to this work.
Abstract
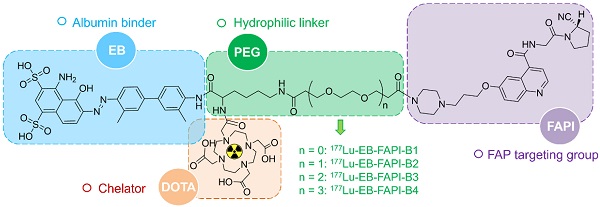
Rationale: Fibroblast activation protein (FAP) targeted molecular imaging radiotracers have shown promising preclinical and clinical results in tumor diagnosis. However, rapid clearance and inadequate tumor retention of these molecules have hindered them for further clinical translation in cancer therapy. In this study, we aimed to develop a series of albumin binder-truncated Evans blue (EB) modified FAP targeted radiotracers, and optimize the pharmacokinetic (PK) characteristics to overcome the existing limitations in order to apply in the radionuclide therapy of cancer.
Methods: A series of compounds with the general structure of EB-FAPI-Bn were synthesized based on a FAP inhibitor (FAPI) variant (FAPI-02) and radiolabeled with 177LuCl3. To verify the binding affinity and FAP targeting specificity of these tracers in vitro, U87MG cell uptake and competition assays were performed. Preclinical PK was evaluated in U87MG tumor-bearing mice using SPECT imaging and biodistribution studies. The lead compound EB-FAPI-B1 was selected and cancer therapeutic efficacy of 177Lu-EB-FAPI-B1 was assessed in U87MG tumor-bearing mice.
Results: 177Lu-EB-FAPI-B1, B2, B3, B4 were stable in PBS (pH 7.4) and saline for at least 24 h. EB-FAPI-B1 showed high binding affinity (IC50 = 16.5 nM) to FAP in vitro, which was comparable with that of FAPI-02 (IC50 = 10.9 nM). SPECT imaging and biodistribution studies of 177Lu-EB-FAPI-B1, B2, B3, B4 have proved their prominently improved tumor accumulation and retention at 96 h post-injection, especially for 177Lu-EB-FAPI-B1, high tumor uptake and low background signal make it the optimal compound. Compared to the saline group, noteworthy tumor growth inhibitions of 177Lu-EB-FAPI-B1 have been observed after administration of different dosages.
Conclusion: In this study, several EB modified FAPI-02 related radiopharmaceuticals have been synthesized successfully and evaluated. High binding affinity and FAP targeting specificity were identified in vitro and in vivo. Remarkably enhanced tumor uptake and retention of EB-FAPI-B1 were found over the unmodified FAPI-02. 177Lu-EB-FAPI-B1 showed remarkable tumor growth suppression in U87MG tumor model with negligible side effects, indicating that 177Lu-EB-FAPI-B1 is promising for clinical application and transformation.
Keywords: fibroblast activation protein (FAP), albumin binder, FAPI, SPECT imaging, radioligand therapy
 Global reach, higher impact
Global reach, higher impact