13.3
Impact Factor
Theranostics 2021; 11(17):8430-8447. doi:10.7150/thno.59661 This issue Cite
Research Paper
Self-assembly of differentiated progenitor cells facilitates spheroid human skin organoid formation and planar skin regeneration
1. Cell Therapy Institute, Spinal Cord Injury and Tissue Regeneration Center Salzburg (SCI-TReCS), University Clinic, Paracelsus Medical University, Salzburg, Austria
2. Department of Transfusion Medicine, University Clinic, Paracelsus Medical University, Salzburg, Austria
3. Accanis Biotech, Biocenter Vienna, Austria
4. Department of Plastic, Aesthetic and Reconstructive Surgery, Hospital Barmherzige Brueder, Salzburg, Austria
5. Stem Cell Core Facility, Charité, Berlin Institute of Health; Berlin, Germany
Abstract
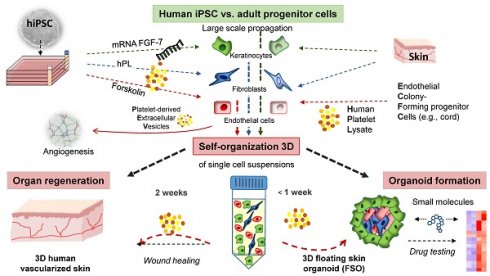
Self-assembly of solid organs from single cells would greatly expand applicability of regenerative medicine. Stem/progenitor cells can self-organize into micro-sized organ units, termed organoids, partially modelling tissue function and regeneration. Here we demonstrated 3D self-assembly of adult and induced pluripotent stem cell (iPSC)-derived fibroblasts, keratinocytes and endothelial progenitors into both, planar human skin in vivo and a novel type of spheroid-shaped skin organoids in vitro, under the aegis of human platelet lysate.
Methods: Primary endothelial colony forming cells (ECFCs), skin fibroblasts (FBs) and keratinocytes (KCs) were isolated from human tissues and polyclonally propagated under 2D xeno-free conditions. Human tissue-derived iPSCs were differentiated into endothelial cells (hiPSC-ECs), fibroblasts (hiPSC-FBs) and keratinocytes (hiPSC-KCs) according to efficiency-optimized protocols. Cell identity and purity were confirmed by flow cytometry and clonogenicity indicated their stem/progenitor potential. Triple cell type floating spheroids formation was promoted by human platelet-derived growth factors containing culture conditions, using nanoparticle cell labelling for monitoring the organization process. Planar human skin regeneration was assessed in full-thickness wounds of immune-deficient mice upon transplantation of hiPSC-derived single cell suspensions.
Results: Organoids displayed a distinct architecture with surface-anchored keratinocytes surrounding a stromal core, and specific signaling patterns in response to inflammatory stimuli. FGF-7 mRNA transfection was required to accelerate keratinocyte long-term fitness. Stratified human skin also self-assembled within two weeks after either adult- or iPSC-derived skin cell-suspension liquid-transplantation, healing deep wounds of mice. Transplant vascularization significantly accelerated in the presence of co-transplanted endothelial progenitors. Mechanistically, extracellular vesicles mediated the multifactorial platelet-derived trophic effects. No tumorigenesis occurred upon xenografting.
Conclusion: This illustrates the superordinate progenitor self-organization principle and permits novel rapid 3D skin-related pharmaceutical high-content testing opportunities with floating spheroid skin organoids. Multi-cell transplant self-organization facilitates development of iPSC-based organ regeneration strategies using cell suspension transplantation supported by human platelet factors.
Keywords: Skin organoids, progenitor cells, human induced pluripotent stem cells, human platelet-derived growth factors, skin regeneration
 Global reach, higher impact
Global reach, higher impact