13.3
Impact Factor
Theranostics 2021; 11(15):7546-7569. doi:10.7150/thno.56482 This issue Cite
Review
Recent strategies for nano-based PTT combined with immunotherapy: from a biomaterial point of view
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing 102488, China.
Received 2020-11-27; Accepted 2021-5-23; Published 2021-6-4
Abstract
Cancer has been a great threat to humans for decades. Due to the limitations of monotherapy, combinational therapies such as photothermal therapy (PTT) and immunotherapy have gained increasing attention with expectation to overcome the shortfalls of each other and obtain satisfactory therapeutic outcomes. PTT can inhibit primary tumors by thermal ablation but usually fails to achieve complete eradication and cannot prevent metastasis and recurrence. Meanwhile, the efficacy of immunotherapy is usually attenuated by the weak immunogenicity of tumor and the immunosuppressive tumor microenvironment (ITM). Therefore, many recent studies have attempted to synergize PTT with immunotherapy in order to enhance the therapeutic efficacy. In this review, we aim to summarize the cutting-edge strategies in combining nano-based PTT with immunotherapy for cancer treatment. Herein, the combination strategies were mainly classified into four categories, including 1) nano-based PTT combined with antigens to induce host immune responses; 2) nano-based PTT in combination with immune adjuvants acting as in situ vaccines; 3) nano-based PTT synergized with immune checkpoint blockade or other regulators to relieve the ITM; 4) nano-based PTT combined with CAR-T therapy or cytokine therapy for tumor treatment. The characteristics of various photothermal agents and nanoplatforms as well as the immunological mechanisms for the synergism were also introduced in detail. Finally, we discussed the existing challenges and future prospects in combined PTT and immunotherapy.
Keywords: photothermal therapy, immunotherapy, combination therapy, nanoplatforms, cancer
Introduction
Due to aging and population growth, there will be a significant rise in the annual number of new cancer cases from 18.1 million in 2018 to 29.4 million in 2040 [1]. Cancer has become a tremendous threat to people worldwide. Currently, the conventional strategies for tumor treatment mainly include surgery, radiotherapy and chemotherapy. All of them showed positive therapeutic effects on early tumors but had limited efficacy for advanced tumors with metastasis [2]. Moreover, various side effects accompanied by these therapies also bring great challenges [3]. Therefore, more effective treatment strategies with high specificity continue to be explored.
Photo-therapy, which harnesses the absorption of near-infrared (NIR) light (650-1350 nm) by photo-agents to induce a therapeutic response has drawn great attention. Upon NIR light irradiation, the hyperthermia generated by photothermal agents is the basis for photothermal therapy (PTT), while the reactive oxygen species (ROS) released by photo-sensitizers is the main mechanism for photodynamic therapy (PDT) [4]. In this review, we mainly focused on recent advances in nano-based PTT combined with immunotherapy. The combination of PDT with immunotherapy was not included this time, but it has been introduced in other articles in details [5-8]. PTT can reduce tumor burden through thermal ablation, and it has become an attractive option in cancer treatment [9]. By locally exerting NIR light irradiation, the hyperthermia (>42 °C) generated by photothermal agents can cause apoptosis of cancer cells by destroying the cell membrane, damaging the cytoskeleton and inhibiting DNA synthesis [10-12]. In addition, PTT was able to trigger host immunity, revealing promising prospects in combination with immunotherapy [13, 14]. Moreover, tumors are more sensitive to heat than normal tissues due to oxygen deficit and the mild acidic microenvironment; thereby, this strengthens the selectivity of PTT to tumors [15]. Research interests have been extensively concentrated on its applications and combination with other therapies, since PTT has advantages including high selectivity, non-resistance and minimal invasiveness [16, 17].
The key to achieve photo-ablation of tumor relies on the design of photothermal agents which should have strong NIR light absorbance, high photothermal conversion efficiency, good biocompatibility and sufficient accumulation within tumors. Currently, various types of inorganic and organic photothermal agents have been exploited for their ability to perform PTT on tumors. Inorganic photothermal agents mainly include gold nanomaterials, like gold nanoparticles (GNPs), gold nanorods (GNRs) and gold nanostars (AuNSs) [18, 19]; carbon based materials, such as single-walled carbon nanotubes (SWCNTs), graphene and carbon dots (CD) [20, 21]; sulfide metallic nanoparticles, for example, CuS and MnS2 [22]; black phosphorus and etc. [23]. Generally, they are characterized by high photothermal conversion efficiency and photothermal stability. Their photothermal property can be adjusted by varying their size, shape or doping with other elements [24]. However, the slow degradation rate in vivo might cause potential toxicity [18]. While the organic photothermal agents typically include small molecular dyes, such as indocyanine green (ICG) and IR780; polydopamine (pD), polyaniline (PANI) and polypyrrole nanoparticles [25]. Those organic photothermal agents are usually degradable and have high biocompatibility; but some of them are still facing drawback of photobleaching. Moreover, photothermal agents are usually designed as nanoplatforms. Due to the nanoscale sizes or surface modification of targeting ligands such as antibodies, folic acid, peptides and hyaluronic acid [26-28]), these photothermal agents could achieve passive or active targeted delivery to tumors, thereby enhancing the accumulation in tumors. Moreover, they can meanwhile serve as nanocarriers to load drugs, antigens or adjuvants, exhibiting potential for combinational therapy with other treatment modalities [29, 30].
Even though PTT could debulk the tumor volume rapidly, it is generally difficult to completely eradicate tumors with PTT alone for some reasons as follows: 1) The penetration depth for NIR light is limited. Typically, the penetration depth of an NIR laser of 808 nm was reported to be within several millimeters (mm) (normally less than 5 mm [31]), which is difficult to reach the very inside of a large tumor. 2) Photobleaching after a short time period of laser irradiation leads to a reduction in photothermal efficacy, especially for organic small molecular dyes. 3) Long-term tumor remission was insufficient, and there are high risks of tumor relapse and metastasis. Therefore, combining PTT with other therapies was expected to overcome the above challenges.
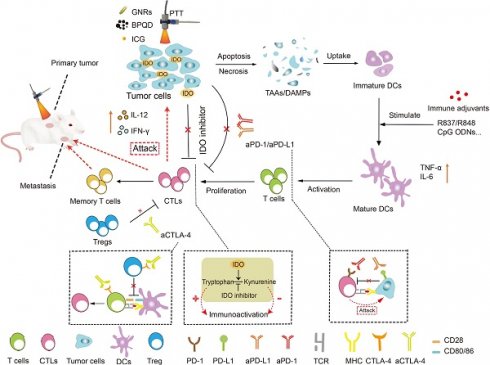
The ability to evade immune system surveillance and passivate immunogenicity is the primary reason for the occurrence and development of tumors [32]. Generally, there are three important phases in cancer immune surveillance: elimination, equilibrium and escape [33, 34]. In the process of elimination, firstly, acute inflammatory responses triggered by tumor-associated antigens (TAAs) can promote the secretion of cytokines such as interleukin-12 (IL-12) and interferon-γ (IFN-γ), and induce the activation of dendritic cells (DCs). Then upon activation, DCs will migrate to the nearby lymph nodes (LNs), where they present tumor antigens and activate tumor-specific CD8+ cytotoxic T lymphocyte (CTLs) to kill tumor cells. During the phase of equilibrium, a long-lasting campaign between the immune system and cancer cells is established. Tumor cells with high immunogenicity are eradicated by the immune system, while others that can lower their immunogenicity by immune editing will survive. Consequently, immune escape occurred. Additionally, certain negative regulators, including the PD-L1 on tumor cells, interleukin 10 (IL-10), transforming growth factor β (TGF-β), regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment (TME) can prevent the activation of immune cells and prevent the tumor infiltration of CTLs and antigen-presenting cells (APCs) [35, 36].
Recently, immunotherapy in which the body immune system is trained to recognize and fight against tumors has shown great potential for cancer treatment [37], especially for aggressive and metastatic tumors. Cancer immunotherapy relies on the efficient presentation of tumor antigens to T-cells to elicit a potent anti-tumor immune response and generate long-term immune memory, thereby inducing the killing of cancer cells and preventing cancer recurrence [38]. Currently, cancer immunotherapy mainly includes the application of tumor vaccines, immune checkpoint blockade (ICB) and chimeric antigen receptor T cell (CAR-T) therapy, which can restrain the growth and metastasis of tumors either by strengthening the immune response or reversing the immunosuppressive microenvironment (ITM). However, despite the advantages of immunotherapy, it also has limitations. 1) Single immunotherapy is not effective for all types of cancer, and the therapeutic responses may vary between different patients. 2) The efficacy of immunotherapy for large tumors is generally limited due to the ITM, loss of immunogenicity for cancer cells and excessive tumor burden [39, 40]. 3) ICB therapies only perform their therapeutic function on their associated pathways instead of priming the immune system to specific response [41]. 4) The activation of anti-tumor responses after vaccination is low due to variations in antigen specificity between different tumors and patients [42]. All of the above factors have led to the attenuated efficacy of single immunotherapy in the treatment of cancers.
On account of the characteristics of immunotherapy and PTT, a combination of these two therapies has been proposed for the following reasons: 1) Nano-based PTT is able to eliminate the primary tumor by thermal ablation and rapidly reduce the tumor burden. In addition, the hyperthermia generated by PTT can induce immunogenic cell death (ICD) and release damage-associated molecular patterns (DAMPs) or TAAs, thereby increasing the immunogenicity of residual tumor tissues to trigger immune responses. Moreover, these nanoplatforms can be easily taken up by DCs via endocytosis and promote the cross-presentation of antigens, which makes them promising vehicles for cancer immunotherapy [43]. 2) Immunotherapy mainly involves the subsequent recruitment and maturation of DCs, promoting the secretion of pro-inflammatory cytokines and stimulating the CTLs to clear up residual tumor cells as well as establishing long-term immune memories to prevent metastasis and tumor recurrence. Typically, the strategies for combining nano-based PTT with immunotherapy mainly include 1) nano-based PTT combined with antigens to elicit immune responses; 2) nano-based PTT in combination with immune adjuvants acting as in situ vaccines; 3) nano-based PTT synergize with ICB or other regulators to relieve ITM. 4) nano-based PTT combined with other immunotherapies such as CAR-T therapy or cytokine therapy for cancer treatment. This article mainly reviewed the recent strategies of nanoplatform-based PTT combined with immunotherapies (Table 1) and discussed challenges and future prospects.
Photothermal materials combined with immune modulators for photothermal-immunotherapy
| Photothermal agent | Type | Nanocarrier | Immunotherapy | Immunotherapy agent | Cancer model | Therapeutic outcome | References |
|---|---|---|---|---|---|---|---|
| pD | organic | MPDA NPs | Immune adjuvant | R837 | B16F10 | Significantly suppressed primary tumor growth and notably prolonged the survival time of mice | [58] |
| PANI | organic | GCS | Immune adjuvant | R848 | CT26 | Almost completely restrained the primary tumor and effectively suppressed the tumor recurrence and metastasis | [61] |
| pD | organic | Al2O3 NPs | Immune adjuvant | Al2O3, CpG ODNs | B16F10 | Significantly inhibited the primary tumor and prolonged the survival time of mice | [67] |
| IR7 | organic | Liposomes | Immune adjuvant | CpG ODNs | CT26 | Effectively eradicated primary tumors in mice and inhibited tumor metastasis. | [69] |
| ICG | organic | Liposomes | Immune adjuvant | Poly I:C | CT26, B16F10 | Almost completely restrained the primary tumor growth and prevented metastasis of cancer | [72] |
| ICG | organic | PLEL hydrogel | Immune adjuvant | R848, CpG ODNs | 4T1 | Inhibited the residual tumor growth after surgery, prevented recurrence and metastasis | [76] |
| ICG | organic | Fe3O4 NPs | Immune adjuvant | R837 | 4T1 | Inhibited tumor growth, metastasis and recurrence | [14] |
| BPQDs | inorganic | Hydrogel | Immune adjuvant, Immune checkpoint inhibition | LPS, GM-CSF, PD-1 antibody | 4T1-luc, B16F10-luc | Inhibited the recurrence and metastasis of tumors and prolonged the survival rate | [23] |
| ICG | organic | OVA | Exogenous tumor antigen | OVA | B16 | Almost totally inhibited the primary tumor growth and prevented occurrence of tumor | [50] |
| BPQDs | inorganic | Exosomes | Exogenous tumor antigen | Exosomes | LLC | Significantly delayed tumor occurrence and almost totally inhibited the primary tumor growth | [52] |
| ICG | organic | Eukaryotic-pr-okaryotic vesicle, PLGA | Exogenous tumor antigen, adjuvant | Melanoma cytomembrane vesicles, attenuated Salmonella outer membrane vesicles | B16 | Almost totally inhibited primary tumor and efficiently suppressed tumorgenesis | [78] |
| MnFe2O4 | inorganic | MnFe2O4 NPs | Exogenous tumor antigen, adjuvant | OVA, R837 | 4T1 | Effectively restrained primary tumor growth and prevented lung metastases, resulting in a prolonged survival time and improved survival rate | [114] |
| Au@Pt NPs | inorganic | Au@Pt NPs | Immune checkpoint inhibition | PD-L1 antibody | 4T1 | Effectively eliminated primary tumors, inhibited the growth of distal tumors and alleviated tumor metastasis | [31] |
| BPQD | inorganic | Nanovesicle | Immune checkpoint inhibition | PD-1 antibody | 4T1 | Significantly inhibited primary and secondary tumor growth | [83] |
| HAuNS | inorganic | PLGA | Immune checkpoint inhibition | PD-1 antibody | CT26, 4T1 | Efficiently eliminated most primary tumors, significantly inhibited the growth of the distant tumors, and induced the longest survival time | [84] |
| PBNP | inorganic | PBNP | Immune checkpoint inhibition | CTLA-4 antibody | Neuro2a | Decreased tumor growth rates and exhibited protection against tumor re-challenge | [87] |
| Melanin nanoparticles | organic | Melanin NPs | IDO inhibition | INCB24360 | 4T1 | Strongly inhibited both primary and abscopal 4T1 tumors | [90] |
| Fe3O4 NPs | inorganic | PLGA | Immune checkpoint inhibition, immune adjuvant | R837, PD-1 antibody | 4T1 | Significantly inhibited both the primary and distant tumors and effectively prevented the lungs/liver metastasis | [94] |
| ICG | organic | Covalent organic frameworks | Exogenous tumor antigen, immune checkpoint inhibition | OVA, PD-L1 antibody | CT26 | Effectively eliminated primary tumor and inhibited the metastasis of cancer cells | [115] |
| Bi2Se3 nanocage | inorganic | Bi2Se3 nanocage | Immune checkpoint inhibition, immune adjuvant | R848, PD-L1 antibody | 4T1 | Totally inhibited both the primary tumor and distant tumor and established long-term immune memory | [95] |
| ICG | organic | Liposome | Immune checkpoint inhibition | PD-1 antibody, TIM-3 antibody | MC38, CT26 | Cleared the primary tumor and inhibited the growth of the distant tumor | [93] |
| Au40C-DOPC | inorganic | PLGA | Immune checkpoint inhibition, immune adjuvant | R837, PD-L1 antibody | 4T1 | Significantly suppressed both the primary tumor and distant tumor | [116] |
| IR1061 | organic | PAAV-SNO | IDO inhibition | IDO inhibitor | 4T1 | Significantly inhibited the primary tumor growth with 4/6 mice became tumor free and suppressed breast lung metastasis | [97] |
| ICG | organic | PLGA | CAR-T therapy | CAR-T cells | WM115 | Significantly inhibited the primary tumor growth with 2 out of 6 mice being completely cured | [100] |
| GNR | inorganic | GNR | Immune checkpoint inhibition, STING | PD-1 antibody, STING | K7 | Strongly inhibited the primary tumors and significantly retarded distant tumors | [103] |
| Au NPs | inorganic | Au NPs and TNF-α | Cytokine therapy | TNF-α | 4T1 | Significantly inhibited the primary 4T1 tumors | [104] |
| CuS | inorganic | MSN | Cytokine therapy | IL-12 | B16F10 | Effectively inhibited both primary and distant tumors | [105] |
Nano-based PTT combined with antigens to induce host immune responses
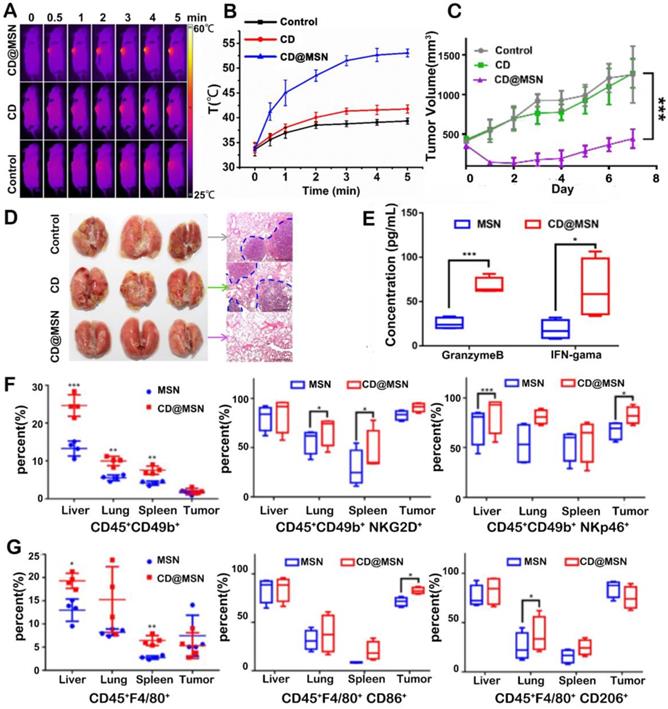
Recent studies have shown that the hyperthermia generated by PTT can induce ICD, a favorable cell death phenotype that can attract immune cells and elicit robust immune responses [44]. Cancer cell death triggered by hyperthermia can lead to the activation of danger signaling pathways and the release of DAMPs or TAAs, thereby markedly increasing the immunogenicity of those dying cells, and this kind of cell death pathway is termed as ICD. According to other reports, the induction of oxidative stress and endoplasmic reticulum (ER) stress play an essential role in this process [45]. DAMPs are critical markers of ICD, including the expression of calreticulin (CRT), heat shock proteins (HSP70, HSP90) and the release of high mobility group box 1 (HMGB1) and ATP. They serve as danger signals to recruit immune cells and are crucial for DC maturation and antigen presentation. TAAs are antigen molecules that are overexpressed when normal cells become cancerous and also play important roles in activating immune responses. In an example reported by Qian et al. [46], the CD-incorporated mesoporous silica nanoparticles (CD@MSN) were prepared for combined photothermal immunotherapy. Herein, CD was incorporated into MSN to avoid rapid excretion and to improve the photothermal performances via CD condensation. After i.v. injection, the hybrid particles could perform PTT (ca. 55 °C within 5 min) on tumor cells upon NIR light irradiation and the hybrid particles could degrade into nanodebris due to CD-induced swelling (Figure 1A-B). Then the generated debris were able to capture the TAAs (H60 and mrn41 proteins) released from the dying tumor cells after photo-ablation and stimulate immune responses after extravasation from the necrotic tumor tissues into the nearby immune organs. As a result, the primary tumor growth was greatly retarded and lung metastasis was significantly inhibited (Figure 1C-D). It was also revealed that CD@MSN mediated PTT promoted the proliferation and activation of NK cells (Figure 1F) and increased the ratio of M1 (CD45+F4/80+CD86+) to M2 (CD45+F4/80+CD206+) type tumor-associated macrophages (Figure 1G). And the secretion of granzyme B and IFN-γ in plasma was also elevated (Figure 1E). However, the tumor inhibition in this study was not persistent probably due to insufficient immune activation by PTT alone. Moreover, despite the gradual degradability in vitro, the effect of CD@MSN on the physiological function of major organs should also be investigated.
In another example by Sweeny et al., Prussian blue nanoparticles (PBNPs), an organic photothermal agent were designed to achieve combined photothermal-immunotherapy against neuroblastoma [47]. Unlike other inorganic photothermal agents, PBNP is able to be degraded under physiological conditions of pH 7.4, which avoids causing potential toxicity. Moreover, PBNPs can efficiently convert NIR light into heat and raise the temperature at tumor site [48]. Results showed that after i.t. injection, PBNPs mediated PTT could effectively eradicate the primary tumor for the neuroblastoma-bearing mice. Additionally, PTT could trigger ICD marked by the secretion of ATP and HMGB1 as well as elevated expression of CRT. After per-vaccination with PTT treated Neuro2a cells, mice were challenged with tumor cells again on the 7th day. It was found that tumor progression became much slower and the long-term survival rate was also elevated. These results demonstrated that PTT could not only kill the primary tumors via hyperthermia but also trigger host immune responses to prevent the occurrence of tumors.
TAAs or DAMPs induced by PTT are a major source of antigens. In this case, antigens were generated after PTT and the combination therapy mainly aimed to eradicate the existing tumors and trigger immune responses to prevent tumor relapse or metastasis. However, there were also studies integrated the exogenous antigens with photothermal agents into one nanoplatform during preparation with aim to prevent tumor occurrence prophylactically. Such exogenous neoantigens could be ovalbumin (OVA), tumor cell derived exosomes or debris of tumor cell membrane, which can activate antigen-specific immune responses and arouse a long-term immune memory [39, 49].
(A) Photothermal images and (B) tumor temperature curves of tumor-bearing mice after intravenous injection of CD or CD@MSN followed by 808 nm laser irradiation (0.75 W cm-2). (C) Changes of tumor volume over time after different treatments. Data were represented as mean ± SD (n = 4). Statistical significance was calculated by one-way ANOVA using the Tukey post-test (***p < 0.001). (D) Photos of the lungs harvested from different treatment groups 14 days after administration (the yellow dotted line circled the metastatic tumor focis) and corresponding H&E stained sections (the blue dotted line presented the border of the metastatic tumors), respectively. Bar = 100 μm. (E) Concentrations of granzyme B and IFN-γ in mice plasma from different groups. (F, G) Representative flow cytometric quantification of proliferation and differentiation of NK cells (F) and macrophages (G) gating on CD45+ cells harvested from different organs. Adapted with permission from [46], copyright 2019 Nano Letters.
(A) Illustration for fabrication and mechanism of OVA-ICG nanovaccine for photothermal-immunotherapy against tumor, DC stimulation/tracking, and tumor prevention. (B) Expression levels of surface molecules (MHC-II, CD80, and CD83) on DC 2.4 cells after activation with pure OVA, pure ICG, and OVA-ICG nanovaccine. (C) TNF- α and IL-6 in the DC 2.4 cell culture supernatant after incubation with OVA, ICG, and OVA-ICG for 24 and 48 h. (D) Schematic illustration of protocol for therapeutic assay and tumor growth curves of the mice with different treatments. (E) Schematic illustration of protocol for tumor prevention assay and tumor growth curves of the mice treated with PBS or OVA-ICG. Adapted with permission from [50], copyright 2018 Advanced Materials.
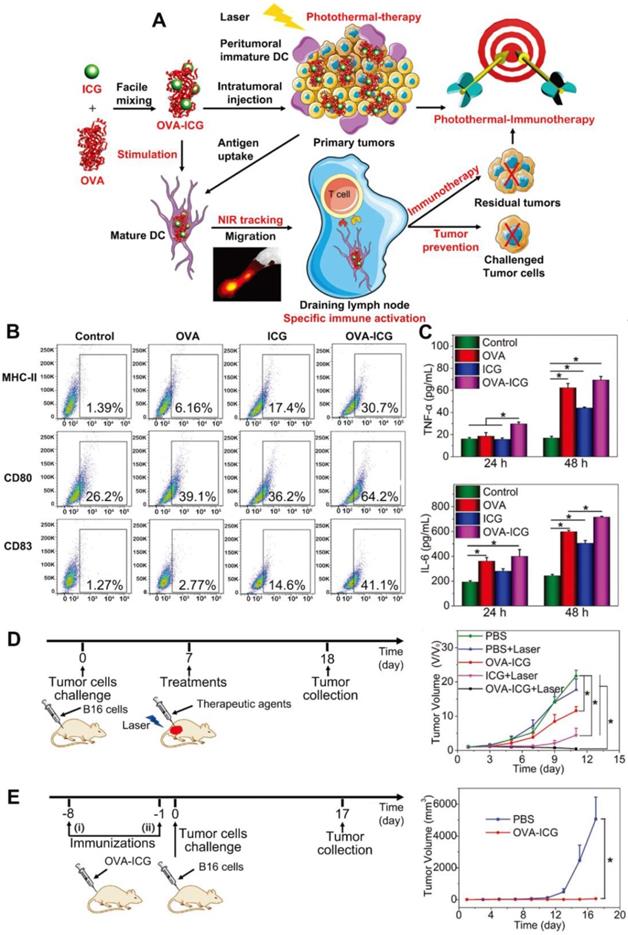
For example, Pan et al. [50] utilized OVA both as a model antigen and a carrier to load ICG (Figure 2A) for combinational therapy. As one kind of organic small molecular dyes, ICG is a promising candidate for cancer theranostic application owing to its proved safety in clinic, ability for NIR fluorescence imaging and PTT. To improve its photo-stability and tumor accumulation, ICG was usually loaded in nanocarriers [51]. This nanovaccine which had high antigen-loading efficiency (80.8%) could exhibit therapeutic and prophylactic dual functions. After treating immature DCs with ICG-OVA, the expression levels of MHC-II, CD80, and CD83 were significantly elevated. This indicates the activation and maturation of DCs (Figure 2B). Additionally, the levels of tumor necrosis factor-α (TNF-α, one marker in cellular immunity) and IL-6 (one marker in humoral immunity) in the cell culture medium were significantly increased (Figure 2C). After a single intratumoral injection of OVA-ICG followed by 808 nm laser irradiation, the tumor temperature increased to 60 °C, and the growth of B16 tumors was strongly suppressed. The anti-tumor efficacy of OVA-ICG plus NIR irradiation was much higher compared to that of single PTT mediated by ICG, which demonstrated the enhanced therapeutic efficacy of combined PTT and immunotherapy (Figure 2D). In addition, the population of CD8+ cytotoxic T cells in tumors and the anti-OVA immunoglobulin G (IgG) levels in the serum also increased. To test the prophylactical effect of this nanovaccine, mice were immunized with OVA-ICG twice before challenging with B16 cells. It was found that tumors barely grew, indicating its effectiveness in tumor prevention (Figure 2E). Also, exosomes which are a set of vesicles secreted by many cells play important biological roles in intercellular communication, body function maintenance and disease emergence. Since tumor cell derived exosomes, which carried tumor-specific antigens, could stimulate immune responses, they were explored to combine with PTT. In the study by Liu and co-workers [52], exosomes obtained from tumor-bearing mice after PTT (hEX) were used as tumor antigens and carriers for black phosphorus quantum dots (BPQDs). In this nanoplatform (hEX@BP), hEX could protect BPQDs, the photothermal agents from rapid degradation and clearance. What's more, hEX could target to tumors via their inherent homing ability. Lastly, hEX carrying tumor antigens was able to trigger immune responses. Mice were immunized with this vaccine every week for 3 times before s.c. inoculation of LLC (Lewis lung cancer) cells. From the results, hEX@BP immunization only inhibited tumor growth partially, indicating that immunotherapy was insufficient to completely prevent tumor occurrence. But once it was followed by BP mediated PTT after i.t. injection, tumor growth was significantly delayed, which again confirmed the improved therapeutic outcome of combinational therapy. However, exosome isolation which consumes lots of time and energy could be a challenge.
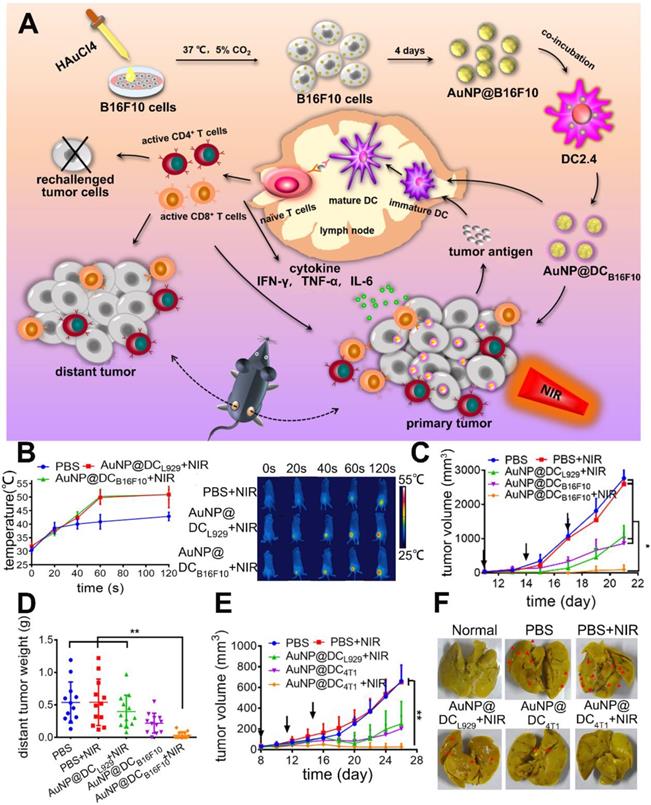
In another article, Zhang's group prepared an immunological Au nanoparticle (Au NPs) via intracellular generation and exocytosis for combined PTT and immunotherapy [53]. Briefly, Au NPs generated within B16F10 cells were trapped in vesicles and secreted into extracellular environment with tumor specific antigens (AuNP@B16F10). To further improve the immunological property and avoid potential metastasis caused by tumor derived vesicles, the AuNP@B16F10 was introduced to DCs and exocytosed as DCs derived vesicles (AuNP@DCB16F10, Figure 3A). After subcutaneous injection, this nanoparticle could gradually migrate to tumor and LNs over time. And the tumor temperature reached ca. 50 °C within 1 min under NIR light, which was high enough to induce tumor ablation (Figure 3B). Moreover, it was confirmed that AuNP@DCB16F10 carrying DAMPs and neoantigens from the B16F10 cells could be identified by the immune cells to trigger anti-tumor immunity. The anti-tumor effect was investigated on murine melanoma model and treatments were given for 3 times with 3 days interval. To elucidate the role of tumor antigen in inducing anti-tumor immunity, AuNP@DCL929 without transferred tumor antigen was prepared as control. In comparison to AuNP@DCL929 + NIR and AuNP@DCB16F10 which represented single PTT and single immunotherapy, AuNP@DCB16F10 + NIR achieved nearly complete tumor elimination with inhibition rate of 96.7% (Figure 3C). Moreover, AuNP@DCB16F10 + NIR could also significantly inhibit distant tumors (Figure 3D) and prevent tumor recurrence due to the effective activation of the CTLs, including the CD8+CD69+, CD8+CD107+ and CD8+ Granzyme B+ T cells in tumors and LNs. Of note, by replacing B16F10 cells with 4T1 breast cancer cells, the obtained AuNP@DC4T1 displayed similar anti-tumor effect on the 4T1 tumor models (Figure 3E-F). However, the clinical use of AuNP@DCB16F10 still faces challenges like the complicated procedure of preparation as well as difficulty in scale-up production.
Nano-based PTT combined with immune adjuvant for tumor treatment
Although PTT alone can trigger host immunity, the efficacy is relatively weak. Therefore, immune adjuvants are often employed to combine with PTT for enhanced immune stimulation. PTT-induced DAMPs and TAAs in conjunction with immune adjuvants can form in situ vaccines. These are able to prime strong immune responses and induce long-term immune memory. Commonly used immune adjuvants are mainly Toll-like receptor (TLR) agonists, including cytosine-phosphate-guanine oligodeoxynucleotides (CpG ODNs, a TLR-9 agonist), resiquimod (R848, a TLR7/8 agonist), polyinosinic:polycytidylic acid (poly I:C, a TLR-3 agonist), and monophosphoryl lipid A (MPLA, a TLR-4 agonist); these are able to imitate the pathogen associated molecular patterns (PAMPs) to promote DC maturation and increase the excretion of immune-related cytokines [54].
(A) Schematic preparation of AuNP@DCB16F10 and mechanism of AuNP@DCB16F10-mediated combinational treatment modality. (B) IR thermal images and corresponding temperature profiles of tumor-bearing mice injected with PBS, AuNP@DCL929, and AuNP@DCB16F10, respectively, with laser irradiation (n = 3). (C) Tumor growth curves after different treatments with or without laser irradiation of 2.0 W cm-2 (n = 7). The arrows represent the injection time. (D) Weight of the distant B16F10 tumor harvested at 19th day. (E) 4T1-tumor growth curve (n = 8). (F) Representative images of stained lungs of different groups. The arrows refer to the 4T1-tumor nodules on surface. Adapted with permission from [53], copyright 2019 Nano Letters.
TLR7/8 which is expressed on the intracellular compartments of many types of immune cells could recognize viral and bacterial RNA [55, 56]. And TLR7/8 agonists such as R848 and R837 are synthetic imidazoquinoline-like molecules that function as immune modulators and possess anti-viral and anti-tumor activity [57]. In the study by Wang and coworkers, R837 loaded mesoporous polydopamine nanoparticles (MPDA NPs) was prepared for combinational therapy (Figure 4A) [58]. The synthesized NPs could encapsulate R837 with high efficiency owing to its porous structure. And after being coated with a PVP layer, these PVP-MPDA@R837 NPs could migrate to draining lymph nodes (DLNs) with long retention time. Consequently, drug exposure within the DLNs was greatly maximized and the off-target side effects of immunotherapy could also be alleviated. Herein, the MPDA acted as not only the carrier for the adjuvant R837 but also a photothermal agent with intrinsic biocompatibility and high photothermal conversion efficiency (up to 40%) [59]. Results revealed that after peritumorally injection, the PVP-MPDA@R837 + NIR treated group exhibited the strongest inhibition effect on B16F10 tumor growth with an inhibition rate of ca. 66.3%, which was much higher compared to the PVP-MPDA + NIR (30.5%) and PVP-MPDA@R837 (41.2%) treated groups (Figure 4B). Moreover, the PVP-MPDA@R837 + NIR treatment also greatly elevated the maturation level of DCs in DLNs as well as the percentage of CD8+ T cells in the spleen of tumor bearing mice (Figure 4C-D). These findings confirmed the synergistic effect of PTT and immunotherapy. But whether the nanosystem could prevent tumor metastasis and recurrence was not investigated further.
(A) Schematic depiction of PVP-MPDA@R837 nanoparticles and the mechanism of intrigued anti-tumor immune responses. (B) Tumor volumes of B16F10-bearing mice receiving different treatments. Footpad injections of various formulations were performed once every 3 d from 0 d for three times and tumor laser irradiation was applied at 24 h after injection. (C) Representative flow cytometry plots of CD80+ or CD86+ among CD11c+ DCs extracted from the popliteal LNs (tumor sentinel LNs). (D) Representative flow cytometry plots illustrated CD3+ CD8a+ T cells in splenocytes. Adapted with permission from [58], copyright 2020 Biomaterials.
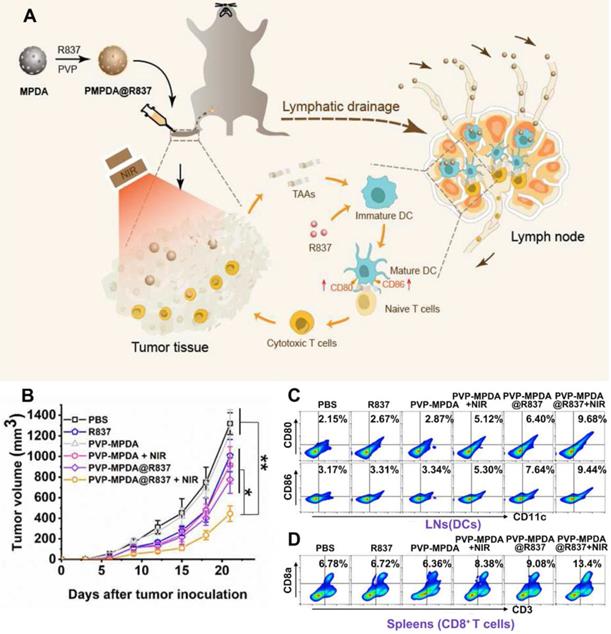
R848 is also a commonly seen TLR7/8 agonist that outperforms R837 in promoting pro-inflammatory cytokine secretion and DC activation [60]. In Chen's study [61], a conjugated polymer polyaniline-glycol-chitosan (PANI-GCS) was synthesized and loaded with R848 (denoted as R848@NPs) to induce mild hyperthermia and generate anti-tumor immunity. Herein, PANI in the polymer structure was used as the photothermal agent. PANI has been regarded as a favorable choice owing to its attractive properties including chemical stability, simple synthesis, as well as good optical property among the organic photothermal agents. It could induce a stable and strong hyperthermia effect due to the extended π-electrons [62, 63]. However, the potential cytotoxicity of intermediates during the polymerization reaction of PANI could not be neglected, as reported by several studies [64, 65]. In this study, hyperthermia was deliberately maintained at a relatively low temperature (ca. 44 °C) to spare the immune cells from heat induced elimination. It was found that the mild hyperthermia caused by R848@NPs upon laser irradiation significantly up-regulated the expression of HSP70, a DAMP molecule on CT26 cells. R848@NPs could be easily uptake by DCs and induce their maturation. The in vivo anti-tumor efficacy of different formulations was tested on subcutaneous CT26 tumor models via i.t. injection every 7 days, with a total of 3 injections. Results showed that hyperthermia induced by empty NPs at high temperatures (approximately 55 °C) caused severe burns at the tumor site. However, tumor inhibition was only transient, indicating the limited efficacy of conventional PTT. Moreover, the mild hyperthermia (ca. 44 °C) or single R848 treated groups only delayed tumor growth, while the low-temperature hyperthermia plus adjuvant (R848@NPs/+NIR) treated group exhibited the strongest tumor recession and the highest survival rate within 30 days of observation. The most encouraging finding was that after re-challenging the surviving mice with CT26 cells on the contralateral side, the second tumor barely grew, suggesting that the R848@NPs/+NIR could effectively prevent tumor recurrence and help to establish a durable anti-tumor immune memory. The underlying immunological mechanism revealed that the infiltration of CTLs within tumor tissue and the secretion level of proinflammatory cytokine IL-6 significantly increased. Also, the population of MDSCs and the level of immunosuppressive IL-10 in the TME decreased markedly after the combinational therapy.
(A) Chemical structure of pD-Al2O3 nanoparticles. (B) Tumor growth curve after the indicated treatments (8 mice per group). (C) Proportions of tumor-infiltrating CD8+ T cells and CD4+ T cells in TDLNs and spleens of mice after the indicated treatments. Proportions were determined using flow cytometry. Pound signs in panel C indicate P values for comparisons between pD-Al2O3 + Laser + CpG and Control using the Student's 2-tailed t test. * P < 0.05; **P < 0.01. (D) Survival of mice after the indicated treatments (8 mice per group). (E) Tumor growth curve (8 mice per group). Adapted with permission from [67], copyright 2018 Theranostics.
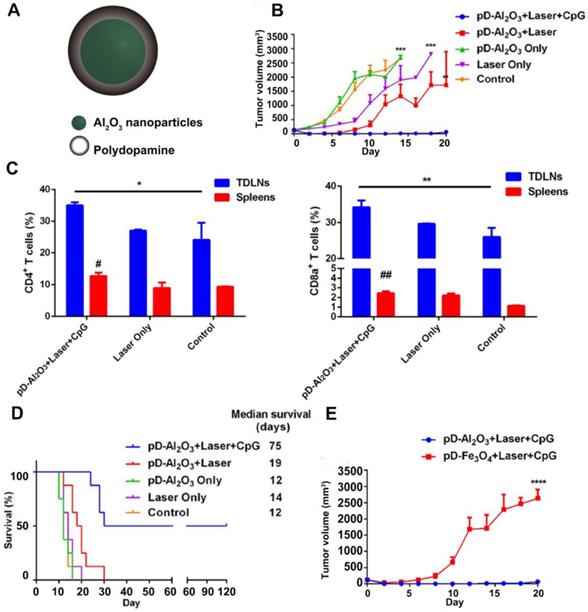
Apart from small molecular adjuvants, CpG ODNs were also used as immune adjuvants in combination with PTT. Unmethylated CpG ODNs that bind to TLR9 can be efficiently internalized by APCs and stimulate the release of various cytokines, thereby initiating a series of innate and adaptive immune responses [66]. For example, Chen and coworkers prepared pD-coated Al2O3 nanoparticles (pD-Al2O3 NPs) in combination with CpG for synergistic photothermal-immunotherapy. Herein, the pD functioned as a photothermal agent, and the inner Al2O3 core together with CpG served as an immune adjuvant [67] (Figure 5A). The results revealed that pD-Al2O3 NPs + CpG could induce the maturation of DCs both in vitro and in vivo. After intratumoral injection of pD-Al2O3 NPs, the temperature of the B16F10 tumor reached 55 °C within 5 min under NIR light. However, PTT alone failed to restrain tumor growth completely. When PTT was followed by s.c. injection of CpG for 3 times, tumors gradually shrank and became undetectable by day 18, demonstrating the importance of CpG in triggering strong anti-tumor responses (Figure 5B). Additionally, the combination of pD-Al2O3 NPs and CpG led to higher levels of CTLs (CD8+/CD3+) and helper T cells (CD4+/CD3+) in the tumor draining lymph nodes (TDLNs) and spleen (Figure 5C). The mice received combinational treatment survived for more than 75 days, revealing effective inhibition on tumor recurrence and metastasis (Figure 5D). Interestingly, the researchers also tried replacing the Al2O3 core with Fe3O4 but found tumor inhibition became less effective (Figure 5E). This indicated that the Al2O3 core and CpG dual played adjuvant functions in this system. There were also other studies using the IR820-conjugated hydrogels loaded with CpG self-crosslinked NPs [68] or the IR7 encapsulated liposomes coated with HA-CpG [69] for combined PTT and immunotherapy.
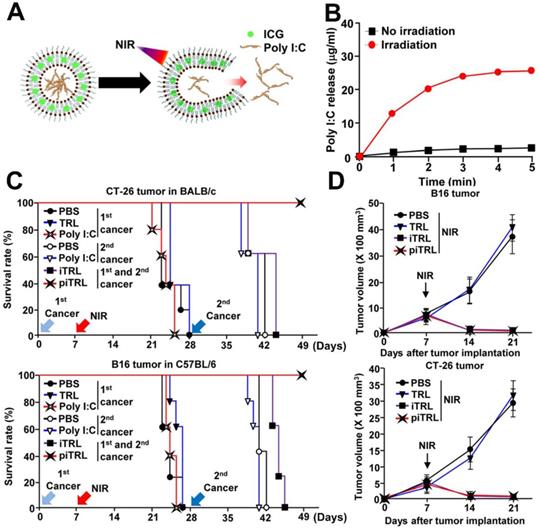
Additionally, Poly I:C is a double stranded RNA which binds to the TLR-3. It favors the cross-presentation of exogenous antigens by APCs [70] and is able to trigger both cellular and humoral immunity [71]. Documented by Xu et al. [72], ICG and poly I:C co-loaded thermoresponsive liposomes (piTRLs) were prepared (Figure 6A) for combinational therapy. In this research, ICG based PTT could not only ablate cancer cells but also trigger the release of poly I:C from piTRLs to activate DCs in the TDLNs (Figure 6B). After intratumoral injection of piTRL into the CT26 or B16F10 tumor-bearing mice followed then by laser irradiation, the tumor temperature rose to ca. 52 °C, and tumor growth was almost completely inhibited. Then, the cured mice were re-challenged with tumor cells through i.v. injection. Results showed that single PTT treated mice all died after the second round of inoculation, probably due to the insufficient activation of immune responses by PTT. On the contrary, the piTRL + laser treated mice all survived, suggesting a key role poly I:C played in protection against the disseminated metastatic tumors (Figure 6C-D). Further experiments proved that such protection originated from the tumor antigen specific immune responses.
(A) Schematic diagram of poly I:C release from piTRL under NIR-laser irradiation. (B) The concentration of released poly I:C from piTRL under NIR-laser irradiation at a power intensity of 1W cm-2. (C) The survival rate of CT-26 challenged BALB/c mice and B16 challenged C57BL/6 mice were monitored, n = 5 for each group. (D) Tumor growth curves for CT-26 and B16 carcinoma with or without laser irradiation. Data are from the analyses of six individual mice. Adapted with permission from [72], copyright 2019 Journal for Immunotherapy of Cancer.
Schematic illustration of A) the preparation process of RIC NPs@PLEL hydrogels and B) photothermal-immunotherapy to prevent post-surgery tumor recurrence. (C) Reversible sol-gel transformation photos of PLEL hydrogels and RIC NPs@PLEL hydrogels. (D) Recurrence-free rate of mice after various treatments. (E) Maturation of BMDCs. (F, G) TNF-α and IL-6 secreted in the BMDC cell culture supernatant after different treatments. **p < 0.01 and ***p < 0.001. (H) Lung metastatic nodules at the end of different treatments. Adapted with permission from [76], copyright 2020 Advanced Functional Materials.
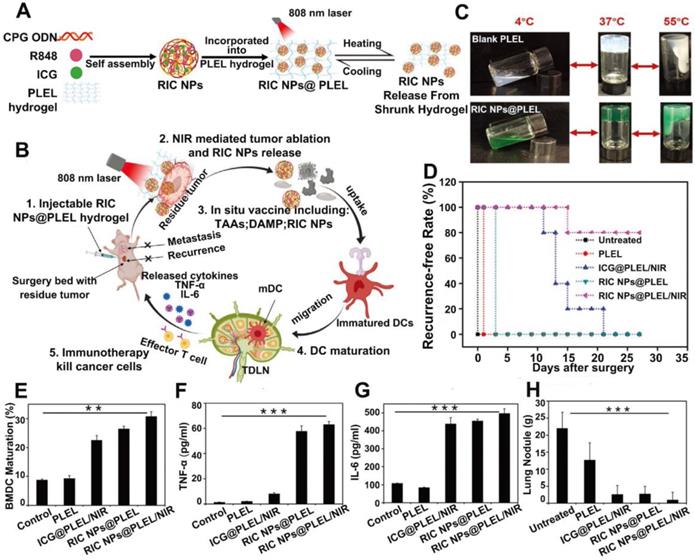
Recent studies also revealed that certain combinations of TLR agonists could strengthen the immune activation. Since different TLR agonists act on different PRRs, combination of them might mimic a natural infection and improve the antigen-specific immune responses [73, 74]. For example, the combination of aluminum salt with a TLR-4 agonist, MPLA, was approved by FDA for usage in the human papillomavirus (HPV) vaccines [75]. Therefore, dual immune adjuvants in combinatioan with PTT was also explored to further strengthen the anti-tumor immunity. Jia et al. [76] prepared a thermosentive PELE hydrogel loaded with RIC NPs which was self-assembled by ICG, R848 and CpG (RIC NPs@PLEL). This designed platform could act as an in situ vaccine to prevent postsurgery recurrence and metastasis (Figure 7A-B). The PLEL hydrogel was in liquid form at temperatures below 4 °C but quickly transformed into gels at body temperature, which greatly prolonged the retention of loading cargos at the injection site (Figure 7C). After incubation with 4T1 cells pretreated with RIC NPs@PLEL/NIR, DCs were successfully activated marked by an increased population of mature DCs (CD11c+CD80+CD86+) and promoted secretion of TNF-α and IL-6 (Figure 7E-G). After tumor resection, RIC NPs@PLEL was injected at the surgical bed followed by three cycles of laser irradiation (808 nm, 5 min), and 80% of the mice were completely cured without recurrence and no metastatic nodules were found in the lungs. In contrast, tumors all relapsed for other treatment groups, indicating the effectiveness of combinational therapy mediated by RIC NPs@PLEL (Figure 7D and 7H). In another similar study, surgically removed tumor cell membrane coated BPQDs (BPQDs-CCNVs) were loaded into a thermalsensitive hydrogel containing dual adjuvants for combined PTT and immunotherapy. BPQDs are biodegradable photothermal agents with high photothermal conversion efficiency and minimal cytotoxicity [28]. Lipopolysaccharide (LPS) together with granulocyte-macrophage colony stimulating factor (GM-CSF) served as adjuvants [23]. After firstly removing tumors by surgery, the Gel-BPQD-CCNVs were subcutaneously injected on the contralateral side and irradiated with NIR light. Tumor relapse and metastasis could be greatly inhibited. Results of flow cytometry revealed that the percentage of mature DCs in DLNs and the population of T cells in relapsed tumors were all markedly elevated. However, there was still a lack of explanation and discussion on the pairing of adjuvants and comparison between single adjuvant vs. dual adjuvants on the anti-tumor effect in these studies. Although these TLR agonists have demonstrated promising prospects and some of them have been evaluated in preclinical or clinical trials, there are still several challenges need to be addressed, including instability and off-target related side effects. For example, poly I:C could be degraded by serum nucleases, which hampers its capability in inducing IFNs or mediating anti-tumor immunity in primates. And there were reports about the severe side effects caused by R837, after oral or systemic administration [77]. What's more, the adjuvanticity of different TLR agonists have not been compared. Further investigation is still needed to evaluate the efficacy and safety of these adjuvants.
Schematic illustration of fabrication of eukaryotic-prokaryotic vesicles coated PI@EPV nanovaccine. Adapted with permission from [78], copyright 2020 Advanced Materials.
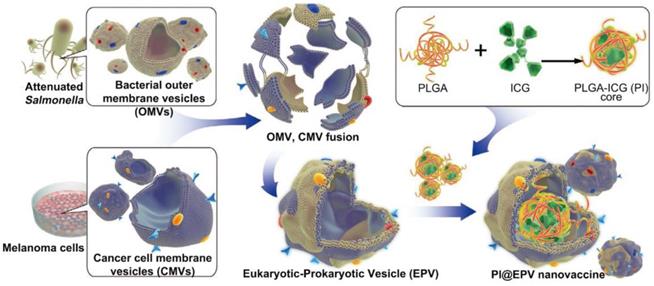
The integration of PTT with adjuvants could also be achieved using a biomimetic approach. For example, Chen et al. have prepared a eukaryotic-prokaryotic vesicle (EPV) nanoplatform by fusing melanoma cytomembrane vesicles (CMVs) with attenuated Salmonella outer membrane vesicles (OMVs) [78] (Figure 8). Herein, CMVs provided melanoma antigens and OMVs played the role of natural adjuvants. To further potentiate the tumor elimination capability, PLGA loaded with ICG(PI) was encapsulated into EPV (PI@EPV) to synergize immunotherapy with PTT. In vitro experiments found that PI@EPV was capable of stimulating DC maturation and T cell proliferation. And such immunoactivation was further boosted upon NIR laser irradiation due to the ICD effect caused by PTT. To test the ability of PI@EPV to prevent tumorgenesis, mice were intradermally immunized with nanovaccine for 3 times before the B16 or 4T1 tumor challenge. The PI@EPV treated group showed the highest tumor suppression rate (78.57%) as well as the longest tumor free time (19 days post-challenge) on the B16 tumor model. But it had no obvious suppression on the 4T1 tumor, revealing the specificity of anti-tumor immunity of melanoma cell vesicle-derived vaccine. Also, the PI@EPV vaccination greatly increased the tumor specific CTLs (CD3+CD8+CD107a) in spleens and enhanced the induction of effector memory T cells (CD3+CD8+CD44+CD62L-). Aside from the prophylactic effect, PI@EPV plus laser irradiation could also effectively eradicate the existing tumor with 80% of the treated mice became tumor free. Taken together, these results once again confirmed the enhanced anti-tumor efficacy after combining immunotherapy with PTT.
Nano-based PTT combined with immune checkpoint inhibitors for tumor treatment
The recurrence and metastasis of tumors could not be well controlled by PTT alone. Although PTT assisted with immune adjuvants could increase tumor immunogenicity and boost immune activation, the ITM may still dampen the final therapeutic outcomes. The ITM is associated with the PD-1/PD-L1 interaction between T cells and tumor cells as well as the presence of some negatively regulating immune cells, including Tregs, MSDCs or M2 phenotype macrophages. Therefore, it is sometimes necessary to utilize immune checkpoint inhibitors or regulators to reverse the ITM in order to improve the therapeutic efficacy of PTT. At present, the PD-1 or PD-L1 antibodies (e.g., pembrolizumab) [79] and the CTLA-4 antibodies (e.g., ipilimumab) [80] are most widely used to reinvigorate the CTLs.
PD-1 is a type of immunosuppressive molecule expressed on the surface of many types of cells, including T cells. And it can specifically interact with a transmembrane protein, PD-L1 which is overexpressed on many malignant tumors [81]. The binding of PD-L1 to PD-1 transmits inhibitory signals which lead to restrained cytokine excretion as well as apoptosis of T cells [82]. Therefore, ICB treatment was proposed by using PD-1 or PD-L1 antibodies to recover the function of T cells by blocking the PD-1/PD-L1 pathway.
In the study by Liang et al., the PD-1 antibody (aPD-1) was chosen to combine with PTT to treat basal like breast cancer [83]. Since this type of cancer has high mutation load and extensive lymphocyte infiltration, it is more likely to respond to ICB. Herein, BPQDs were photothermal agents and the erythrocyte membrane (RM) was coated on BPQDs (BPQD-RMNV) to achieve long blood circulation and high tumor accumulation. In the animal experiments, Balb/c mice were inoculated with 4T1-luc cancer cells on both flanks with an interval of 7 days. The first one represented the primary tumor and the second one represented the metastatic tumor. On the 8th day post-inoculation, mice were given different treatments through i.v. injection and subjected to NIR light irradiation. Subsequently, aPD-1 were i.v. injected every 3 days for 4 times. From the tumor size and bioluminescence signals, the combinational therapy (BPQD-RMNVs + laser + aPD-1) completely restricted the tumor growth on both sides and achieved the highest survival rate which significantly outperformed the aPD-1 or PTT treatment alone. The above results manifested the potential of combined PTT and immunotherapy in treating original and metastatic tumors.
Apart from combining ICB with PTT in the dose regimen, immune checkpoint inhibitors and photothermal agents could also be incorporated into one nanosystem for combinational therapy. Luo et al. encapsulated the anti-PD-1 peptide (APP) together with hollow gold nanoshell (HAuNS) into PLGA nanoparticles (denoted as AA@PN). It was found that a slow and continuous release of APP from nanoparticles could be obtained within 40 days [84]. After single i.t. injection followed by multiple times of laser irradiation, AA@PN + laser significantly inhibited the growth of both primary and untreated distant tumors on 4T1 or CT26 tumor models. Moreover, the survival rate maintained above 80% which was much higher compared to immunotherapy or PTT monotherapy. Notably, they also discovered that multi-dose APP treatment (0.2 mg per injection for 10 times) was much more effective in tumor inhibition compared to single APP treatment (1 time injection of 2 mg). This finding revealed that perdurable PD-1 blocking was very important to the efficacy of immunotherapy. Since AA@PN could release APP in a sustained manner, a better therapeutic effect was therefore obtained as a result of long-term PD-1 blocking. Also, the number of CD8+ CTLs and the production of IFN-γ in distant tumors were markedly increased after AA@PN + laser treatment. To further restrict the action site of ICB, there was another study used a LMDP peptide-conjugated Au@Pt NPs (Au@Pt-LMDP) for combinational therapy. The LMDP peptide was synthesized by connecting a hydrolysis-resistant D-peptide (DPPA-1, a PD-L1 antagonist) with a tumor-targeting peptide LyP-1 (CGNKRTRGC) via an MMP2-responsive linker (PLGVRG) [53]. After i.v. injection, this nanoplatform could accumulate at the tumor site and achieve on demand release of DPPA-1 induced by the abundant MMP2 in the TME. Such site specific delivery of immune checkpoint inhibitors could greatly minimize the unwanted side effects. Results showed that the inhibition rates for the primary and distal tumors after Au@Pt-LMDP plus laser treatment reached 100% and 88.52%, respectively. This demonstrated that the combination of PTT with PD1/PD-L1 blockage could effectively eliminate primary tumor and prevent tumor metastasis. Similarly, the enhanced therapeutic outcome was due to higher population of CD8+ CTLs in tumor and spleen as well as more secretion of pro-inflammatory cytokines, including IL-6, TNF-α, IFN-γ and IL-12p70.
Aside from the PD-1/PD-L1 pathway, CTLA-4, a receptor mainly expressed on Tregs, is another critical negative regulator of immune responses. It opposes the actions of CD28 mediated co-stimulation by competing for CD80 and CD86 [85]. Inhibitors that block the CTLA-4 signaling pathway can destroy the interaction between CTLA-4 and CD80/CD86, thereby facilitating the binding of CD28 with CD80/CD86 to strengthen the T-cell responses and induce T cell mediated tumor rejection [86]. In a study conducted by Juliana et al., PBNP mediated PTT was combined with anti-CTLA-4 checkpoint inhibition for the treatment of neuroblastoma [87]. The PBNP was i.t. injected into neuro2a tumor-bearing mice and irradiated with NIR light. The PTT treated tumors rapidly shrank at the beginning but soon recurred. While the PBNP based PTT + anti-CTLA-4 treated group exhibited complete tumor regression, with a survival rate of 55.5% over 100 days post treatment, which was significantly higher than that of the groups receiving anti-CTLA-4 alone (12.5%) or single PBNP-based PTT (0%). Moreover, the PTT + anti-CTLA-4 treatment also provided effective protection against secondary tumor growth after re-challenging the surviving mice with Neuro2a cells.
(A) Schematic illustration of M@C NPs for cancer immunotherapy by ICD induction and immune checkpoint blockade. (B) Expression of CRT proteins on the surface of 4T1 cells. (C) Changes of primary tumor growth. (D) The changes of distance tumors growth. (E) Quantification of fluorescent signals from immunofluorescent staining of CD3+ T cells and (F) CD8+ T cells in tumors. (G) ELISA analysis of IL-6 and (H) IL-12. i-vi represent mice treated with: (i)M@C + L + IDOi, (ii) M@C + L, (iii) M + L + IDOi, (iv) M@C + IDOi, (v) L, (vi) PBS. P values were calculated using the t-test (***P < 0.001, **P < 0.01, *P < 0.05) to compare other groups with group i. Adapted with permission from [90], copyright 2020 Chemical Communications.
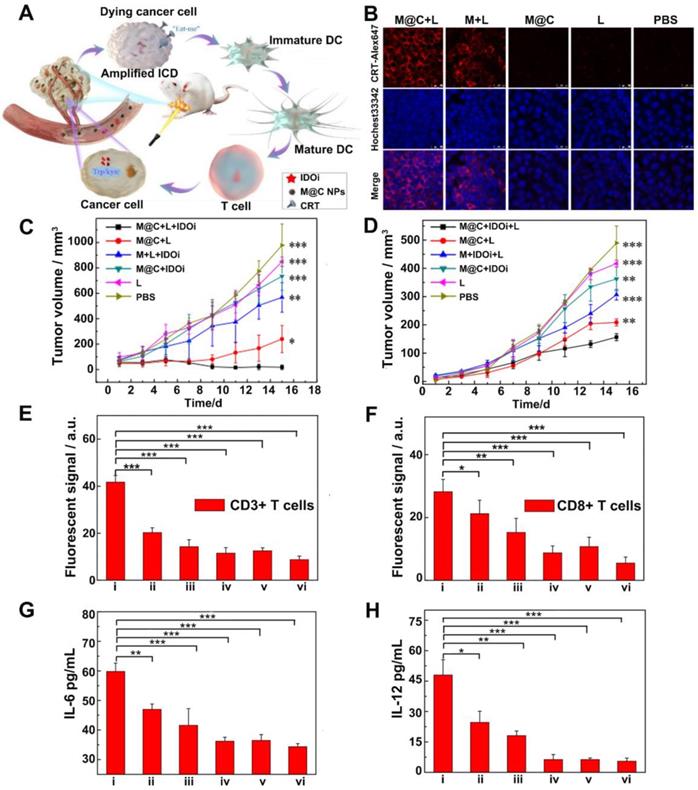
Indoleamine 2, 3-dioxygenase (IDO) is overexpressed in the TME for many types of cancer. It has been found to be another potential immune checkpoint modulator that participates in the escape of cancer cells from immune surveillance during tumor progression [5]. IDO is able to achieve enzymatic conversion of tryptophan to kynurenine, resulting in tryptophan deficiency, which disables the multiplication and functions of T lymphocytes and produces immune tolerance to tumor cells [88]. Therefore, IDO inhibitors have drawn wide attention as another alternative for ICB to relieve ITM [89]. For example, Li and coworkers [90] prepared cancer cell membrane-coated melanin nanoparticles (M@C NPs), in which melanin NPs served as photothermal agents with high photothermal conversion efficiency and good biocompatibility while the 4T1 cancer cell membrane helped the NPs evade clearance from circulation and target to the tumor site. Herein, the IDO inhibitor INCB24360 was used in combination with M@C NPs based PTT to enhance immune responses (Figure 9A). The results showed that PTT successfully induced ICD, as confirmed by the expression of CRT on the 4T1 cell membrane (Figure 9B). In vivo experiments revealed that the treatment with M@C NPs (i.v.) and IDO inhibitor (i.p.) plus laser irradiation strongly inhibited the growth of both primary and abscopal 4T1 tumors (Figure 9C-D). And the secretion levels of IL-12 and IL-6 as well as the percentage of CD3+CD8+ T cells within tumors were also the highest among all the treatment groups (Figure 9E-H). This indicated the synergistic effect of PTT and immunotherapy in provoking anti-tumor immune responses.
Scheme of the preparation pathway of NLG919(IODI)/IR780 co-loaded micelles and the mechanism by which the NLG919/IR780-micelle mediated PTT combined with immunotherapy suppressed the growth of the tumor margin beyond effective PTT and the distal (or secondary) tumor. Adapted with permission from [91], copyright 2018 Advanced Science.
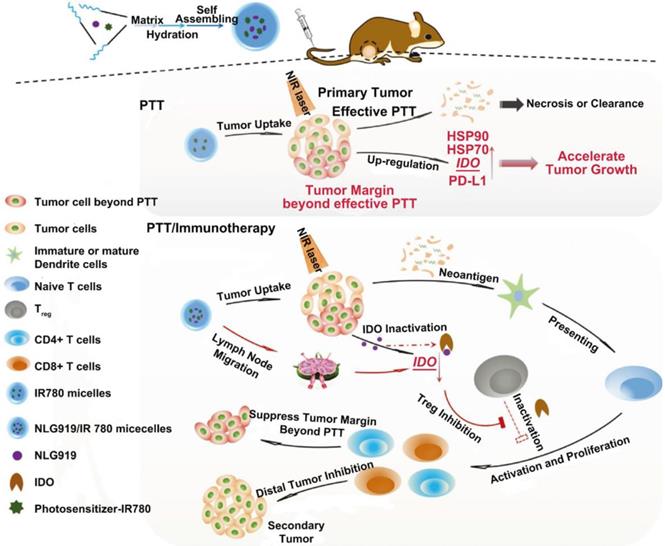
In another study by Peng et al., MPEG-PCL micelles co-loaded with an IDO inhibitor (NLG919) and a photosensitizer (IR780) were prepared for combinational therapy [91] (Figure 10). Researchers found that the penetration of PTT was rather limited. Around the heat generating source, the radius of tumor tissue with temperature above 43 °C was less than 6 mm. What's more, the tumor margin beyond effective PTT proliferated even faster due to the up-regulated HSP, PD-L1 and IDO which participate in the protection and evasion of cancer cells. Despite the enhanced tumor accumulation of micelles after i.v. injection, PTT mediated by IR780 micelles failed to restrain tumor growth compared to the control group; but once it combined with NLG919, significant tumor regression was observed. They also found that the combinational treatment could suppress the growth of secondary tumors as well as lung metastasis on 4T1 tumor models due to the inhibited IDO activity and elevated ratio of CD8+ T cells to Tregs.
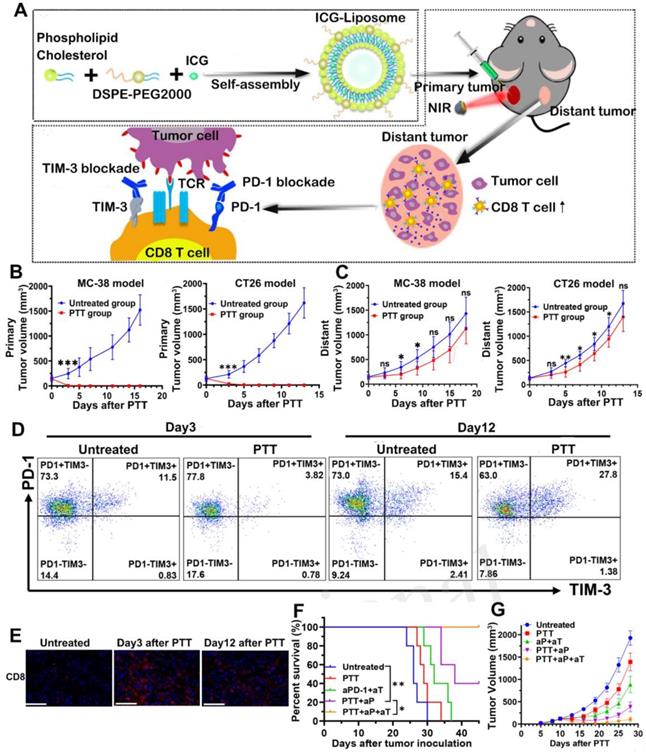
Notably, there were also studies using two kinds of ICBs combined with PTT to enhance the therapeutic outcome. T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) is an indicator of antigen-specific T cell exhaustion and involves in the negative regulation of Th1 response. Therefore, anti-TIM-3 antibody has been used to relieve T cell anergy [92]. For example, Huang et al. [93] prepared ICG encapsulated liposomes to perform PTT and combined it with dual PD-1/TIM-3 blockade for effective cancer treatment (Figure 11A). In this study, the anti-tumor efficacy was tested on the bilateral MC-38 and CT26 tumor models. From the results, local PTT raised the tumor temperature to near 60 °C and completely ablated the primary tumors (Figure 11B). PTT also exhibited modest inhibition on distant tumors at an early stage after treatment, which was due to the trafficking of activated CD8+ T cells from peripheral immune organs to tumor tissues (Figure 11C and 11E). However, PTT alone was insufficient to maintain long-term recession of distant tumors, since the CD8+ T cells gradually underwent functional exhaustion as a result of compensatory up-regulation of PD-1 and TIM-3 at a later stage after PTT (Figure 11D). After combining PTT with dual blockade of PD-1 and TIM-3 (PTT + aP + aT), the distant tumor growth was significantly delayed with a tumor-free survival of 40% (Figure 11F-G). Such superior anti-tumor efficacy proved the synergistic effect of PTT with immunotherapy.
(A) The schematic illustration for ICG-loaded liposome as a theranostic nanoplatform and PTT in the primary tumor and immunological changes in the distant tumor after PTT with dual blockade of PD-1 and TIM-3. (B) Primary tumor growth profile of MC-38 and CT26 bearing mice from each group. (C) Distant tumor outgrowth curves of double MC-38 tumor-bearing mice and double CT-26 tumor-bearing mice. (D) Representative flow cytometric plots showing PD-1 and TIM-3 expression in tumor infiltrating CD8+ T cells on days 3 and 12 after PTT. (E) CD8+ T cells infiltration of each group in the MC-38 TME on day 3 and day 12 after treatment were examined by immunofluorescence. (F) Kaplan-Meier survival curves and (G) distant tumor growth of tumor-bearing mice. Adapted with permission from [93], copyright 2020 Frontiers in Chemistry.
Furthermore, the integration of ICBs, immune adjuvants and PTT was also explored to obtain a better therapeutic effect. For example, mPEG-PLGA polymer co-loaded with Fe3O4 superparticles and R837 (Fe3O4-R837 SPs) was prepared to combine with PD-L1 checkpoint inhibitor for the treatment of 4T1 murine breast cancer [94]. Herein, the Fe3O4 SPs could not only perform PTT but also increase tumor accumulation via magnetic targeting. The in vivo results revealed that single PTT mediated by Fe3O4 SPs could directly eradicate the primary tumors but had little effect on the distant tumors. With the involvement of immune adjuvant R837, the inhibitory effect on distant tumors was modestly enhanced. When further combined with ICB, the Fe3O4-R837 SPs + anti-PD-L1 + laser showed significant inhibition on both the primary and distant tumors and effectively prevented lung/liver metastasis. And it is worth mentioning that ICB therapy alone could not well control the secondary tumor growth, which suggested that only when the tumors had turned from “cold” to “hot” after PTT and became immunogenic enough, the benefits of ICB therapy could be obtained. In another example, Song and coworkers have developed a PEGylated Bi2Se3 nanocage loaded with R848 (NC-PEG/R848) to combine with ICB [95]. The Bi2Se3 NC possessed distinguished photothermal conversion ability, and the hollow structure favored R848 loading with high efficiency. And PEGylation facilitated the tumor accumulation of NC via EPR effect. Likewise, the combination therapy was the most effective in inhibiting the primary and distant tumors.
(A) Schematic illustration of NIR (II) PTT combined with immunotherapy for cancer treatment. (B) Schematic showing controllable aggregation of AuNPs on fluidic liposomes. Local DOPC clusterings were shown in red color. AuNPs were absorbed on gel-phased liposomes when the temperature was below melting temperature and aggregated when the temperature was above melting temperature. (C) Schematic diagram of the in vivo CALR exposure at different depths inside the tumor under the NIR(I) or NIR(II) laser irradiation. (D) Percentage of CALR positive areas of dissected tumor tissues at different depths (0, 3, 6 and 9 mm). Data are shown as mean ± SD (n = 3) **p < 0.005, ***p < 0.001, ****p < 0.0001. (E) Immunohistochemical staining of CALR exposure in 4T1 tumors received 660, 808, and 1064 nm PTT. (F) Schematic showing the structure and therapeutics releasing process of erythrocyte membrane-camouflaged nanobullets. Adapted with permission from [96, 97], copyright 2020 and 2019 ACS Nano.
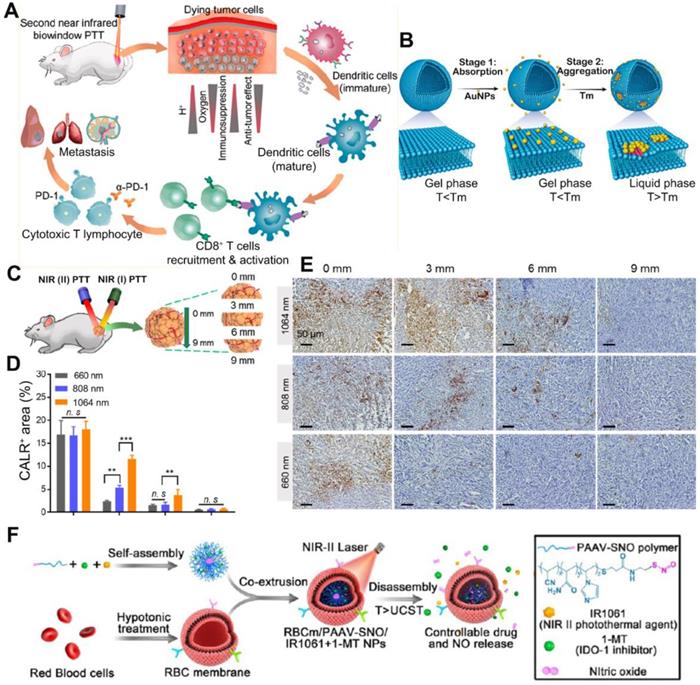
Other strategies to synergize nano-based PTT with immunotherapy
Recently, the exploration of PTT in the second near infrared (NIR II, 950-1350 nm) window has drawn great attention. Compared to the first NIR light (NIR I 650-950 nm), the NIR II biowindow with minimal photo scattering, low tissue background, and deep tissue penetration depth to ca. 3-5 cm is more attractive for PTT [96]. Photothermal agents which could respond to the NIR II light can induce more homogeneous and extensive ICD, and reverse the TEM from “cold” to “hot”. In the research by Ma et al., citrate coated Au NPs with 40 nm were attached to the DOPC liposomes(Au40C-DOPC) to achieve the NIR II PTT, since the aggregation of Au NPs on the lipid membrane could lead to the redshift of LSPR as a result of interparticle plasmon coupling (Figure 12A-B). Compared to the light of 660 nm and 808 nm, the NIR II light of 1064 nm had deeper penetration depth within tumor tissues (Figure 12C-E). Upon irradiation by 1064 nm laser, Au40C-DOPC effectively induced ICD at the very inside of tumor and significantly elevated the infiltration of CD8+ T cells. To test the tumor prevention effect, mice were s.c. injected with 4T1 cells pre-treated by Au40C-DOPC mediated PTT twice with 1 week apart. After re-challenging the mice with live tumor cells at the 7th day post vaccination, the tumor growth was significantly delayed with 5/8 of the mice remained tumor free. Researchers also tested the anti-tumor efficacy of NIR II PTT combined with immune adjuvant (R837 loaded in PEG-PLGA micelle) and immune checkpoint inhibitors (anti-PD-L1) on the bilateral tumor models. Results showed that both the primary tumor and distant tumor was suppressed significantly after the combinational therapy. Similarly, Yang and co-workers explored the anti-tumor effect of IR1061 mediated NIR II PTT combined with IDO inhibitor (1-MT) [97]. IR1061 and 1-MT were co-encapsulated into nanoparticles self-assembled by a poly (acrylamide-co-acrylonitrile-co-vinylimidazole)-S-nitrosothiols copolymer, PAAV-SNO). Then the nanoparticles were camouflaged with an erythrocyte membrane for long circulation (Figure 12F). Herein, SNO is a thermal sensitive nitric oxide (NO) donor, which could release NO upon the breakage of S-NO bond by PTT [98]. After further being camouflaged with an erythrocyte membrane, the final obtained nanoparticles were denoted as RBCm/PAAV-SNO NPs. From the bio-distribution study, the RBCm facilitated the tumor accumulation of nanoparticles due to its ability to avoid phagocytic clearance and extend blood circulation. The IR1061 mediated NIR II PTT could abate the tumor cells deeply and effectively induce ICD marked by increased CRT exposure and HMGB1 release. Upon 1064 nm laser irradiation, the release of NO and 1-MT within tumor tissue was triggered as a result of chemical bond cleavage and particle disassembly. Moreover, with the vessel normalization and hypoxia relief mediated by NO and the IDO inhibition by 1-MT, the ITM was efficiently reversed. To test the therapeutic effect, 4T1-tumor bearing mice were i.v. injected 3 times with different formulations and subjected to laser irradiation or not at 24 h post injection. Results revealed that single IDO inhibition only had limited anti-tumor efficacy, once combined with PTT and NO gas therapy, the tumor growth was significantly inhibited with 4/6 mice became tumor free. By analyzing the intratumoral cell population, an increase of tumor infiltration of CD8+ T cells as well as decreased number of Tregs and M2-like macrophages were found after the combinational treatment. Also, the level of TNF-α and IFN-γ was greatly augmented.
Other than immune adjuvants and ICB, CAR-T therapy is also an appealing option for combination with PTT. The principle of CAR-T therapy is that by modifying T cells with chimeric antigen receptors which specifically target to tumor, the tumor cells could be easily identified and eliminated by T cells without the involvement of major histocompatibility complex (MHC) [99]. However, the desmoplastic structure of tumor and the presence of ITM usually attenuated the efficacy of CAR-T therapy. To overcome these challenges, Chen et al. combined the PLGA-ICG (PI) mediated PTT with CAR T therapy to obtain a better therapeutic outcome [100]. In this study, CAR T cells which target to the chondroitin sulfate proteoglycan-4 (CSPG4) antigen overexpressed in melanoma (e.g., WM115 cell line) were prepared. It was found that WM115 cells pre-treated by PTT could induce a vigorous proliferation of CSPG4.CAR T cells as well as an increased production of IL-2 and IFN-γ. Also, PTT was able to increase tumor perfusion, relieve the hypoxia and facilitate the accumulation of CAR CSPG4+ T in tumors on the WM115 xenograft model. The combinational anti-tumor activity was also verified. After i.t. injection of PI followed by laser irradiation (808 nm, 0.3W cm-2, 20 min), 1 × 107 CAR.CSPG4+ T cells were i.v. injected. The combined treatment significantly inhibited tumor growth with 2 out of 6 mice being completely cured. This result confirmed the superior anti-tumor efficacy after combining PTT with CAR-T therapy.
Recently, the stimulator of interferon gene (STING) mediated immunotherapy has been focused on. STING is a kind of ER membrane signaling protein, which can sense cyclic dinucleotides (CDNs) and activate immune response as well [101]. STING agonists have been exploited as new immune adjuvants. Several studies have shown that STING signaling is vital to anti-tumor immunity in triggering the production of type I IFN and activating both the innate and adaptive immunity [102]. Zhao et al. have prepared a nano-complex composed of cyclic dimeric guanosine monophosphate-adenosine monophosphate (cGAMP, a STING agonist) and the polyethyleimine modified golden nanorod (GNR-PEI) for combined treatment [103]. It was revealed that after co-incubation with the osteosarcoma K7 cells pre-treated by GNR-PEI/cGAMP with or without laser irradiation, the BMDCs was effectively activated via a STING dependent signaling pathway marked by the up-regulation of phosphorylated interferon regulatory factor 3 (IRF3), IFN-β and CXC chemokine ligand-10 (CXCL10). And the treatment of GNR-PEI/cGAMP + laser successfully elicited ICD evidenced by augmented CRT exposure, HMGB1 release, and ATP secretion. Even though GNR-PEI/cGAMP + laser could strongly inhibit the primary tumors on K7 xenograft model, it was insufficient in restraining the secondary tumor growth. After further complemented with anti-PD-1, the growth of distant tumors was significantly retarded. Also, an increased population of CD8+ T cells, M1-like macrophages and NK cells together with a decreased percentage of MDSC, Tregs and M2-like macrophages were observed within tumors.
Additionally, PTT combined with cytokine therapy for the oral treatment of tumors has been reported as well. Fan and co-workers [104] have creatively developed a thermally-sensitive programmable bacteria (TPB) based on the non-invasive bacterium, E. coli MG1655. The TPB was transformed with the plasmid pBV200 which contained a thermally-sensitive promoter TcI and the gene of a therapeutic protein, TNF-α. Then bio-mineralized Au NPs were decorated on TPB obtaining TPB@Au to achieve PTT assisted immunotherapy. Results showed that TPB could prevent the decomposition of loaded genes in stomach, enter systemic circulation from the gastrointestinal tract and target to tumor tissues due to their homing ability to TME. Upon laser irradiation, the heat generated by PTT relieved the TcI repression and initiated the expression of TNF-α to induce the apoptosis of tumor cells. Results revealed that the 4T1 tumors were significantly inhibited after the treatment by TPB@Au plus laser mainly as a result of intratumoral release of TNF-α. However, the anti-tumor effect of single PTT was not compared in parallel. Although it was shown that TPB@Au had negligible impact on intestinal microflora and homeostasis, the safety of this system still needs further confirmation. Similarly, in the study by Lin et al., a MSN co-loading CuS within the pore and a plasmid encoding IL-12 gene on the outer surface (CSP@IL-12) was prepared for combinational treatment [105]. IL-12 is a kind of pro-inflammatory cytokine mainly secreted by APCs and plays a vital role in the proliferation of CTLs and NK cells. It could also trigger the production of IFN-γ, TNF-α, IL-2, GM-CSF perforin and granzyme B to enhance the anti-tumor immune responses. From the results, the secretion of IL-12 by B16F10 cells could be detected after effective gene delivery by the CSP nanoparticles. Upon laser irradiation, the CSP@IL-12 significantly induced ICD on B16F10 cells and promoted the maturation of DCs. However, the influence of heat generation on the IL-12 gene expression was not investigated. Also, the CSP@IL-12 + laser showed the strongest inhibition on both the primary and distant tumors compared to single PTT or IL-12 cytokine treatment.
Conclusions
Overall, nano-based PTT could effectively debulk primary tumors by thermal ablation and induce ICD to trigger host immune responses. However, increasing evidence suggests that PTT alone cannot control distant tumor growth and prevent metastasis. After combining PTT with immune adjuvants, the maturation of DCs was significantly promoted, as was the secretion of pro-inflammatory cytokines, which favors the activation and infiltration of CTLs to the tumor site. Also, the introduction of exogenous tumor antigens could further strengthen the immunogenicity of tumors. Based on the results of many research articles, immune adjuvant-assisted PTT showed stronger inhibition on both primary and distant tumors. A growing number of studies have begun to realize that the modulation of ITM is of equal importance compared to immune stimulation. PTT was alternatively combined with immune checkpoint inhibitors, such as anti-PD-1/PD-L1, anti-CTLA-4 or IDO inhibitors. Results showed that ITM was effectively relieved after the combined treatment, marked by an increased ratio of CTLs to immunosuppressive cells (Tregs, M2 phenotype macrophages or MDSCs) within tumor tissues. This combination was found to be effective in complete tumor elimination and metastasis prevention since those inhibitors could unleash the CTLs to kill tumor cells by shutting down the negative regulating pathways. Moreover, enhanced anti-tumor efficacy could also be obtained after combining PTT with CAR-T therapy or cytokine therapy. Since PTT was able to increase the tumor infiltration of immune cells and to control the release of immune activating cytokines. In view of limited penetration depth of NIR I PTT, photothermal materials which in the NIR II biowindow were also explored, and the combination of NIR II PTT with immunotherapy have achieved satisfied therapeutic effect on animals.
Until now, a variety of nanoplatforms have been developed. And they played important roles in the combination of PTT with immunotherapy, such as 1)serving as carriers to encapsulate photothermal agents and immune modulators, sometimes nano-sized photothermal agents could be carriers by themselves; 2)protecting the loaded cargo from rapid degradation or clearance; 3)enabling the therapeutic agents to accumulate within tumors or migrate to LNs to alleviate systemic side effects; 4)facilitating the cellular uptake of antigens and the controlled release of immune modulators. Apart from these capabilities, an ideal nanoplatform should also have good biodegradability and biocompatibility, which is the prerequisite for clinical use in the future.
In summary, the combination of nano-based PTT with immunotherapy has shown great potential and efficacy in fighting against cancer and more therapeutic strategies and nanomaterials are yet to be explored in order to obtain better therapeutic effect and benefit the patient clinically.
Perspectives
Even though satisfactory therapeutic outcomes were obtained after combining PTT with immunotherapy in lots of studies, there are still several concerns that need to be addressed. Firstly, the optimal temperature for PTT remains to be confirmed. Conclusions from different articles were sometimes contradictory. In the example reported by Sweeney and coworkers [47], PBNP-mediated PTT within an optimal thermal window from 50 to 60 ℃ could trigger ICD more effectively and demonstrated a much higher survival rate on the neuroblastoma tumor model compared to that within the lower temperature range (<50 °C) or higher temperature range (>80 °C). And many studies introduced in this review achieved effective tumor ablation with PTT at temperatures above 50 °C. However, there were also studies emphasizing the importance of low-temperature hyperthermia. For example, many researchers [61, 106] argued that high temperature (>50 °C) would cause damage to normal issues, including immune cells, while mild hyperthermia (ca. 45 °C) was beneficial to induce DAMPs such as HSP70, which was necessary to trigger immune responses. Moreover, the slightly elevated temperature also facilitates the migration of various immune cells to the tumor site due to accelerated blood and lymph flow. Taken together, higher temperatures are favorable for tumor ablation but may adversely affect immune cells. While low temperature might be insufficient for tumor eradication, but it can modulate the local TME and better activate the immune system. Therefore, the optimal thermal dose remains to be explored in the future. Secondly, the administration form of the nanoplatforms also needs to be carefully considered. Hydrogels could be a good option for local drug delivery co-encapsulating photothermal agents and immune regulators in treatment of superficial tumors (e.g., melanoma). And many studies applied the hydrogels on the surgical bed after tumor resection to eliminate the residual tumor cells [76, 107, 108]. The biocompatible hydrogels with high loading efficiency can achieve controlled or sustained release of immune modulators. Moreover, hydrogels can prolong retention time of photothermal agents which can reduce the times of administration. For example, in Jia and coworkers' research [76], which was mentioned before, after one intratumoral injection of RIC NPs@PLEL on day 0, the tumor site could be repeatedly irradiated on days 1, 4 and 7, which was highly convenient. However, it is regretful that hydrogel so far was not suitable for systemic administration. Thirdly, the dose regimen or schedule varied greatly between studies, including the dosage of photothermal agents and immune modulators as well as the timing, frequency and route of administration. However, there were rare studies compared the effect of dose regimen on the anti-tumor efficacy of combinational treatment. And the optimal dose regimen should be further screened. Lastly, the immune modulating effect of biomaterials involved in these nanoplatforms should be further explored. Sometimes, the nanomaterial could demonstrate immune stimulatory or suppressive effect, for example, nanomaterials composed of Al2O3 or polysaccharides were reported to have adjuvant effect [109-111].
Despite the excellent therapeutic effect of combined PTT and immunotherapy on animal studies, clinical translation still faces many challenges. The specific mechanisms of immune responses induced by PTT are complicated, and the intensity and controllability of immune responses during combination therapy are also continually to be studied. Moreover, the potential risks of PTT to patients caused by toxicity of nanomaterials, hyperthermia or laser power density should not be ignored. For example, the high temperature or prolonged irradiation time aiming to obtain stronger thermal ablation might result in unexpected damage to noncancerous tissue nearby. The safe power density limit is ~0.33 W cm-2 for the 808 nm NIR laser and ~0.726 W cm-2 for the 980 nm NIR laser as indicated by American National Standard for the Safe Use of Lasers, but there was higher power density being used in the above mentioned studies [112]. Also, the safety for immunotherapy should be explored further. The commonly seen immune related side effects (irAE) including various inflammation, neurological toxicity, anaphylaxis and cytokine release syndrome should be also be noted [113]. Currently, all of these studies are in the laboratory stage, and the feasibility and safety of these innovatively designed nanoplatforms need to be further studied before they are used in the clinic. Even so, the combination of PTT and immunotherapy still shows great potential to become an important complement for tumor treatments.
Abbreviations
APCs: antigen presenting cells; AuNSs: gold nanostars; BPQDs: black phosphorus quantum dots; CAR-T: chimeric antigen receptor T cells; CD: carbon nanodots; CRT: calreticulin; CpG ODNs: cytosine-phosphate-guanine oligodeoxynucleotides; CTLs: cytotoxic T lymphocyte; CTLA-4: cytotoxic T lymphocyte-associated antigen 4; CXCL10: CXC chemokine ligand-10; DAMPs: damage-associated molecular patterns; DCs: dendritic cells; Dox: doxorubicin; DLNs: draining lymph nodes; ER: endoplasmic reticulum; GCS: glycol-chitosan; GM-CSF: granulocyte-macrophage colony stimulating factor; GNPs: gold nanoparticles; GNRs: gold nanorods; HMGB1: high mobility group box 1; HSP: heat shock proteins; IDO: indoleamine 2,3-dioxygenase; IFN-γ: interferon-γ; ICB: immune checkpoint blockade; ICD: immunogenic cell death; ICG: indocyanine green; IRF3: interferon regulatory factor 3; ITM: immunosuppressive tumor microenvironment; LPS: lipopolysaccharide; IgG: immunoglobulin G; IL-10: interleukin-10; IL-12: interleukin-12; ITM: immunosuppressive tumor microenvironment; LNs: lymph nodes; MDSC: myeloid-derived suppressor cell; MSN: mesoporous silica nanoparticles; MHC: major histocompatibility complex; MPDA NPs: mesoporous polydopamine nanoparticles; MPLA: monophosphoryl lipid A; NIR: near-infrared; OVA: ovalbumin; PAMPs: pathogen associated molecular patterns; PANI: polyaniline; PBNP: prussian blue nanoparticle; pD: polydopamine; PD-1/PD-L1: programmed cell death 1/programmed death ligand 1; PDT: photodynamic therapy; poly I:C: polyinosinic:polycytidylic acid; TRL: thermo-responsive liposomes; PTT: photothermal therapy; RM: erythrocyte membrane; TAAs: tumor-associated antigens; TDLNs: tumor draining lymph nodes; TGF-β: transforming growth factor β; TIM-3: T cell immunoglobulin and mucin domain containing protein 3; TME: tumor microenvironment; TLR: Toll-like receptor; Tregs: regulatory T cells; STING: stimulator of interferon gene; SWCNT: single-walled carbon nanotubes.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant number 81803737); the Fundamental Research Funds for the Central Universities (Grant number 2019-JYB-XJSJJ008); and the Young Scientist Cultivation Program of Beijing University of Chinese Medicine. The authors also thank Jeffrey Silverberg and Yihua Pei for assistance with language editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wild CP. The global cancer burden: necessity is the mother of prevention. Nat Rev Cancer. 2019;19:123-4
2. Li W, Yang J, Luo L, Jiang M, Qin B, Yin H. et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. 2019;10:3349
3. Ducassou A, Gambart M, Munzer C, Padovani L, Carrie C, Haas-Kogan D. et al. Long-term side effects of radiotherapy for pediatric localized neuroblastoma: results from clinical trials NB90 and NB94. Strahlenther Onkol. 2015;191:604-12
4. Shang T, Yu X, Han S, Yang B. Nanomedicine-based tumor photothermal therapy synergized immunotherapy. Biomater Sci. 2020;8:5241-59
5. Ng CW, Li J, Pu K. Recent progresses in phototherapy-synergized cancer immunotherapy. Adv Funct Mater. 2018 28
6. Yu X, Zheng H, Chan MTV, Wu WKK. Immune consequences induced by photodynamic therapy in non-melanoma skin cancers: a review. Environ Sci Pollut Res Int. 2018;25:20569-74
7. Hwang HS, Shin H, Han J, Na K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J Pharm Investig. 2018;48:143-51
8. Xu X, H Lu, R Lee. Near infrared light triggered photo/immuno-therapy toward cancers, Front Bioeng Biotechnol. 2020; 8: 488.
9. Ahmad R, Fu J, He N, Li S. Advanced gold nanomaterials for photothermal therapy of cancer. J Nanosci Nanotechnol. 2016;16:67-80
10. Yata T, Takahashi Y, Tan M, Nakatsuji H, Ohtsuki S, Murakami T. et al. DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials. 2017;146:136-45
11. Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9:1074
12. Oei AL, Vriend LEM, Crezee J, Franken NAP, Krawczyk PM. Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol. 2015 10
13. Zhang X, Tang J, Li C, Lu Y, Liu J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioactive Materials. 2021;6:472-489
14. Zhang F, Lu G, Wen X, Li F, Ji X, Li Q. et al. Magnetic nanoparticles coated with polyphenols for spatio-temporally controlled cancer photothermal/immunotherapy. J Control Release. 2020;326:131-9
15. Wang S, Zhang Q, Luo XF, Li J, He H, Yang F. et al. Magnetic graphene-based nanotheranostic agent for dual-modality mapping guided photothermal therapy in regional lymph nodal metastasis of pancreatic cancer. Biomaterials. 2014;35:9473-83
16. Yang H, He H, Tong Z, Xia H, Mao Z, Gao C. The impact of size and surface ligand of gold nanorods on liver cancer accumulation and photothermal therapy in the second near-infrared window. J Colloid Interface Sci. 2020;565:186-96
17. Liao Z, Zhang W, Qiao Z, Luo J, Ai Niwaer AE, Meng X. et al. Dopamine-assisted one-pot synthesis of gold nanoworms and their application as photothermal agents. J Colloid Interface Sci. 2020;562:81-90
18. Li J, Zhang C, Gong S, Li X, Yu M, Qian C. et al. A nanoscale photothermal agent based on a metal-organic coordination polymer as a drug-loading framework for effective combination therapy. Acta Biomater. 2019;94:435-46
19. Wang Y, Barhoumi A, Tong R, Wang W, Ji T, Deng X. et al. BaTiO3-core Au-shell nanoparticles for photothermal therapy and bimodal imaging. Acta Biomater. 2018;72:287-94
20. Gong P, Guo L, Pang M, Wang D, Sun L, Tian Z. et al. Nano-sized paramagnetic and fluorescent fluorinated carbon fiber with high NIR absorbance for cancer chemo-photothermal therapy. J Mater Chem B. 2018;6:3068-77
21. Yang J, Su H, Sun W, Cai J, Liu S, Chai Y. et al. Dual chemodrug-loaded single-walled carbon nanohorns for multimodal imaging-guided chemo-photothermal therapy of tumors and lung metastases. Theranostics. 2018;8:1966-84
22. Cao W, Cao W, Liu R, Zhu J, Liu R, Zhu J. Synthesis and applications of two-dimensional transition metal sulfide nanomaterials in photothermal therapy. General Chemistry. 2018 4
23. Ye X, Liang X, Chen Q, Miao Q, Chen X, Zhang X. et al. Surgical tumor-derived personalized photothermal vaccine formulation for cancer immunotherapy. ACS Nano. 2019;13:2956-68
24. Fernandes N, Rodrigues CF, Moreira AF, Correia IJ. Overview of the application of inorganic nanomaterials in cancer photothermal therapy. Biomater Sci. 2020;8:2990-3020
25. Ruiz-Perez L, Rizzello L, Wang J, Li N, Battaglia G, Pei Y. Polypyrrole and polyaniline nanocomposites with high photothermal conversion efficiency. Soft Matter. 2020;16:4569-73
26. Meng F, Wang J, Ping Q, Yeo Y. Camouflaging nanoparticles for ratiometric delivery of therapeutic combinations. Nano Lett. 2019;19:1479-87
27. Huang Z-g, Lv F-m, Wang J, Cao S-j, Liu Z-p, Liu Y. et al. RGD-modified PEGylated paclitaxel nanocrystals with enhanced stability and tumor-targeting capability. Int J Pharm. 2019;556:217-25
28. Agrawal S, Dwivedi M, Ahmad H, Chadchan SB, Arya A, Sikandar R. et al. CD44 targeting hyaluronic acid coated lapatinib nanocrystals foster the efficacy against triple-negative breast cancer. Nanomedicine. 2018;14:327-37
29. Wu H, Gu D, Xia S, Chen F, You C, Sun B. One-for-all intelligent core-shell nanoparticles for tumor-specific photothermal-chemodynamic synergistic therapy. Biomater Sci. 2020
30. Kumar S, Mongia A, Gulati S, Singh P, Diwan A, Shukla S. Emerging theranostic gold nanostructures to combat cancer: novel probes for combinatorial immunotherapy and photothermal therapy. Cancer Treat Res Commun. 2020;25:100258
31. Yang Q, Peng J, Shi K, Xiao Y, Liu Q, Han R. et al. Rationally designed peptide-conjugated gold/platinum nanosystem with active tumor-targeting for enhancing tumor photothermal-immunotherapy. J Control Release. 2019;308:29-43
32. Conniot J, Silva JM, Fernandes JG, Silva LC, Gaspar R, Brocchini S. et al. Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front Chem. 2014;2:105
33. Topfer K, Kempe S, Muller N, Schmitz M, Bachmann M, Cartellieri M. et al. Tumor evasion from T cell surveillance. J Biomed Biotechnol. 2011;2011:918471
34. Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16-25
35. Bates JP, Derakhshandeh R, Jones L, Webb TJ. Mechanisms of immune evasion in breast cancer. BMC Cancer. 2018 18
36. Razavi S-M, Lee KE, Jin BE, Aujla PS, Gholamin S, Li G. Immune evasion strategies of glioblastoma. Frontiers in Surgery. 2016 3
37. Xu Z, Ramishetti S, Tseng Y-C, Guo S, Wang Y, Huang L. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J Control Release. 2013;172:259-65
38. Paulis LE, Mandal S, Kreutz M, Figdor CG. Dendritic cell-based nanovaccines for cancer immunotherapy. Curr Opin Immunol. 2013;25:389-95
39. Sayour EJ, Mendez-Gomez HR, Mitchell DA. Cancer vaccine immunotherapy with RNA-loaded liposomes. Int J Mol Sci. 2018 19
40. Song H, Yang P, Huang P, Zhang C, Kong D, Wang W. Injectable polypeptide hydrogel-based co-delivery of vaccine and immune checkpoint inhibitors improves tumor immunotherapy. Theranostics. 2019;9:2299-314
41. Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29-39
42. Wei X, Chen F, Xin K, Wang Q, Yu L, Liu B. et al. Cancer-testis antigen peptide vaccine for cancer immunotherapy: progress and prospects. Transl Oncol. 2019;12:733-8
43. Gao S, Yang D, Fang Y, Lin X, Jin X, Wang Q. et al. Engineering nanoparticles for targeted remodeling of the tumor microenvironment to improve cancer immunotherapy. Theranostics. 2019;9:126-51
44. Lu J, Liu X, Liao Y. P, Salazar F, Sun B, Jiang W, et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8:1811
45. Garg AD, Dudek-Peric AM, Romano E, Agostinis P. Immunogenic cell death. Int J Dev Biol. 2015;59:131-40
46. Qian M, Chen L, Du Y, Jiang H, Huo T, Yang Y. et al. Biodegradable mesoporous silica achieved via carbon nanodots-incorporated framework swelling for debris-mediated photothermal synergistic immunotherapy. Nano Lett. 2019;19:8409-17
47. Sweeney EE, Cano-Mejia J, Fernandes R. Photothermal therapy generates a thermal window of immunogenic cell death in neuroblastoma. Small. 2018;14:e1800678
48. Dacarro G, Taglietti A, Pallavicini P. Prussian blue nanoparticles as a versatile photothermal tool. Molecules. 2018 23
49. Zhang J, Mai J, Li F, Shen J, Zhang G, Li J. et al. Investigation of parameters that determine Nano-DC vaccine transport. Biomed Microdevices. 2019 21
50. Pan J, Wang Y, Zhang C, Wang X, Wang H, Wang J. et al. Antigen-directed fabrication of a multifunctional nanovaccine with ultrahigh antigen loading efficiency for tumor photothermal-immunotherapy. Adv Mater. 2018 30
51. An F, Yang Z, Zheng M, Mei T, Deng G, Guo P. et al. Rationally assembled albumin/indocyanine green nanocomplex for enhanced tumor imaging to guide photothermal therapy. J Nanobiotechnology. 2020;18:49
52. Liu Q, Fan T, Zheng Y, Yang SL, Yu Z, Duo Y. et al. Immunogenic exosome-encapsulated black phosphorus nanoparticles as an effective anticancer photo-nanovaccine. Nanoscale. 2020;12:19939-52
53. Zhang D, Wu T, Qin X, Qiao Q, Shang L, Song Q. et al. Intracellularly generated immunological gold nanoparticles for combinatorial photothermal therapy and immunotherapy against tumor. Nano Lett. 2019;19:6635-46
54. Kansy B, Lei Y, Li J, Srivastava RM, Stephenson R, Brandau S. et al. The role of toll-like receptor (TLR) agonists in combinational immunotherapy for head and neck cancer. Oral Oncol. 2015 51
55. Lee M, Park CS, Lee YR, Im SA, Song S, Lee CK. Resiquimod, a TLR7/8 agonist, promotes differentiation of myeloid-derived suppressor cells into macrophages and dendritic cells. Arch Pharm Res. 2014;37:1234-40
56. Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Front Immunol. 2014;5:461
57. Van Haren SD, Dowling DJ, Foppen W, Christensen D, Andersen P, Reed SG. et al. Age-specific adjuvant synergy: dual TLR7/8 and mincle activation of human newborn dendritic cells enables Th1 Polarization. J Immunol. 2016;197:4413-24
58. Wang L, He Y, He T, Liu G, Lin C, Li K. et al. Lymph node-targeted immune-activation mediated by imiquimod-loaded mesoporous polydopamine based-nanocarriers. Biomaterials. 2020;255:120208
59. Zeng X, Luo M, Liu G, Wang X, Tao W, Lin Y. et al. Polydopamine-modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments. Adv Sci (Weinh). 2018;5:1800510
60. Ding Y, Liu J, Lu S, Igweze J, Xu W, Kuang D. et al. Self-assembling peptide for co-delivery of HIV-1 CD8+ T cells epitope and Toll-like receptor 7/8 agonists R848 to induce maturation of monocyte derived dendritic cell and augment polyfunctional cytotoxic T lymphocyte (CTL) response. J Control Release. 2016;236:22-30
61. Chen PM, Pan WY, Wu CY, Yeh CY, Korupalli C, Luo PK. et al. Modulation of tumor microenvironment using a TLR-7/8 agonist-loaded nanoparticle system that exerts low-temperature hyperthermia and immunotherapy for in situ cancer vaccination. Biomaterials. 2020;230:119629
62. Nguyen HT, Phung CD, Thapa RK, Pham TT, Tran TH, Jeong JH. et al. Multifunctional nanoparticles as somatostatin receptor-targeting delivery system of polyaniline and methotrexate for combined chemo-photothermal therapy. Acta Biomater. 2018;68:154-67
63. Tian Q, Li Y, Jiang S, An L, Lin J, Wu H. et al. Tumor pH-responsive albumin/polyaniline assemblies for amplified photoacoustic imaging and augmented photothermal therapy. Small. 2019;15:e1902926
64. Ćirić-Marjanović G. Recent advances in polyaniline research: polymerization mechanisms, structural aspects, properties and applications. Synth Met. 2013;177:1-47
65. Ibarra LE, Tarres L, Bongiovanni S, Barbero CA, Kogan MJ, Rivarola VA. et al. Assessment of polyaniline nanoparticles toxicity and teratogenicity in aquatic environment using Rhinella arenarum model. Ecotoxicol Environ Saf. 2015;114:84-92
66. Hanagata N. Structure-dependent immunostimulatory effect of CpG oligodeoxynucleotides and their delivery system. Int J Nanomedicine. 2012;7:2181-95
67. Chen W, Qin M, Chen X, Wang Q, Zhang Z, Sun X. Combining photothermal therapy and immunotherapy against melanoma by polydopamine-coated Al2O3 nanoparticles. Theranostics. 2018;8:2229-41
68. Dong X, Liang J, Yang A, Qian Z, Kong D, Lv F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials. 2019;209:111-25
69. Li L, Yang S, Song L, Zeng Y, He T, Wang N. et al. An endogenous vaccine based on fluorophores and multivalent immunoadjuvants regulates tumor micro-environment for synergistic photothermal and immunotherapy. Theranostics. 2018;8:860-73
70. Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14:546-58
71. Bardel E, Doucet-Ladeveze R, Mathieu C, Harandi AM, Dubois B, Kaiserlian D. Intradermal immunisation using the TLR3-ligand Poly (I:C) as adjuvant induces mucosal antibody responses and protects against genital HSV-2 infection. npj Vaccines. 2016 1
72. Xu L, Zhang W, Park HB, Kwak M, Oh J, Lee PCW. et al. Indocyanine green and poly I:C containing thermo-responsive liposomes used in immune-photothermal therapy prevent cancer growth and metastasis. J Immunother Cancer. 2019;7:220
73. Hou Y, Wang Y, Tang Y, Zhou Z, Tan L, Gong T. et al. Co-delivery of antigen and dual adjuvants by aluminum hydroxide nanoparticles for enhanced immune responses. J Control Release. 2020;326:120-30
74. Kuai R, Sun X, Yuan W, Ochyl LJ, Xu Y, Hassani Najafabadi A. et al. Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy. J Control Release. 2018;282:131-9
75. Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H. et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186-97
76. Jia YP, Shi K, Yang F, Liao JF, Han RX, Yuan LP. et al. Multifunctional nanoparticle loaded injectable thermoresponsive hydrogel as NIR controlled release platform for local photothermal immunotherapy to prevent breast cancer postoperative recurrence and metastases. Adv Funct Mater. 2020 30
77. Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2011;29:3341-55
78. Chen Q, Huang G, Wu W, Wang J, Hu J, Mao J. et al. A hybrid eukaryotic-prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv Mater. 2020;32:e1908185
79. Reck M. Pembrolizumab as first-line therapy for metastatic non-small-cell lung cancer. Immunotherapy. 2018;10:93
80. Ariyan CE, Brady MS, Siegelbaum RH, Hu J, Bello DM, Rand J. et al. Robust antitumor responses result from local chemotherapy and CTLA-4 blockade. Cancer Immunol Res. 2018;6:189-200
81. Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol. 2015;23:32-8
82. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125:3384-91
83. Liang X, Ye X, Wang C, Xing C, Miao Q, Xie Z. et al. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J Control Release. 2019;296:150-61
84. Luo L, Yang J, Zhu C, Jiang M, Guo X, Li W. et al. Sustained release of anti-PD-1 peptide for perdurable immunotherapy together with photothermal ablation against primary and distant tumors. J Control Release. 2018;278:87-99
85. Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717-25
86. Hou X, Tao Y, Pang Y, Li X, Jiang G, Liu Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer. 2018;143:3050-60
87. Cano-Mejia J, Burga RA, Sweeney EE, Fisher JP, Bollard CM, Sandler AD. et al. Prussian blue nanoparticle-based photothermal therapy combined with checkpoint inhibition for photothermal immunotherapy of neuroblastoma. Nanomedicine. 2017;13:771-81
88. Lu J, Liu X, Liao YP, Wang X, Ahmed A, Jiang W. et al. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano. 2018;12:11041-61
89. Wachowska M, Stachura J, Tonecka K, Fidyt K, Braniewska A, Sas Z. et al. Inhibition of IDO leads to IL-6-dependent systemic inflammation in mice when combined with photodynamic therapy. Cancer Immunol Immunother. 2020;69:1101-12
90. Li Y, Liu X, Pan W, Li N, Tang B. Photothermal therapy-induced immunogenic cell death based on natural melanin nanoparticles against breast cancer. Chem Commun (Camb). 2020;56:1389-92
91. Peng J, Xiao Y, Li W, Yang Q, Tan L, Jia Y. et al. Photosensitizer micelles together with IDO inhibitor enhance cancer photothermal therapy and immunotherapy. Adv Sci (Weinh). 2018;5:1700891
92. Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540-51
93. Huang TY, Huang GL, Zhang CY, Zhuang BW, Liu BX, Su LY. et al. Supramolecular photothermal nanomedicine mediated distant tumor inhibition via PD-1 and TIM-3 blockage. Front Chem. 2020;8:1
94. Ge R, Liu C, Zhang X, Wang W, Li B, Liu J. et al. Photothermal-Activatable Fe3O4 superparticle nanodrug carriers with PD-L1 immune checkpoint blockade for anti-metastatic cancer immunotherapy. ACS Appl Mater Interfaces. 2018;10:20342-55
95. Song Y, Wang Y, Wang S, Cheng Y, Lu Q, Yang L. et al. Immune-adjuvant loaded Bi2Se3 nanocage for photothermal-improved PD-L1 checkpoint blockade immune-tumor metastasis therapy. Nano Res. 2019;12:1770-80
96. Huang B, Hu J, Li H, Luo M-Y, Chen S, Zhang M. et al. Near-infrared IIb emitting nanoprobe for high-resolution real-time imaging-guided photothermal therapy triggering enhanced anti-tumor immunity. ACS Applied Bio Materials. 2020;3:1636-45
97. Yang Z, Gao D, Guo X, Jin L, Zheng J, Wang Y. et al. Fighting immune cold and reprogramming immunosuppressive tumor microenvironment with red blood cell membrane-camouflaged nanobullets. ACS Nano. 2020 14
98. Zhang Y, Song T, Feng T, Wan Y, Blum NT, Liu C. et al. Plasmonic modulation of gold nanotheranostics for targeted NIR-II photothermal-augmented immunotherapy. Nano Today. 2020 35
99. June R.S.O.C. Carl H, Kawalekar Omkar U, Ghassemi Saba, Milone Michael C, CAR T cell immunotherapy for human cancer, Science. 2018; 359:1361-5.
100. Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn S, Wang C. et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater. 2019;31:e1900192
101. Wang ZB, Xu J. Better adjuvants for better vaccines: progress in adjuvant delivery systems, modifications, and adjuvant-antigen codelivery. Vaccines (Basel). 2020 8
102. Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY. et al. Author correction: Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2019;570:E53
103. Zhao X, Han Y, Sun Y, Feng W, Liu J, Li D. et al. Combining photothermal ablation-based vaccine with immune checkpoint blockade for synergistic osteosarcoma immunotherapy. Mater Des. 2021 198
104. Fan JX, Li ZH, Liu XH, Zheng DW, Chen Y, Zhang XZ. Bacteria-mediated tumor therapy utilizing photothermally-controlled TNF-alpha expression via oral administration. Nano Lett. 2018;18:2373-80
105. Lin X, Wang X, Li J, Cai L, Liao F, Wu M. et al. Localized NIR-II photo-immunotherapy through the combination of photothermal ablation and in situ generated interleukin-12 cytokine for efficiently eliminating primary and abscopal tumors. Nanoscale. 2021;13:1745-58
106. Huang L, Li Y, Du Y, Zhang Y, Wang X, Ding Y. et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat Commun. 2019;10:4871
107. Zhuang B, Chen T, Xiao Z, Jin Y. Drug-loaded implantable surgical cavity-adaptive hydrogels for prevention of local tumor recurrence. Int J Pharm. 2020;577:119048
108. Fang L, Zhao Z, Wang J, Zhang P, Deng YP, Jiang YY. et al. Engineering autologous tumor cell vaccine to locally mobilize antitumor immunity in tumor surgical bed. Sci Adv. 2020;6:eaba4024
109. Carroll EC, Jin L, Mori A, Munoz-Wolf N, Oleszycka E, Moran HBT. et al. The vaccine adjuvant cChitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. 2016;44:597-608
110. Moran HBT, Turley JL, Andersson M, Lavelle EC. Immunomodulatory properties of chitosan polymers. Biomaterials. 2018;184:1-9
111. Sun BN, Yu S, Zhao DY, Guo SH, Wang XH, Zhao K. Polysaccharides as vaccine adjuvants. Vaccine. 2018;36:5226-34
112. Chen T, Cen D, Ren Z. et al. Bismuth embedded silica nanoparticles loaded with autophagy suppressant to promote photothermal therapy. Biomaterials. 2019;221:119419
113. Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Vareki SM. et al. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors-a systematic review and meta-analysis. Cancer Treat Rev. 2021 92
114. Zhou B, Wu Q, Wang M, Hoover A, Wang X, Zhou F. et al. Immunologically modified MnFe2O4 nanoparticles to synergize photothermal therapy and immunotherapy for cancer treatment. Chem Eng J. 2020;396:125239
115. Zhou Y, Liu S, Hu C, Cai L, Pang M. A covalent organic framework as a nanocarrier for synergistic phototherapy and immunotherapy. J Mater Chem B. 2020;8:5451-9
116. Ma Y, Zhang Y, Li X, Zhao Y, Li M, Jiang W. et al. Near-infrared II phototherapy induces deep tissue immunogenic cell death and potentiates cancer immunotherapy. ACS Nano. 2019;13:11967-80
Author contact
![]() Corresponding authors: N, Han, E-mail: hanning1989com, Tel: 18500083932; SY, Du, E-mail: dusyedu.cn, Tel: 17812165051.
Corresponding authors: N, Han, E-mail: hanning1989com, Tel: 18500083932; SY, Du, E-mail: dusyedu.cn, Tel: 17812165051.
 Global reach, higher impact
Global reach, higher impact