13.3
Impact Factor
Theranostics 2021; 11(15):7425-7438. doi:10.7150/thno.60211 This issue Cite
Research Paper
Generation of triacyl lipopeptide-modified glycoproteins by metabolic glycoengineering as the neoantigen to boost anti-tumor immune response
The school of Medicine, Nankai University, Tianjin 300071, China.
Abstract
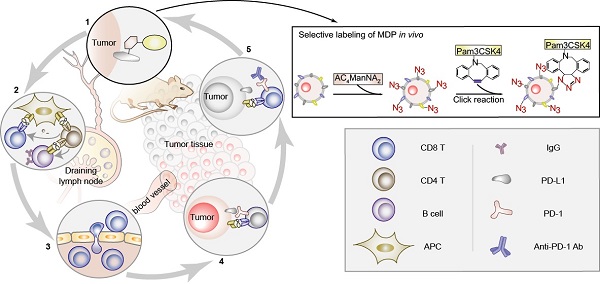
The lack of tumor specific antigens (TSA) and the immune tolerance are two major obstacles for the immunotherapy of cancer. Current immune checkpoint inhibitors (ICIs) show clinical responses in only limited subsets of cancer patients, which, to some extent, depends on the mutation load of tumor cells that may generate neoantigens. Here, we aimed to generate a neoantigen MDP to exhibit stronger anti-tumor efficacy.
Methods: In this study, we utilized chemically modified sialic acid precursor tetra acetyl-N-azidoacetyl-mannosamine (AC4ManNAZ) to engineer the glycoproteins on the membranes of tumor cells for the covalent ligation of hapten adjuvant Pam3CSK4 in vivo, which eventually generated a neoantigen, i.e., ManNAZ-DBCO-Pam3CSK4 (MDP), on tumor cells. The high labeling efficiency, relatively specific biodistribution in tumor tissues and the anti-tumor efficacy were confirmed in the syngeneic murine models of the breast cancer and the lung cancer.
Results: The generation of MDP neoantigen in tumor-bearing mice significantly evoked both the humoral and the T-cell-dependent antitumor immune responses, resulting in a strong inhibition on the growth of the breast cancer and the lung cancer allografts and significantly prolonged survival of tumor-bearing mice. Interestingly, MDP neoantigen was able to dramatically increase the sensitivity of cancer cells to ICIs and greatly enhance the anti-tumor efficacy in the murine models of both breast cancer and the lung cancer, which showed no or low responses to the immunotherapy with anti-PD1 antibody alone.
Conclusions: We developed a simple metabolic glycoengineering method to artificially generate neoantigens on tumor cells to enhance tumor cell immunogenicity, which is able to significantly improve the response and the clinical outcome of ICIs.
Keywords: tumor immunotherapy, glycoengineering, tetra acetyl-N-azidoacetyl-mannosamine, Pam3CSK4, PD1/PD-L1
 Global reach, higher impact
Global reach, higher impact