13.3
Impact Factor
Theranostics 2021; 11(15):7379-7390. doi:10.7150/thno.58781 This issue Cite
Research Paper
TRPV2-spike protein interaction mediates the entry of SARS-CoV-2 into macrophages in febrile conditions
1. Key Laboratory of the Ministry of Education for Conservation and Utilization of Special Biological Resources in the Western, Yinchuan 750021, China.
2. College of Life Science, Ningxia University, Yinchuan 750021, Ningxia, China.
3. Faculty of Basic Medicine, Shanghai Jiao tong University School of Medicine, Shanghai, China.
*These authors contributed equally to this paper.
Abstract
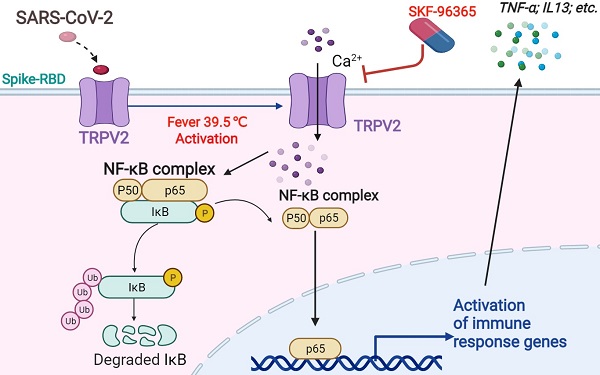
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel strain of highly contagious coronaviruses that infects humans. Prolonged fever, particularly that above 39.5 °C, is associated with SARS-CoV-2 infection. However, little is known about the pathological effects of fever caused by SARS-CoV-2.
Methods: Primary bovine alveolar macrophages (PBAMs), RAW264.7 mouse macrophages, and THP-1 human cells were transfected with plasmids carrying the genes encoding the SARS-CoV-2 spike (S) protein or receptor-binding domain (RBD). Proteins in the macrophages interacting with S-RBD at 39.5 °C or 37 °C were identified by immunoprecipitation-mass spectrometry. Glutathione S-transferase pulldown, surface plasmon resonance, and immunofluorescence were performed to evaluate the transient receptor potential vanilloid 2 (TRPV2) interaction with SARS-CoV-2-S-RBD at 39.5 °C. Using an RNA sequencing-based approach, cytokine gene expression induced by SARS-CoV-2 S transfection at 39.5 °C and 37.5 °C in primary alveolar macrophages was measured. Fluo-4 staining and enzyme-linked immunosorbent assays were used to assess the regulatory function of TRPV2 in intracellular Ca 2+ and cytokines under SARS-CoV-2-S-RBD at 39.5 °C. Additionally, cytokine release was examined after TRPV2 knockdown with shRNA oligonucleotides or inhibition using the SKF-96365 antagonist.
Results: We identified an interaction between the primary alveolar macrophage receptor TRPV2 and S-RBD under febrile conditions. Febrile temperature promotes Ca2+ influx through SARS-CoV-2 infection in PBAMs, further activates the NF-κB p65 signaling pathway, and enhances the secretion of cytokines. Furthermore, knockdown or antagonist (with SKF-96365) of TRPV2 significantly decreased the release of cytokines that drive the inflammatory response.
Conclusion: Collectively, our findings identified TRPV2 as a receptor of SARS-CoV-2 in conditions of febrile temperature, providing insight into critical interactions of SARS-CoV-2 with macrophages, as well as a useful resource and potential drug target for coronavirus disease 2019.
Keywords: SARS-CoV-2, Spike protein receptor-binding domain, TRPV2, SKF-96365, primary bovine alveolar macrophage
 Global reach, higher impact
Global reach, higher impact