13.3
Impact Factor
Theranostics 2021; 11(15):7337-7359. doi:10.7150/thno.57804 This issue Cite
Research Paper
HIF-1-induced mitochondrial ribosome protein L52: a mechanism for breast cancer cellular adaptation and metastatic initiation in response to hypoxia
1. Department of Breast Surgery, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China.
2. Department of Cardiology, Shanghai Institute of Cardiovascular Diseases, Zhongshan Hospital, Fudan University, Shanghai, China.
3. Department of Cardiology, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China.
4. Department of Surgical Oncology and General Surgery, Key Laboratory of Precision Diagnosis and Treatment of Gastrointestinal Tumors, Ministry of Education, The First Affiliated Hospital of China Medical University, Shenyang, Liaoning, China.
# Xinyan Li and Mengshen Wang contributed equally to this work.
Abstract
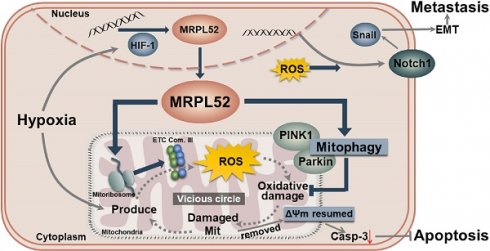
Background: Hypoxia is a hallmark of the physical microenvironment of solid tumors. As a key factor that regulates tumor development and progression, hypoxia can reprogram the expression of multiple genes, whose biological function and molecular mechanism in cancer remain largely unclear. The mitochondrial ribosome protein family consists of nuclear-encoded mitochondrial proteins that are responsible for protein synthesis in the mitochondria.
Methods: A high-throughput RNA sequencing assay was carried out to identify differentially expressed mRNAs between breast cancer tissues and adjacent normal tissues as well as breast tumors with metastasis and those without metastasis. Our clinical samples and TCGA database were analyzed to observe the clinical value of mitochondrial ribosome protein L52 (MRPL52) in human breast cancer. Potent hypoxia response elements in the promoter region of MRPL52 were identified and validated by chromatin immunoprecipitation and luciferase reporter assays. Functional experiments were performed using breast cancer cell lines with MRPL52 ectopic expression and knockdown cultured in a 20% or 1% O2 environment.
Results: MRPL52 expression was upregulated in human breast cancer and was significantly associated with aggressive clinicopathological characteristics and a higher metastatic risk of breast cancer patients. We found that the overexpression of MRPL52 in breast cancer is induced by hypoxia-inducible factor-1 in response to hypoxic exposure. The role of MRPL52 in suppressing apoptosis and promoting migration and invasion of hypoxic breast cancer cells was demonstrated by our experimental evidence. Mechanistically, MRPL52 promoted PTEN-induced putative kinase 1 /Parkin-dependent mitophagy to remove oxidatively damaged mitochondria and prevent uncontrolled reactive oxygen species (ROS) generation, thus repressing activation of the mitochondrial apoptotic cascade. Additionally, MRPL52 augmented epithelial-mesenchymal transition, migration and invasion of hypoxic breast cancer cells by activating the ROS-Notch1-Snail signaling pathway. Benefited from this bidirectional regulatory mechanism, MRPL52 is responsible for maintaining ROS levels in a window that can induce tumorigenic signal transduction without causing cytotoxicity in hypoxic breast cancer cells.
Conclusions: This work elucidates the molecular mechanism by which MRPL52 mediates hypoxia-induced apoptotic resistance and metastatic initiation of breast cancer, and provides new insights into the interplay between cancer and the tumor microenvironment.
Keywords: Breast cancer, Hypoxia, Mitochondrial ribosome, Mitophagy, Metastasis
 Global reach, higher impact
Global reach, higher impact