13.3
Impact Factor
Theranostics 2021; 11(15):7322-7336. doi:10.7150/thno.56990 This issue Cite
Research Paper
Engineering circular RNA regulators to specifically promote circular RNA production
1. Second Affiliated Hospital, Institute of Cancer Stem Cell, Dalian Medical University, Dalian, China 116044.
2. Department of Pathology, First Affiliated Hospital, Dalian Medical University, Dalian, China 116044.
#These authors contributed equally to this work.
Abstract
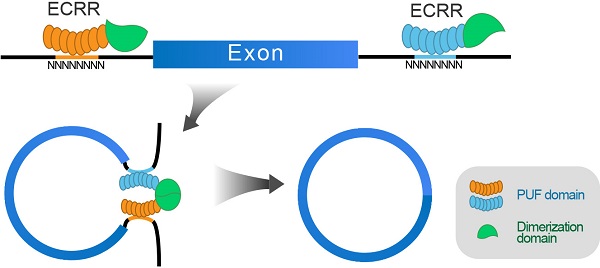
Background: A large number of circular RNAs (circRNAs) have been discovered in the mammalian transcriptome with high abundance, which play vital roles in gene regulation, thereby participating in the development of multiple diseases. However, the biogenesis, regulation, and especially manipulation of circRNAs still remain largely unknown.
Methods: Engineering circRNA regulators (ECRRs) were developed to promote circRNA biogenesis. Multiple circRNA mini-gene reporters were generated to evaluate the regulatory role of ECRRs. RT-PCR, qRT-PCR, northern blot, western blot, and flow cytometry assays were applied to assess the efficiency of artificial circRNA regulators on circRNA production in the presence or absence of RNase R treatment.
Results: We engineered circRNA regulators by combining sequence-specific RNA binding motifs of human Pumilio 1 with functional domains that could form dimerization. We applied these engineered regulators to promote the circRNA production of the exogenous circRNA minigene reporter circGFP, thereby stimulating the functional GFP protein generation. Crucially, such regulation is in time-course dependent and dose-dependent manners with designed specificity. Moreover, the application of ECRRs could also stimulate circRNA biogenesis of another minigene reporter circScreen, suggesting that ECRRs can be commonly used to promote circRNA generation of exogenous reporters. Most importantly, ECRRs could be utilized to specifically promote the production of the endogenous circRNAs circ10720 and circBIRC6 as well.
Conclusion: Our approach allows the creation of engineered regulators to target virtually any pre-mRNA in vivo, offering a novel avenue to investigate circRNA biogenesis and manipulate disease-related circRNA production.
Keywords: circRNA, back-splicing, PUF domain, dimerization, ECRRs
 Global reach, higher impact
Global reach, higher impact