13.3
Impact Factor
Theranostics 2021; 11(15):7276-7293. doi:10.7150/thno.54630 This issue Cite
Research Paper
Closed-loop trans-skull ultrasound hyperthermia leads to improved drug delivery from thermosensitive drugs and promotes changes in vascular transport dynamics in brain tumors
1. School of Mechanical Engineering, Georgia Institute of Technology, Atlanta, USA.
2. Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta, GA, USA.
3. Department of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, GA, USA.
4. Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
5. Department of Pediatrics, Medical University of South Carolina, Charleston, SC, USA.
6. Department of Radiology, Stanford University, Stanford, CA, USA.
Abstract
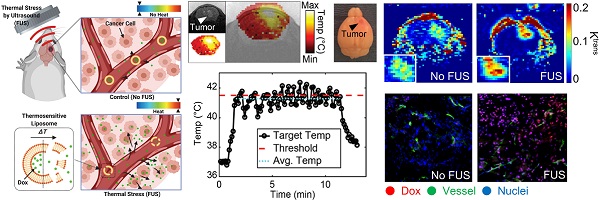
Effective drug delivery in brain tumors remains a major challenge in oncology. Although local hyperthermia and stimuli-responsive delivery systems, such as thermosensitive liposomes, represent promising strategies to locally enhance drug delivery in solid tumors and improve outcomes, their application in intracranial malignancies remains unexplored. We hypothesized that the combined abilities of closed-loop trans-skull Magnetic Resonance Imaging guided Focused Ultrasound (MRgFUS) hyperthermia with those of thermosensitive drugs can alleviate challenges in drug delivery and improve survival in gliomas.
Methods: To conduct our investigations, we first designed a closed loop MR-guided Focused Ultrasound (MRgFUS) system for localized trans-skull hyperthermia (ΔT < 0.5 °C) in rodents and established safety thresholds in healthy mice. To assess the abilities of the developed system and proposed therapeutic strategy for FUS-triggered chemotherapy release we employed thermosensitive liposomal Dox (TSL-Dox) and tested it in two different glioma tumor models (F98 in rats and GL261 in mice). To quantify Dox delivery and changes in the transvascular transport dynamics in the tumor microenvironment we combined fluorescent microscopy, dynamic contrast enhanced MRI (DCE-MRI), and physiologically based pharmacokinetic (PBPK) modeling. Lastly, to assess the therapeutic efficacy of the system and of the proposed therapeutic strategy we performed a survival study in the GL261 glioma bearing mice.
Results: The developed closed-loop trans-skull MRgFUS-hyperthermia system that operated at 1.7 MHz, a frequency that maximized the brain (FUS-focus) to skull temperature ratio in mice, was able to attain and maintain the desired focal temperature within a narrow range. Histological evidence (H&E and Nissl) suggests that focal temperature at 41.5 ± 0.5 °C for 10 min is below the threshold for tissue damage. Quantitative analysis of doxorubicin delivery from TSLs with MRgFUS-hyperthermia demonstrated 3.5-fold improvement in cellular uptake in GL261 glioma mouse tumors (p < 0.001) and 5-fold increase in delivery in F98 glioma rat tumors (p < 0.05), as compared to controls (TSL-Dox-only). Moreover, PBPK modeling of drug transport that was calibrated using the experimental data indicated that thermal stress could lead to significant improvement in the transvascular transport (2.3-fold increase in the vessel diffusion coefficient; P < 0.001), in addition to promoting targeted Dox release. Prospective experimental investigations with DCE-MRI during FUS-hyperthermia, supported these findings and provided evidence that moderate thermal stress (≈41 °C for up to 10 min) can promote acute changes in the vascular transport dynamics in the brain tumor microenvironment (Ktrans value for control vs. FUS was 0.0097 and 0.0148 min-1, respectively; p = 0.026). Crucially, survival analysis demonstrated significant improvement in the survival in the TSL-Dox-FUS group as compared to TSL-Dox-only group (p < 0.05), providing supporting evidence on the therapeutic potential of the proposed strategy.
Conclusions: Our investigations demonstrated that spatially controlled thermal stress can be attained and sustained in the mouse brain, using a trans-skull closed-loop MRgFUS system, and used to promote the effective delivery of chemotherapy in gliomas from thermosensitive drugs. This system also allowed us to conduct mechanistic investigations that resulted in the refinement of our understanding on the role of thermal stress in augmenting mass and drug transport in brain tumors. Overall, our study established a new paradigm for effective drug delivery in brain tumors based on closed-loop ultrasound-mediated thermal stress and thermosensitive drugs.
Keywords: focused ultrasound, hyperthermia, thermosensitive drugs, thermal stress, brain cancer
 Global reach, higher impact
Global reach, higher impact