13.3
Impact Factor
Theranostics 2021; 11(15):7199-7221. doi:10.7150/thno.57745 This issue Cite
Review
Triptolide: pharmacological spectrum, biosynthesis, chemical synthesis and derivatives
1. School of Traditional Chinese Medicine, Capital Medical University, Beijing, 100069, China.
2. Beijing Shijitan Hospital, Capital Medical University, Beijing, 100038, China.
3. State Key Laboratory Breeding Base of Dao-di Herbs, National Resource Center for Chinese Materia Medica, Chinese Academy of Chinese Medical Sciences, Beijing, 100700, China.
4. Basic Medical College, Henan University of Chinese Medicine, Zhengzhou 450046, China.
5. Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, 100069, China.
Received 2021-1-1; Accepted 2021-4-29; Published 2021-5-24
Abstract
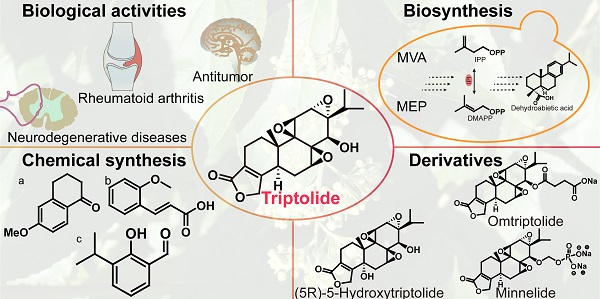
Triptolide, an abietane-type diterpenoid isolated from Tripterygium wilfordii Hook. F., has significant pharmacological activity. Research results show that triptolide has obvious inhibitory effects on many solid tumors. Therefore, triptolide has become one of the lead compounds candidates for being the next "blockbuster" drug, and multiple triptolide derivatives have entered clinical research. An increasing number of researchers have developed triptolide synthesis methods to meet the clinical need. To provide new ideas for researchers in different disciplines and connect different disciplines with researchers aiming to solve scientific problems more efficiently, this article reviews the research progress made with analyzes of triptolide pharmacological activity, biosynthetic pathways, and chemical synthesis pathways and reported in toxicological and clinical studies of derivatives over the past 20 years, which have laid the foundation for subsequent researchers to study triptolide in many ways.
Keywords: Tripterygium wilfordii, Triptolide, Biological activities, Biosynthesis, Chemical synthesis
1. Introduction
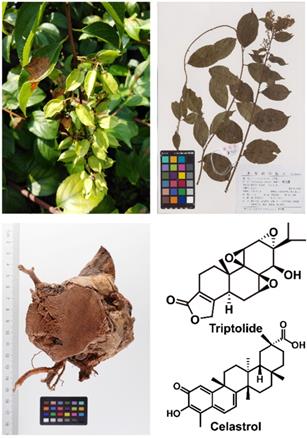
Tripterygium wilfordii Hook. F. is a traditional Chinese medicinal herb, commonly known as "Thunder God Vine" or "Lei Gong Teng" (Figure 1). It is mainly used to treat autoimmune and inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus [1], and its main active compounds are terpenoids including triptolide and celastrol. Triptolide (Figure 2) is currently considered to be one of the active compounds most likely to translate from traditional to modern medicine [2].
Triptolide is an important diterpene active compound in T. wilfordii. Since 1972, when Kupchan et al. first isolated triptolide and found its significant anti-leukaemic effects [3], it has also been proven to have significant anti-inflammatory, immunosuppressive, anticancer and other important biological activities (Figure 3) [4]. Currently, triptolide is mainly obtained from its extraction from medicinal plants and separation from other compounds. Due to the extremely low content of triptolide in medicinal plants (~66.5 μg/g) [5], chemical extraction and separation are insufficient to meet the needs of industrialization. Triptolide is an abietane-type diterpene with 3 epoxy groups and an α, β-unsaturated five-membered lactone ring structure, which makes the chemical synthesis process challenging and not commercially viable on an industrial scale. Therefore, the current research focus is the biosynthesis of triptolide and its precursor. In recent years, with increasingly intensive study into traditional Chinese medicine (TCM), researchers have developed medications based on active compounds such as artemisinin, Taxol and other effective compounds used in TCM. Moreover, artemisinin and paclitaxel are also successful examples of using the principles of synthetic biology used to produce natural products or their precursor compounds at high yields.
In addition, an increasing number of scientific research problems can be solved by interdisciplinary contributions. For example, predicting protein folding structure through AI technology considered among the top ten scientific breakthroughs of science. This example provides a reference for scientific researchers seeking breakthroughs of technical bottlenecks. By combining the ideas used in different disciplines to study triptolide, researchers may generate additional novel ideas.
Therefore, to obtain a deeper understanding of triptolide through the combination of multiple disciplinary approaches, we analyzed its biosynthetic pathway. Triptolide and its precursors were efficiently synthesized using the principles of synthetic biology, which laid the foundation for pharmacological research on triptolide, the precursor compounds used in triptolide biosynthesis and triptolide derivatives. This article reviews the research progress on triptolide in terms of its pharmacological activity, biosynthesis, chemical synthesis, and toxicology and discusses recent clinical trials of its derivatives. This review will help researchers better understand all aspects of triptolide and provides constructive suggestions for the further study of triptolide.
2. Biological functions
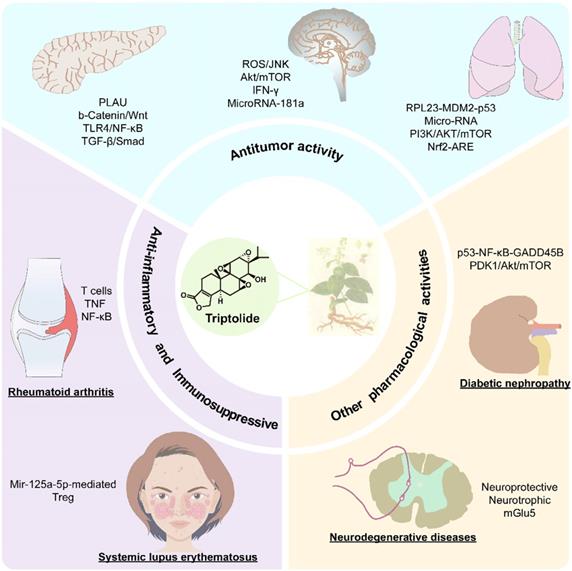
2.1. Anti-inflammatory and immunosuppressive effects of triptolide
As a TCM herb, T. wilfordii has long been used to treat conditions characterized by rheumatism, including rheumatoid arthritis, nephritis and systemic lupus erythematosus. Its main effective component, triptolide, has obvious anti-inflammatory and immunosuppressive effects [1]. Recent studies have shown that triptolide has a positive therapeutic effect on a variety of autoimmune and inflammatory diseases. It not only can induce apoptosis by inhibiting the proliferation of immune cells and inflammation-related cells but can also reduce the release of cytokines and pro-inflammatory mediators, thus inducing anti-inflammatory and immunosuppressive effects [4].
2.1.1. Rheumatoid arthritis
Rheumatoid arthritis (RA) is an inflammatory, autoimmune disease. Multiple studies have shown that triptolide can be effectively used to treat RA through various mechanisms. These findings suggest that triptolide is one of the main compounds critical for the therapeutic effect of traditional Chinese herbal remedies on RA. The current research on the mechanism of RA treatment with triptolide mainly includes the following aspects: (1) Reduction in joint inflammation in RA by inhibiting T cell secretion of inflammatory cytokines [6], (2) amelioration of inflammation in RA by inhibiting angiogenesis at the sites of inflammation [7], (3) induction of fibroblast apoptosis to inhibit the inflammatory response in RA [8], (4) reduction in the degree of inflammation by inhibiting multiple signaling pathways (e.g., the TREM1 signaling pathway) [9] , (5) induction of OCP cell apoptosis by mediating the degradation of clap2, inhibiting OC formation, which has a therapeutic effect on RA [10].
Tripterygium wilfordii and its important active ingredients.
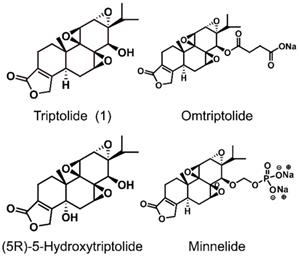
The structures of triptolide (1) and its derivatives.
The pharmacological activity and mechanism of triptolide.
To study the mechanisms by which triptolide exerts its effects in the treatment of rheumatoid arthritis, network pharmacology and molecular docking were used. Network pharmacology is a new discipline based on the theory of system biology, which analyzes the network of biological system and selects specific signal nodes for multi-target drug molecular design. Molecular docking is a method of drug design based on the characteristics of receptors and the interaction between receptors and drug molecules. First, considering network pharmacology, Yunbin Jiang et al. analyzed the anti-RA active compounds in T. wilfordii and concluded that triptolide and celastrol are the key active compounds. The data confirmed that the key molecular mechanism is related to the inhibition of the inflammatory response by inactivating the TNF and NF-κB signaling pathways [11]. Xinqiang Song et al. organized the genes and proteins related to RA in public databases through a creative approach, interpretative phenomenological analysis (IPA). Subsequently, molecular docking was used to predict the binding pockets of the six top candidate triptolide target proteins: CD274, RELA, MCL1, MAPK8, CXCL8 and STAT1 [12]. However, network pharmacology is mainly used to analyze big data for predicting potential genes, targets, proteins or signaling pathways. This approach can only provide a certain degree of referent information for the treatment of RA with triptolide. Therefore, researchers need to be cautious and rigorous in the analysis of network pharmacology results.
Triptolide has a significant therapeutic effect on RA, but due to the own toxicity it induces, the current research hotspot involves technology using nanomaterials to carry triptolide to target the release to the lesion. Studies have shown that the use of poly-γ-glutamic acid-grafted di-tert-butyl L-aspartate hydrochloride (PAT) to prepare a TP-containing nanodrug carrier system can reduce the toxicity of triptolide ensuring the therapeutic effect of triptolide and revealing its potential as an effective drug candidate for RA [13]. The use of amphiphilic pH-sensitive galactosyl dextran-retinal (GDR) nanoparticles to encapsulate triptolide may enhance the anti-inflammatory effect of CIA mouse models [14]. The latest results confirmed that by encapsulating triptolide in the star-shaped amphiphilic block copolymer POSS-PCL-b-PDMAEMA, the constructed pH-sensitive triptolide nanomedicine can achieve significant anti-inflammatory effects at ultra-low doses to treat RA [15]. The use of nanomaterials to carry triptolide has many advantages, such as targeted drug delivery and reduced triptolide dose. Nanomaterials provide effective solutions for accessing the narrow treatment window of triptolide. Nanomaterial carriers are examples of the combination of material chemistry and natural drugs, which in this case was used to address the limitations of triptolide.
2.1.2. Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disease. T. wilfordii has been used in the treatment of SLE for centuries, and has achieved remarkable results. Modern research shows that triptolide can alleviate SLE through miR-125a-5p-mediated upregulation of the Treg ratio [16].
2.1.3 Mechanisms of the anti-inflammatory and immunosuppressive effects of triptolide
Research indicates that triptolide exerts its anti-inflammatory and immunosuppressive effects via multiple targets. First, triptolide can regulate signaling pathways, such as by inhibiting the nuclear factor-κB (NF-κB) signaling pathway [17], or inhibiting the IL-6/signal transducer and transcription 3 (STAT3)-activated signaling pathway and downregulating IL-17 [18]. Second, triptolide can inhibit the expression of pro-inflammatory molecules, such as VCAM-1, TGF-β, C3 and CD40 [19, 20]. In addition, it can also inhibit myofibroblast infiltration, thereby improving multiple organ fibrosis [21]. With the continuous development of technology, researchers are clarifying the anti-inflammatory and immunosuppressive mechanisms of triptolide at the cellular, molecular and genetic levels.
2.2. Antitumor activity
Triptolide has significant, broad-spectrum antitumor and sensitizing effects. In recent years, increasing evidence has shown that triptolide has significant antitumor activity, with potential therapeutic effects on lung cancer, liver cancer, pancreatic cancer and other tumors [22, 23] (Table 1). The antitumor activity of triptolide is reflected in its strong cytotoxicity, which can nullify drug resistance, and inhibit neovascularization and tumor metastasis. Triptolide primarily exerts anticancer effects by inducing apoptosis [4]. In recent years, studies have shown that autophagy and cell senescence are also involved in this process [24, 25]. Triptolide is effective not only in ordinary tumor cells, but also in multidrug resistant cancer cells and some tumor stem cells [26, 27].
2.2.1. Lung cancer
Lung cancer is a malignancy with some of the highest mortality rates in the world. Studies have shown that triptolide can regulate the ribosomal RPL23-MDM2-p53 signaling pathway to disintegrate the nucleolus and inhibit rRNA synthesis, ultimately inducing cell cycle arrest and apoptosis to inhibit cell proliferation and tumor growth [28]. Reno et al. confirmed that triptolide can change the expression profile of miRNAs in lung cancer cells and inhibit the migration, invasion and metastasis of cancer cells [29]. This research has provided new ideas for the treatment of lung cancer and confirmed that triptolide can be used as a potential lung cancer treatment drug.
Studies have shown that triptolide has a potential therapeutic effect on non-small cell lung cancer (NSCLC). It can induce NSCLC cell apoptosis; downregulate Akt, mTOR and P70S6K phosphorylation levels [30]. At the same time, some researchers found that triptolide can reduce the Wnt signaling pathway, thereby reducing the proliferation of lung cancer cells, tumor formation and metastasis, to treat NSCLC. [31]. In addition to its anticancer effect on NSCLC, triptolide can also target the Nrf2 pathway to reduce the chemotherapy resistance of cancer cells, which provides a new potential therapeutic strategy for NSCLC [32].
At this stage, the combination of triptolide was a hot issue concerning researchers. In one regimen, triptolide is combined with the low-dose anti-inflammatory drug aspirin to prevent lung cancer. Studies have shown that triptolide can activate p53 and inhibit NF-κB at the same time, which has the potential to treat human cancer, and aspirin can improve the efficacy of triptolide [33]. The combination of anticancer drugs and anti-inflammatory drugs may be a promising method for the prevention and treatment of inflammation related cancers (such as lung cancer). In another combination of anticancer drugs, researchers designed lipid-polymer hybrid nanoparticles to serve as a coadministration system. Through in vivo and in vitro experiments, it was confirmed that the two drugs paclitaxel and triptolide in combination with LPN carriers had a synergistic effect in lung cancer transplantation and exhibited few systemic side effects [34]. There are obvious differences between the two methods. One way is to improve the efficacy of anticancer drugs by inhibiting the pathological process of the cancer response. Another way is to combine different anticancer drugs to form a new drug delivery system, improve the synergy of drugs, and reduce the side effects of drugs and drug resistance.
Anticancer effect of triptolide on different cancers.
| Tumor model | Pathway | Mechanism | Cell or animal model | Refs |
|---|---|---|---|---|
| Lung cancer | RPL23-MDM2-p53 | Induce apoptosis, cell cycle arrest and inhibition of cell proliferation | Lung cancer A549 cells (CCL‑185™) BALB/cAnNCr‑nu/nu mice | [28] |
| Micro-RNA | Reduce cancer cell migration and invasion | H460, A549, and H358 cells Nondiabetic severe combined immune deficiency γ mice | [29] | |
| PI3K/AKT/mTOR | Induce apoptosis, inhibition of cell proliferation, glycolysis and energy metabolism | H1299 and NCI-H460 cell | [30] | |
| Nrf2-ARE | Enhance cancer cell sensitivity to chemotherapy drugs | Non-small-cell lung cancer (A549) Mouse lung carcinoma 3LL cell liver carcinoma (HepG2) cell | [32] | |
| Liver cancer | c-Myc/micro-RNA | Induce apoptosis | Liver cancer cell lines SMMC-7721, MHCC-97H, and LM3 HepG2 and Hep3B | [35] |
| gene p53 | Inhibit the vitality of cancer cells Induce apoptosis | HepG2 and QSG7701 liver cancer cell | [36] | |
| Nerve tumor | ROS/JNK Akt/mTOR | Induced G2/M phase arrest, apoptosis, and autophagy | U251, U87-MG and C6 cells BALB/c-nu/nu nude mice | [37] |
| IFN-γ | Inhibits PD-L1 expression Reversing the inhibition of CD4+ T cells by cancer cells | Glioma cell lines, U251-MG, T98G, U87-MG, A172, LN229 and LN18 | [38] | |
| MicroRNA‐138 PI3K/AKT Notch | Induces apoptosis and inhibits cancer cell migration and proliferation | Human medulloblastoma Daoy cells and human embryonic kidney HEK293 cells | [39] | |
| MicroRNA-181a p38MAPK NF-kB | Inhibit the proliferation and migration of cancer cells | Human neuroblastoma SH-SY5Y cells | [40] | |
| Pancreatic cancer | b-Catenin/Wnt | Induces apoptosis of cancer cells | The pancreatic cancer cell line MIA PaCa-2 The S2-VP10 cell lines The BxPC-3, Capan-1 and Human pancreatic ductal epithelial cells Genetically engineered KPC (KrasG12D, P53R172HPDXCre) mice | [177] |
| TLR4/NF-κB | Enhances the sensitivity of pancreatic cancer PANC-1 cells to GEM | The human pancreatic cancer cell line PANC-1 Balb/c nude mice | [42] | |
| TGF-β/Smad | Modulate tumor microenvironment Reduces extracellular matrix (ecm) production | The human pancreatic cancer cell line PANC-1 Mouse embryonic fibroblast cell line NIH3T3 The human pancreatic cancer cell PANC-1-Luc2 Pancreatic tumor-bearing nude mice | [45] | |
| Prostate cancer | Caveolin-1/CD147/MMPs | Inhibition of cancer cell migration and invasion | PC-3 and DU145 human prostate cancer cell | [46] |
2.2.2. Liver cancer
In hepatocellular carcinoma (HCC), triptolide can inhibit the expression of miR-17-92 and miR-106b-25 by inhibiting c-Myc and upregulating their common target genes, thus leading to the death of HCC cells [35]. Moreover, at different concentrations, triptolide was found to induce the phosphorylation of p53 at the serine-15 residue in HepG2 cells. Activating the tumor suppressor gene p53 can induce the apoptosis of liver cancer cells [36].
Nanomaterial preparation for use with triptolide have consistently been the research focus of scholars. In recent years, researchers have designed galactosylated chitosan TP-nanoparticles (GC-TP-NPs) to induce tumor cell apoptosis by blocking the TNF/NF-κB/BCL2 signaling pathway [23]. These are promising drug candidates that prevent the progression of liver cancer while minimizing systemic toxicity.
2.2.3. Neural tumors
Gliomas are common and lethal malignant primary brain tumors that exhibit strong invasion, rapid progression and susceptibility to relapse, leading to a poor prognosis for patients. It has been proven that triptolide not only can inhibit the proliferation of glioma cells and block the cell cycle in the G2/M phase but can also induce apoptosis and protective autophagy. Moreover, triptolide-induced apoptosis and autophagy of glioma cells can inhibit each other. Triptolide can regulate the cell cycle, apoptosis and autophagy by activating ROS / JNK inhibitory functions and the Akt / mTOR signaling pathway [37]. In addition, triptolide can reverse the inhibitory effect of glioma cells on T cells and downregulate the expression of PD-L1 induced by IFN - γ [38]. Therefore, triptolide can be used as an alternative molecule for glioblastoma research and drug development.
Furthermore, triptolide can also achieve anticancer effects by regulating microRNAs. Haifang Zhang et al. found that triptolide can inhibit the PI3K/AKT and Notch pathways, thereby exerting an anticancer effect on medulloblastoma cells [39]. Similarly, studies have shown that triptolide can increase the expression of microRNA-181a, which participates in the proliferation, migration and apoptosis of SH-SY5Y cells, thereby exerting a tumor-suppressing effect [40].
2.2.4. Pancreatic cancer
Triptolide has obvious inhibitory effects on pancreatic cancer. Triptolide, as a super-enhancer (SE) interacting agent, may exhibit its antitumor activity by interfering with cell cross-talk and signal transduction in pancreatic ductal adenocarcinoma [41].
In addition to inhibiting malignant tumors, triptolide can enhance tumor sensitivity to drugs. For example, triptolide was found to enhance the sensitivity of pancreatic cancer PANC-1 cells to GEM [42]. Therefore, combined treatment modalities can offer better drug development prospects for pancreatic cancer. Studies have shown that triptolide can activate autophagy and enhance the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sensitivity of pancreatic cancer cells [43]. The drug resistance of malignant tumors is a limiting factor in the clinical application of many anticancer drugs. As a broad-spectrum anticancer drug, triptolide can inhibit the drug resistance of cancer cells, which provides a new research idea for the clinical application of triptolide and its derivatives.
In recent years, an increasing number of researchers have used nanotechnology to modify natural products to improve the efficacy of drugs and reduce side effects. For example, silk fibroin nanoparticles loaded with triptolide and celastrol have a certain synergistic effect, which includes reducing cell viability and significantly increasing the cell apoptosis rate, and may be used in a promising treatment strategy for pancreatic cancer [44]. The latest research shows that triptolide can be loaded onto CRPPR peptide-modified tumor-targeting acid-triggered micelles, which can improve the therapeutic effect of triptolide and reduce damage to off-target organs [45]. Therefore, it is believed that nontoxic nanomedicines based on active substances in traditional Chinese herbs have great potential as targeted and adjuvant chemotherapy for pancreatic cancer. Currently, the construction of TCM nanoformulations is providing new choices for antitumor drugs.
2.2.5. Other tumors
Triptolide also has antitumor activity in other solid tumors. For example, triptolide inhibits the proliferation, invasion and migration of prostate cancer cells. When shRNA is used to silence the expression of CAV-1, triptolide can reduce the propensity of human prostate cancer cells to migrate and invade tissue [46]. Triptolide inhibits the proliferation, invasion, migration and angiogenesis of oral cancer and oesophageal squamous cell carcinoma (ESCC) cells [47, 48]. Triptolide can trigger the death of colon cancer cells including through apoptosis and in vitro experiments indicate that triptolide is effective against colon cancer stem cells (CSCs) [49]. In addition, triptolide can reduce tumor-associated macrophage infiltration and inhibit the migration of colon cancer cells [50]. Triptolide is a potent Nrf2 inhibitor that can inhibit the transcriptional activity of Nrf2, leading to the apoptosis of isocitrate dehydrogenase (IDH)-mutant cells, providing an operable strategy for the treatment of malignant tumors with IDH1 mutations [51]. Triptolide can induce the apoptosis of cisplatin-resistant ovarian cancer cells and sensitize them to cisplatin [52]. Various transcription factors, proteins and signaling pathways are involved in the antitumor effects of triptolide, but its anticancer effect is mainly achieved by inducing apoptosis.
In addition to the solid tumors mentioned above, triptolide also has a strong effect on haematological malignancies. Studies indicate that triptolide can induce cell morphological changes and exert cytotoxic effects through G0/G1 phase arrest, as well as induce apoptosis, which may be related to cross talk between components involved in apoptosis and autophagy in vitro [53].
Multidrug resistance (MDR) is the main obstacle to chemotherapy in the treatment of cancer, and triptolide is expected to solve this problem. Triptolide can inhibit the proliferation of A549 lung adenocarcinoma cells resistant to paclitaxel through the MAPK/PI3K/AKT signaling pathway [54]. These studies indicate that triptolide has high-efficiency and broad-spectrum antitumor activity in multidrug resistant tumor cells. Triptolide also plays an important role in certain tumor cells that are resistant to radiotherapy. Triptolide can inhibit the growth and induce the apoptosis of radiotherapy-resistant nasopharyngeal carcinoma cells [55].
In addition to its roles described in the aforementioned studies, triptolide has an obvious inhibitory effect on the proliferation of pancreatic cancer, ovarian cancer, leukaemia, prostate cancer, lung cancer, liver cancer, colorectal cancer and other tumor cells, showing broad-spectrum antitumor activity. These studies have provided a theoretical basis for the pharmacological activity studies and clinical application of triptolide derivatives. At the same time, the biosynthesis of triptolide can provide a variety of precursor compounds similar to triptolide. Through interdisciplinary biosynthetic studies and pharmacological research, such as those providing precursor compounds of triptolide biosynthesis for functional research, it is possible to identify precursor compounds with anticancer effects and promote the research progress into related topics.
2.2.6. The mechanism of triptolide anticancer effects
Triptolide exerts its anticancer effects by influencing apoptosis, senescence, proliferation, invasion, migration, and angiogenesis by regulating multiple signal transduction pathways and gene expression levels, as well as interactions with miRNAs and chaperones [56-59]. Early studies have shown that triptolide mostly achieves anticancer effects by inducing apoptosis. Current research data show that apoptosis plays a pivotal role in the development of many tumors [60, 61]. The mechanism of triptolide induced apoptosis varies by cell type. In addition to inducing apoptosis, triptolide can also affect the metabolism of tumor cells by reducing cell viability, affecting cell growth and cell cycle arrest [62, 63]. Increasing evidence shows that in addition to the ability of triptolide to induce apoptosis, it can also achieve anticancer effects by inducing autophagy and the combined effects of apoptosis and autophagy. However, the relationship between apoptosis and autophagy is very complicated. Currently, there are three main reported relationships between apoptosis and autophagy: autophagy and apoptosis can cooperate to promote cell death; autophagy and apoptosis can inhibit each other; and autophagy can promote the progression of apoptosis. In addition, autophagy has a dual role in cancer cells. On the one hand, it can provide energy for cells or effective compounds to promote cell survival. On the other hand, excessive autophagy can promote the process of apoptosis [64]. However, the mechanism by which triptolide induces autophagy in cancer cells and the relationship between apoptosis and autophagy have not been clearly elucidated.
In addition to apoptosis and autophagy, cell senescence, which is a form of irreversible cell growth arrest, is related to tumor treatment. Triptolide can inhibit tumor growth by inducing cell senescence [25]. However, research on this effect of triptolide is still relatively limited. As a multitarget anticancer compound, triptolide can also reduce changes in mitochondrial membrane potential and lysosomal membrane permeability (LMP) [65, 66].
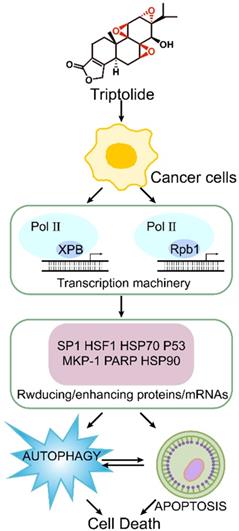
Recent research shows that the molecular target of triptolide is the XPB1 subunit of the transcription factor TFIIH [67]. It primarily covalently binds to Cys342 of the XPB1 subunit via the 12,13-epoxy group, thus inhibiting RNA polymerase II-mediated transcription [68]. (Figure 4) It has been reported that triptolide can degrade TFIIH in a CDK7-dependent manner, thereby causing tumor cell death [69]. There are also reports that in multidrug resistant tumor cells, triptolide achieves antitumor and multidrug resistance effects through cyclin-dependent kinase 7 (CDK7), not XPB [70]. Many other important transcription factors, such as Sp1 and HSF1, are also involved in the survival, development and angiogenesis of tumor cells. Cellular signal transduction pathways such as NF-κB, PI3K/Akt and CaMKK/β-AMPK are also essential for the antitumor activity of triptolide [63]. In tumor cells, triptolide can effectively activate or degrade proapoptotic proteins such as heat shock proteins 90 and 70 (HSP90 and HSP70), caspase-3, caspase-9 and poly-ADP ribose polymerase (PARP) [71].
Mechanism of triptolide induced apoptosis and autophagy.
2.3. Other pharmacological activities
In addition to its obvious antitumor, anti-inflammatory and immunosuppressive effects, triptolide has many additional pharmacological effects that are under study. For instance, triptolide has a good effect on some neurodegenerative diseases, and it was found to improve glomerular sclerosis in patients with diabetic nephropathy.
Although the pathogenesis of the most common neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD) has not been clearly elucidated. Studies have confirmed that triptolide has certain neuroprotective and neurotrophic effects in AD [72]. In addition, triptolide can upregulate mGlu5 to inhibit the activation of microglial cells and induce reactive astrocytes, which in turn protect dopaminergic neurons in a PD model [73].
Triptolide can inhibit the binding of p53 to the promoter of GADD45B to downregulate its transcription. Inhibiting p53-NF-κB-GADD45B signaling to maintain glomerular barrier function provides new research ideas for the anti-proteinuria effect of triptolide in glomerular diseases [74, 75]. In addition, triptolide may improve the proteinuria of diabetic rats by inhibiting the PDK1/Akt/mTOR pathway [76]. The latest research shows that triptolide can inhibit the PI3K/AKT signaling pathway and the interaction between miR-188-5p and PTEN to treat diabetic nephropathy [77].
3. Biosynthesis of triptolide
Following the rapid development of new tools in recent years, synthetic biology has been successfully applied to the production of artemisinin, paclitaxel (Taxol®) and other active compounds isolated from TCM materials. The use of synthetic biology principles to design and modify microbial strains to produce natural active substances has become a very promising method for obtaining sufficient quantities of natural products. This approach is also expected to enable the efficient industrial production of triptolide precursors, triptolide and its derivatives in the future.
3.1. Genomic and transcriptomic studies of T. wilfordii
To explore the key genes of triptolide biosynthesis, our team analyzed tissue samples of T. wilfordii leaves, flowers, stem bark, peeled stem, root bark, root phloem and root xylem, as well as suspensions of cells treated with methyl jasmonate (MeJA) for different times. The samples were classified according to the principle of three biological repeats. The total RNA of each sample was extracted by a modified cetyltrimethylammonium bromide (CTAB) method for sequencing analysis, and finally the transcriptome data were obtained [78]. The results showed that roots and leaves had the highest triptolide content [79]. Therefore, the key genes of triptolide biosynthesis can be screened according to the correlation of their differential expression in different tissues with the triptolide content. Moreover, induction with MeJA increased the content of triptolide in suspension cells. By analysing the expression of genes in suspension cells induced by MeJA at different times, the key genes that regulate triptolide biosynthesis were identified.
In addition, our research team sequenced the genome of T. wilfordii. A total of 28321 protein coding genes were annotated, with an average sequence length of 3338 bp. On average, each predicted gene contained 5.44 exons, with a total of 182.52 Mb of annotated repeats, accounting for 52.36% of the T. wilfordii genome [78]. Based on the genomic data, evolutionary events in the life history of T. wilfordii were analyzed. It was found that the most recent WGT events included the duplication of genes in the upstream metabolism of isoprene. These results suggested that recent WGT events are of great significance to the evolution of triptolide biosynthesis.
The genome and transcriptome, as the main tools for screening biosynthetic pathway genes, have some limitations. In the genome, when identifying genes of the same family, it is possible to merge the genes with high similarity into one gene, which is likely to lead to mistakes in the screening process. In the process of cloning target genes, the gene sequence provided by the genome is mainly the open reading frame (ORF) of the gene. Therefore, if the expression level of the gene is low, the target gene may not be identified due to the limitations of the primers. In addition, the gene sequences provided by the transcriptome may have splicing errors or gene sequence deletion problems. Therefore, it is necessary to integrate the gene information provided by the transcriptome and genome for better screening and cloning of target genes.
3.2. Analysis of the upstream terpenoid biosynthesis pathway
The carbon backbone of terpenes is mainly formed by condensation of isoprene pyrophosphate (IPP) and dimethylallyl diphosphate (DMAPP). In nature, IPP and DMAPP are synthesized in two different biochemical pathways, the 2C-methyl-D-erythritol-4-phosphate (MEP) pathway and the mevalonate (MVA) pathway (Figure 4). Moreover, the MEP and MVA pathways provide precursors for different terpenoids. The MEP pathway mainly produces mono-, di- and tetraterpenes, while the MVA pathway mainly produces sesquiterpenes, sterols, triterpenes and their saponin derivatives.
3.2.1. The mevalonic acid (MVA) pathway
The MVA pathway was first discovered by Lynen et al. [80] and elaborated by Newman et al. [81]. This pathway mainly produces IPP through a series of 6 enzymatic reactions, as follows: ⅰ) The pathway is initiated with acetyl-CoA thiolase (ACAT) catalyzing the formation of acetoacetyl-CoA from two acetyl-CoA units [82]. ⅱ) HMG-CoA is generated from acetoacetyl-CoA and a third acetyl-CoA unit catalyzed by 3-hydroxy-3-methylglutaryl-CoA synthase (HMGS) [83]. ⅲ) The main regulatory step of the MVA pathway is the reduction of HMG-CoA to mevalonate by HMG-CoA reductase (HMGR) on the surface of the endoplasmic reticulum (ER) [84]. ⅳ) Mevalonate is phosphorylated by mevalonate kinase to mevalonate-5-phosphate [84]. ⅴ) Subsequently, phosphomevalonate kinase phosphorylates mevalonate-5-phosphate to mevalonate-5-diphosphate [85]. ⅵ) The final step of the MVA pathway is the formation of IPP from mevalonate diphosphate by mevalonate diphosphate decarboxylase [86]. IPP can be reversibly isomerized to DMAPP by IPP isomerase (IPI).
Two AACT genes, one HMG gene, two HMGR genes, one MVK gene, one PMK gene and one MVD gene were cloned from T. wilfordii. The predicted number of amino acid residues, the ORF sequence, theoretical isoelectric point (pI), molecular weight and the number of genes were analyzed [87]. In addition, some scholars confirmed the gene function by introducing cloned TwHMGS into a suitable yeast strain, and then studying the inducible expression and tissue expression patterns [88].
3.2.2. The 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway
In 1999, Rohmer et al. discovered another route for the synthesis of terpenoids, the methylerythritol phosphate (MEP) pathway. This pathway was elucidated by Lichtenthaler et al. [89] and is also called the 1-deoxy-D-xylulose-5-phosphate (DXP) pathway. Under the catalysis of 7 enzymes, D-3-phosphoglyceraldehyde (GAP) and pyruvate are condensed and reduced to produce IPP and DMAPP via the following steps: ⅰ) Pyruvate and GAP are condensed to form 1-deoxy-d-xylulose 5-phosphate (DXP) under the catalytic action of DXP synthase (DXS). DXS is a key enzyme that controls the flux of the MEP pathway [90]. ⅱ) DXP reductoisomerase (DXR) catalyzes the isomerization of DXP to form MEP [91]. ⅲ) 2-C-methyl-d-erythritol 4-phosphate cytidyltransferase (MCT) activates MEP to form CDP-ME with a cytidine diphosphate linkage at the C4 position [92]. ⅳ) CDP-ME is phosphorylated into CDP-MEP under the action of 4-diphosphocytidyl-2-C-methyl-D-erythritol kinase (CMK). ⅴ) Subsequently, MDS cyclizes the activated CDP-MEP to 2-C-methyl-d-erythritol-2,4-cyclodiphosphate (MEcDP) [93]. ⅵ) MEcDP is reduced to 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate (HMBDP) by 4-hydroxy-3-methylbut-2-enyl diphosphate synthase (HDS) [94]. ⅶ) In the final step, HMBDP is converted by 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR) to form IPP and DMAPP at a product ratio of approximately 5:1 [95]. In addition, IPI is also present in plastids, and can catalyze the isomerization of IPP to maintain the optimal ratio of IPP to DMAPP [96].
Two TwDXS and one TwDXR gene were cloned from T. wilfordii, and the researchers verified their functions through colour complementation experiments [97]. Further overexpression (OE) and RNA-interference experiments confirmed that the expression of TwDXR affects the production of triptolide in T. wilfordii [98]. The TwHDR gene encodes the final enzyme of the MEP pathway, which is very important for regulating isoprene biosynthesis. The function of TwHDR was verified by a complementation assay in a mutant strain of E. coli with a defective HDR gene, and the expression of TwHDR in the cells in suspension was analyzed, and the results contributed to further analysis of the triptolide biosynthesis pathway [99].
3.3. Analysis of the triptolide biosynthesis pathway
The biosynthesis of triptolide is mainly divided into three steps. The first step encompasses the production of IPP and DMAPP through the MVA and MBP pathways [100, 101]. The second step consists of the formation of geranylgeranyl diphosphate (GGPP) by geranylgeranyl diphosphate synthase (GGPPS), catalyzing the continuous addition of IPP to DMAPP, geranyl pyrophosphate (GPP) and farnesyl pyrophosphate (FPP) [102]. In the final step, GGPP is converted to various terpene intermediates under the catalysis of diterpene synthases (diTPSs), and the intermediates are then modified by different postmodification enzymes to generate the corresponding diterpene products (Figure 5) [103].
GGPPS can catalyze the generation of the common diterpene precursor GGPP and is considered to be one of the key synthetases in the diterpene biosynthesis pathway. Five putative GGPPS genes have been cloned from T. wilfordii. The cloned GGPPS and SmCPS/KSL genes were introduced into E. coli with miltiradiene serving as a marker. Finally, it was determined identified that the proteins encoded by the three TwGGPPS exhibited function of GGPPs. By analysing the expression in TwGGPPS in MeJA-induced cells in suspension, researchers showed that the accumulation of triptolide is enhanced with the increase of TwGGPPS1 and TwGGPPS4 expression, suggesting that these two genes may be the main genes that control triptolide synthesis [104]. The latest research shows that TwGGPPS8 exhibits an expression pattern similar to that of TPS7v2, TPS9v2 and TPS27v2, and the highest transcription levels were found in roots rich in triptolide. Based on this observation, it was speculated that TwGGPPS8 may be involved in the biosynthesis of triptolide in roots [105].
After obtaining the common linear diterpene precursor GGPP, researchers further studied the biosynthetic pathway of triptolide. Hansen et al. found that TwTPS27 coupled with TwTPS9 converted normal copalyl diphosphate to miltiradiene by screening diterpene synthase family genes in T. wilfordii [106]. Su et al. added miltiradiene to the culture medium of suspended cells, and the accumulation of triptolide after 5 days exhibited a statistically significant increase compared with the level in the control group [79]. This is the first evidence that miltiradiene is indeed a precursor of triptolide. Through transcriptome sequencing of cells in suspension induced with MeJA, 8 putative diterpene synthase genes were identified, and 6 full-length diterpene synthase genes were cloned. Using GGPP as a substrate, the functional identification was carried out in E. coli, and TwTPS7v2 and TwTPS9v2 were confirmed to generate CPP from GGPP [79]. It was confirmed that CPP is a precursor of miltiradiene. The results showed that TwTPS27v2 can catalyze the formation of miltiradiene from CPP, and RNAi experiments showed that decreased expression of TwTPS7v2, TwTPS9v2 and TwTPS27v2 affects the accumulation of triptolide.
Analysis of the biosynthetic pathway of triptolide. The green dashed box shows the common upstream pathways of terpenoids in T. wilfordii. The solid arrow and red gene indicate the route of identified function, while the dotted arrow and blue gene indicate the possible route.
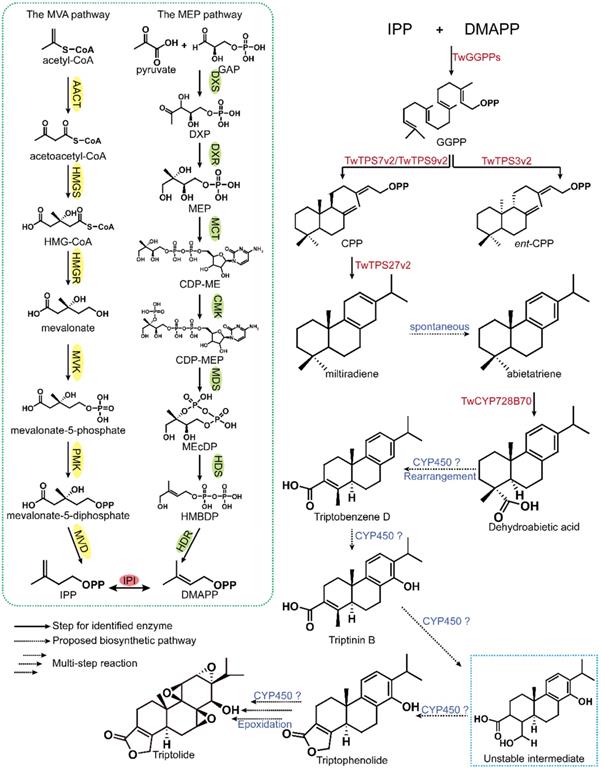
Previous studies had elucidated the biosynthesis of the abietane-type diterpene core skeleton miltiradiene, which laid the foundation for further investigation of cytochrome P450 (CYP450) genes in the downstream synthesis pathway. NADPH-cytochrome P450 reductase acts as the electron donor of CYP450 proteins to support their catalytic function. Four TwCPR genes were cloned from T. wilfordii and soluble proteins were successfully expressed. The activity of TwCPR enzymes was verified by combining them with kaurene oxidase. The results showed that although TwCPR3 was expressed at lower levels in certain tissues, it was a more efficient electron donor [107]. Therefore, it was speculated that TwCPR3 is more suitable for the study of other CYP450 monooxygenases in T. wilfordii. Studies have reported that CYP720B4 in Sitka spruce (Picea sitchensis) can convert miltiradiene to dehydroabietic acid, and it was speculated that dehydroabietic acid may be an important intermediate in the triptolide biosynthesis pathway [108]. The latest research indicates that CYP728B70 is the first CYP450 in the triptolide biosynthesis pathway and that it converts miltiradiene and abietatriene in two consecutive oxidation steps to form the corresponding diterpene alcohol and diterpene acid (dehydroabietic acid) products. Interference and OE analysis indicated that CYP728B70 is involved in triptolide biosynthesis [78].
Currently, there has been a breakthrough in the understanding of the triptolide biosynthesis pathway, and the first CYP450, TwCYP728B70, was discovered. However, there are still many difficulties to be resolved. First, compared with triptolide, the position of the carboxyl group of dehydroabietic acid is problematic. Transfer of the carboxyl group to the three position is an urgent problem for researchers. On the one hand, after decarboxylation, a methyl group may be attached to the third position, and then the three-step oxidation proceeds. On the other hand, there may be an enzyme that can directly transfer the carboxyl group at position 18 to position 3. In addition, the mechanism involved in forming the three epoxy groups in triptolide has not been extensively studied. As suggested in the current literature, CYP450s and dioxygenase may catalyze the formation of these functional groups. Therefore, we hope to solve the problems of carboxyl transfer and epoxy group formation during biosynthesis by combining biosynthesis with chemical synthesis, and ultimately enable the industrial production of triptolide.
3.4. Metabolic engineering for achieving triptolide biosynthesis
Studies have shown that the content of triptolide in T. wilfordii is low, reaching only 66.5 μg/g [5]. To study triptolide better, researchers used Agrobacterium rhizogenes to induce hairy roots from the calli of T. wilfordii roots. The concentrations of triptolide in hairy root, callus, adventitious root and natural root samples were 39.98, 1.76, 47.86 and 21.4 μg/g, respectively, and after MeJA induction, the triptolide concentration increased approximately 2-fold [109]. In addition, by optimizing the growth and induction conditions for T. wilfordii adventitious root culture, a comparatively large quantity of triptolide was obtained. The yield of triptolide was increased to 648.3 μg per flask by adding inducers XAD-7 and MeJA and optimizing the growth conditions [110].
T. wilfordii cells in suspension are also important sources of triptolide for research. Suspension cells are also suitable for a variety of experiments, such as RNAi and overexpression studies. In one study, the triptolide concentrations in T. wilfordii cells in suspension and culture medium were 53±3 μg/g and 4.0±0.2 mg/L, respectively. After 240 h of MeJA induction, the triptolide content was increased 3-fold to 200 μg/g [79, 111]. Furthermore, researchers found that cambial meristematic cells (CMCs) also effectively accumulate triptolide, which has great potential in triptolide biosynthesis research. Studies have shown that CMCs contain triptolide at a concentration of 138.1 μg/g, and after induction with MeJA for 480 h, the content of triptolide can reach 405.1 μg/g [112]. Subsequently, researchers confirmed that MeJA can induce the expression of terpene biosynthesis-related genes in CMCs through real-time reverse transcription-polymerase chain reaction (qRT-PCR). CMCs can therefore be used for the analysis of the triptolide biosynthesis pathway.
3.5. Metabolic engineering of bacteria for triptolide production
Microbial metabolic engineering is a very promising method for obtaining natural products. Miltiradiene is an important intermediate compound of triptolide biosynthesis. The synthesis of miltiradiene by microorganisms is the first step to efficiently produce triptolide. Studies have shown that modular engineering, encompassing the integration of SmCPS and SmKSL together with the integration of BTS1 and ERG20, significantly contributed to the increased output of miltiradiene. Finally, the best synthetic route was introduced into the diploid yeast strain YJ2X, and the resulting engineered strain produced 365 mg/L miltiradiene in a 15-L bioreactor [113]. In addition, Dai et al. increased the yield of miltiradiene to 488 mg/L through various methods, such as overexpression of key enzymes and the use of antibiotic markers to replace auxotrophic markers in plasmids. However, due to the use of antibiotics in the fermentation process to enhance the stability of the plasmid, it cannot be used in large-scale industrial production [114]. Recently, Tianyuan Hu et al. investigated the production capacity of diterpenoid synthases from different species, and selected a class II diterpene synthase (di-TPS) CfTPS1 from Coleus forskohlii (Plectranthus barbatus) and class I di-TPS SmKSL1 from Salvia miltiorrhiza to use for producing a final titre of miltiradiene of 3.5 g/L in a 5-L bioreactor [115].
In addition, researchers knocked out rox1, ypl062w, and yjl06w4, downregulated ERG9, and overexpressed genes such as tHMG1 and ERG20 to increase GGPP production. Subsequently, they introduced double SmMS-SmCPS1 fusion modules to produce miltiradiene and finally co-expressed the TwCYP728B70 and TwCPR3 genes to produce dehydroabietic acid [78]. This series of experiments laid the foundation for the subsequent identification of key enzyme-coding genes in the triptolide biosynthesis pathway.
Although a microbial metabolic plant model has been constructed to produce dehydroabietic acid, it is difficult to meet the needs of subsequent research because of its low yield. Currently, there are several ways to improve the yield of synthetic biology: 1. Genes that do not affect the growth of microorganisms are knocked out or weakened in other ways to increase the accumulation of precursor compounds. 2. The yield of target compounds is increased by the overexpression of genes. 3. Genes with the same function but with higher activity are used to replace genes with lower expression or mutation technology is used to identify mutant genes that produce higher yields. 4. Through the technology of protein fusion or substrate channelization, we can connect the active pockets of proteins to improve the yield of target compounds.
4. Total synthesis of triptolide
Over decades, relatively slow progress has been made toward the goal of total triptolide synthesis, with many of the advancements made attributed the efforts of Berchtold [116], van Tamelen [117] and Yang [118]. Currently, there are four principal ways to synthesize triptolide: Ⅰ) synthesis from tetralinone, α-abietic acid or α-dehydroabietic acid as starting materials, Ⅱ) synthesis via the Diels-Alder reaction, Ⅲ) synthesis of the core skeleton via a polyene cyclization reaction, and IV) synthesis via metal catalysis [119].
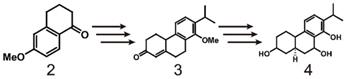
Early research on the synthesis of triptolide using 6-methoxy-1-tetraketone (2) as starting material.
Synthesis of triptolide (1) through 5 and 6. ⅰ) NaH, DMF 25℃, 12h; ⅱ) Me2NH 25 ℃, 12 h; ⅲ) CrO3.py, CH2Cl2 25 ℃, 15 min; ⅳ) grade 3 neutral alumina EtOAc, 25 ℃, 48 h; ⅴ) pˑTosOH (catalyst) C6H6, reflux, 2 h; ⅵ) NaBH4, EtOH, 25 ℃, 2 h aqueous HCl (workup); ⅶ) MeO-, MeOH 25 ℃, 15 min; ⅷ) CrO3, HOAc-H2O (9:1) 25 ℃,6 h; ⅸ) BBr3, CH2Cl2 25 ℃,10 h; ⅹ) NaBH4, EtOH 25 ℃,1 h.
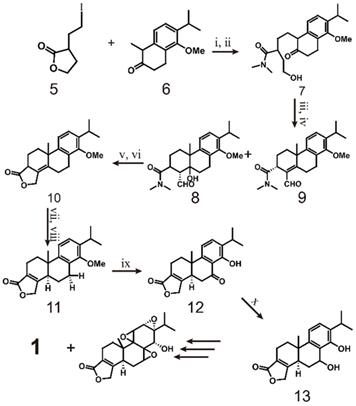
As early as 1977, the Berchtold team began research on the total synthesis of triptolide (scheme 1). They used 6-methoxy-1-tetralone (2) as the starting material and converted it to tricyclo-enone 3 in 10 steps with a yield of 33%. Then, 3 was transformed into 4 in 5 steps, which laid the foundation for the total synthesis of triptolide [120]. In 1980, the team completely outlined the process of synthesizing racemic triptolide (scheme 2) [116]. The reaction is initiated with the alkylation of tetralone (5) with 3-(2-iodoethyl) dihydrofuran-2(3H)-one (6), and bonds in the product are broken with dimethylamine to form a 1:1 enantiomer mixture 7. The mixture of 8 and 9 is formed by oxidation of 7 with a CrO3.py complex with aldol condensation catalyzed by Al2O3. To obtain pure compound 10, the mixture is treated with acid to dehydrate 8, the aldehyde is reduced with NaBH4 during the acid treatment, and an acid-catalyzed lactonization reaction is performed. The isomerization of 10 catalyzed by methoxide results in compound 11, which is then oxidized by benzoic acid 11 and demethylated to obtain 12. Reduction of 12 with NaBH4 provides pure C-7β alcohol (13). Finally, triptolide (1, 21%) and 14-epitriptolide (68%) are obtained through an Alder periodate reaction (NaIO4, 74%), a sequencing m-CPBA oxygenation and basic hydrogen peroxide oxygenation (H2O2/OH-) procedure, and sodium borohydride reduction [119]. In summary, the first total synthesis of racemic triptolide was completed from 5 in 16 steps. Although the final yield was low (1.6%), this work undoubtedly laid the foundation for the study of triptolide synthesis.
Synthesis of triptolide (1) through Compound 14. ⅰ) H2, Pd/C, NH(OMe)MeˑHCl, CDI, Isopropenyl magnesium bromide; ⅱ) NaBH4, CeCl3ˑ7H2O, CH3C(OEt)3, CH3CH2COOH, reflux; ⅲ) LiOH, NH(OMe)MeˑHCl, CDI; ⅳ) Trimethylsilyl lithium acetylide, -78 ℃ to -20 ℃; ⅴ) compound 19, HCOOH, TEA, THF; ⅵ) K2CO3, TBSCI, imidazole; ⅶ) InBr3 (20%), -20 ℃; ⅷ) BH3ˑTHF, H2O2, 3N NaOH; ⅸ) NaH, PMBCI, PTSA; ⅹ) Jones reagent, LiHMDS, Tf2NPh, -78 ℃ to rt; ⅹⅰ) DDQ, CO, Pd(PPh3)4, Bu3N, LiCl, 80 ℃; ⅹⅱ) Rh(PPh3)3Cl, Et3SiH, toluene reflux; ⅹⅲ) I2, AgOTf; ⅹⅳ) Pb(dba)2, TBAF, compound 29, 5% Pd/C.
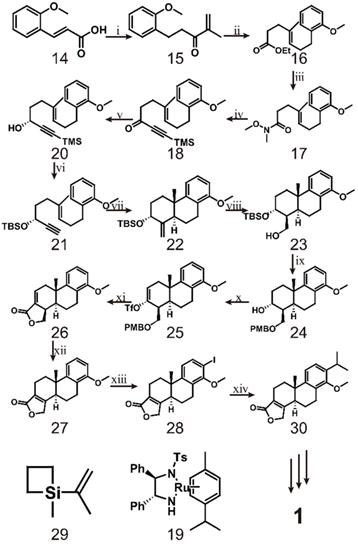
Later, researchers mostly borrowed from the research ideas of Berchtold et al. The innovation of the synthetic route was mainly focused on different treatment methods of tetralone. However, Li et al. developed a different route to synthesize triptolide in 2014 [121] (scheme 3). The route starts from the hydrogenation of common compound 14, which is converted to the corresponding Weinreb amide and finally reacts with isopropenyl magnesium bromide to form enol 15. Compound 15 is then reacted with sodium borohydride in the presence of CeCl3, after which Johnson Claisen rearrangement produces ester 16. This ester is hydrolysed to form amide 17, which is then reacted with trimethylsilyl lithium acetylide to obtain acetylene 18. Compound 18 and 2.5 mol% of (R, R)-19 are incubated in triethylamine and formic acid for 1.5 h to produce alcohol 20. Compound 21 is obtained by protecting the hydroxyl group with a tert-butyldimethylsilyl ether during the potassium carbonate/methanol repair process and then cleaving the acetylenic trimethylsilyl group. The key to this synthetic pathway is that indium-(III) catalyzes the cationic cascade reaction of compound 21. This reaction proceeds via slow addition of 21 to an intensely stirred suspension of InBr3 in dichloromethane at -20 °C. Under these conditions, key intermediate 22 is formed as a single isomer. Subsequently, the authors completed the synthesis of the lactone D-ring through a four-step reaction. In the first step, 22 was subjected to hydroboration using a BH3·THF complex and then oxidized with basic hydrogen peroxide to obtain alcohol 23 as a single isomer. In the second step, PMB ether was formed to protect the free hydroxyl group of alcohol 23, and then p-TsOH was used to remove the silyl protecting group to obtain alcohol 24. The third step is to oxidize 24 with Jones reagent and then convert the resulting carbonyl group to the corresponding trifluorovinyl compound 25. Finally, the PMB ether of 25 was oxidatively cleaved, and tetracyclic lactone 26 was obtained by palladium-catalyzed carbonylation and in situ lactonization. After obtaining intermediate 26, compound 27 was obtained by reacting 26 with a catalytic concentration of tris(triphenylphosphine)rhodium and triethylsilane in refluxing toluene. Ortho-iodination of 27 gave intermediate 28, which was subjected to palladium-catalyzed cross-coupling with 1-methyl-1-(prop-2-enyl) silacyclobutane 29 and then hydrogenated to form triptophenolide methyl ether 30. Triptophenolide methyl ether, as an important intermediate, has been used in the total synthesis of triptolide many times [118, 122]. The difficulty of this approach is the preparation of optically active propargyl alcohol (R)-20. Through several attempts by researchers, the conditions for incubation of 18 and 2.5 mol% of (R,R)-19 in triethylamine and formic acid for 1.5 h were finally discovered [123]. It is speculated that these conditions may a result of the instability of the trimethylsilyl group in the reaction system. Research mainly utilize InBr3-mediated cationic polyene cyclization and palladium-catalyzed carbonylation with lactone formation, which enables the large-scale synthesis of intermediate 26 [121].
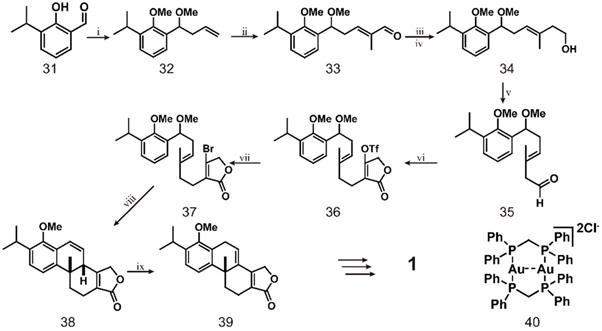
Recent studies have used dimeric gold complex [Au2(dppm)2] Cl2 combined with ultraviolet A (UVA, 365 nm) light irradiation to catalyze the formation of radical intermediates from non-activated bromoalkanes/arenes in a mild photoredox catalysis process [124] (scheme 4). The first step of this reaction is treating aldehyde 31 with allyl magnesium bromide and then adding dimethyl sulfate to obtain allyl 32. Compound 32 is subjected to a metathesis reaction with methacrolein under the catalytic action of a Grubbs second-generation catalyst and a certain amount of copper iodide to obtain a single isomer of aldehyde 33. The aldehyde groups of compound 33 are converted to a diene by the Wittig reaction and then to the corresponding primary alcohol 34 by a borohydride/oxidation sequence. Dess Martin oxidation of 34 produces aldehyde 35, which is subjected to a one-pot proline-catalyzed coupling reaction with tetronic acid in the presence of Hantzsch ester and triflation to afford triflate 36. The mixture is directly heated with lithium bromide in tetrahydrofuran to obtain bromobutene lactone 37, which reacts under optimal conditions, followed by treatment with sulfuric acid to obtain tetracycle 38, and the configuration is confirmed by X-ray analysis. Subsequently, 38 is isomerized using a catalytic amount of RuCl2(PPh3)3 and DIPEA in toluene at 120 °C to obtain 39. Finally, the conversion of 39 to triptolide requires 8 more steps [125]. This route can be used to access the important intermediate 39 for triptolide synthesis, which is converted from compound 31 in eight simple steps. However, the difficulty of the entire route is maintaining the optimal conditions for the reaction of bromo-butyrolactone 37. The researchers finally determined that, vinyl bromide (0.1 M in MeCN), 1 (10 mol%), Na2CO3 (2 equiv.), subjected to, 365-nm LED light for 12 h at room temperature constituted the best reaction conditions. This study has made important contributions to the large-scale synthesis of polycyclic compounds and can be used in the synthesis of many other terpenoids.
The total synthesis of triptolide mainly includes the following three aspects: i) the synthesis of the tricyclic scaffold; ii) the formation of the butenolide (D-ring), and iii) the construction of the three active epoxy groups. Previous research on the total synthesis of triptolide has solved these three problems in a satisfactory manner and achieved important research results on a laboratory scale. However, in view of the complex chemical structure of triptolide, even as researchers continue to optimize the synthetic pathway and reduce the number of steps required for its total synthesis, the final yield of triptolide remains too low. Therefore, researchers need to make unremitting efforts to develop new approaches for triptolide synthesis.
5. Toxicity of triptolide
With the increasing clinical application of T. wilfordii and triptolide, increasing numbers of studies and clinical case reports indicate that triptolide has serious adverse effects. Currently, triptolide has a narrow therapeutic window and induces serious toxicity and side effects, which limits its clinical application. Considering this information, we have summarized the research progress on the hepatotoxicity, nephrotoxicity, cardiotoxicity and reproductive toxicity of triptolide, hoping to contribute to better clinical prospects of this compound.
Total synthesis of triptolide (1) through simple compound 31. ⅰ) allylMgBr, THF, Me2SO4, 70 ℃ ,12 h; ⅱ) Grubbs Ⅱ (1 mol%), Cul (2.5 mol%), Acrolein, Et2O, 40 ℃, 4 h; ⅲ) Ph3PMel, KHMDS, THF, 2 h; ⅳ) Cy2BH, THF, NaBO3ˑ4H2O, 4 h; ⅴ) DMP, CH2Cl2; ⅵ) L-proline (5 mol%), tetronic acid, Hantzch ester CH2Cl2, DIPEA, Tf2O, -78 ℃; ⅶ) LiBr, THF, 70 ℃, 12 h; ⅷ) compound 40, Na2CO3, 365 nm, MeCN, 12 h, H2SO4, 6 h; ⅸ) RuCl2(PPh3)3, DIPEA, PhMe, 120 ℃, 4 d.
5.1. Hepatotoxicity
Liver injury is the most common adverse reaction caused by triptolide, and has caused widespread concern. Many studies have been carried out to explain the mechanism of triptolide-induced liver toxicity, mainly focusing on common phenomena such as oxidative stress and inflammation [126, 127]. In recent years, researchers have discovered that mitotic phagocytosis associated with mitochondrial fission may be a new mechanism of induced triptolide hepatotoxicity [128]. Jie Zhao et al. analyzed triptolide-induced changes in the serum and liver metabolome in mice, identified 30 metabolites that were significantly changed, and selected 29 of these metabolites as potential biomarkers related to triptolide-induced hepatotoxicity, with the aim of helping researchers better understand the mechanism of triptolide-induced toxicity [129]. In addition, proteomics and targeted fatty acid analyzes were also used to reveal the mechanism of triptolide hepatotoxicity.
Yan Lu et al. found that triptolide can reduce the transcription of CYP3A, CYP2C9, CYP2C19 and CYP2E1, and the substrate affinity of the proteins leads to liver toxicity [130]. CYP3A is the main isozyme involved in triptolide metabolism; it facilitates the detoxification of triptolide. Experiments show that catalpol (CAT), the main component of Rehmannia glutinosa, can increase the expression of detoxification enzymes CYP3A2/4, CYP2C9 and UGT1A6, increase the metabolic transformation of triptolide, and thereby reduce its liver toxicity [131]. Knocking out hepatic cytochrome P450 caused a significant increase in triptolide levels, which aggravated its hepatotoxic effects.
In recent years, researchers have used high-content analysis (HCA) to measure the overall cytotoxicity phenotype of HepG2 cells treated with triptolide and finally confirmed that inhibition of global transcription associated with RNA Ⅱ is the core trigger of hepatotoxicity induced by triptolide [132]. The latest research found that propionate produced by the intestinal flora can promote the protective effect of intestinal flora against triptolide by reducing inflammation levels [133].
5.2. Nephrotoxicity
The nephrotoxicity of triptolide also limits its clinical application. However, the mechanism of this toxicity has not been fully elucidated. Researchers used collagen-induced arthritis (CIA) model rats as the research objects and found that triptolide transport is mediated by OTC2 in rat kidney slices and HEK-293T cells. TNF-α can increase the toxicity of triptolide and regulate the expression and function of OTC2, thus indicating that OCT2 mediates the nephrotoxicity of triptolide in vitro [134].
Wei Huang et al. combined network pharmacology and targeted metabolomics, and identified 61 action targets related to renal toxicity induced by triptolide, including 39 direct targets and 22 indirect targets, and finally confirmed dihydroorotate, thymidine, 2-deoxyinosine, uric acid, adenosine and xanthine as biological markers of renal toxicity [135]. In addition, the purine metabolism pathway, toll like receptor signaling pathway and NF-κB signaling pathway play key roles in the nephrotoxicity induced by triptolide.
5.3. Cardiotoxicity
Research by Shurong Wang et al. showed that triptolide caused an increase in the expression of more than 108 microRNAs in the heart of male rats by more than twofold and reduced AhR levels in the myocardium and circulation, inducing acute cardiotoxicity [136]. Therefore, circulating AhR levels and microRNA levels can be used as early warning biomarkers for triptolide-induced cardiotoxicity.
Researchers have studied the role of p53 in triptolide-induced cardiotoxicity in H9c2 cells, primary cardiomyocytes, and C57BL/6-derived p53 mouse models [137]. The results showed that Bax, a target protein of p53, leads to important mitochondrial dysfunction and apoptosis in triptolide-induced cardiotoxicity and can block the permeability of the mitochondrial membrane to protect against triptolide-induced myocardial toxicity.
5.4. Reproductive toxicity
Triptolide has strong reproductive toxicity, mainly in males. Triptolide can inhibit spermatogenesis and testosterone marker enzymes, reduce sperm count, lower the gonadal index and destroy the testicular microstructure [138]. Bo Ma et al. evaluated the mechanism of triptolide-induced reproductive toxicity and identified possible new biomarkers [138]. They reported that triptolide-mediated downregulation of PPAR caused abnormal testicular lipid and energy metabolism, which led to sperm damage, revealing the mechanism of the reproductive toxicity induced by triptolide.
Recently, researchers analyzed the expression profiles of lncRNAs/circRNAs/mRNAs and revealed the mechanism of the reproductive toxicity induced by triptolide relating to lncRNAs/circRNAs [139]. The results show that triptolide can reduce sperm production, lead to abnormal testicular and sperm morphology, and induce mature sperm dysfunction. After stopping the use of triptolide, male fertility recovery was slow, indicating that triptolide not only destroys germ cells in the testes but also damages epididymal sperm. Data analysis show that the potential mechanism of reproductive toxicity induced by triptolide may involve the interference of genes related to spermatogenesis.
6. Derivatives of triptolide
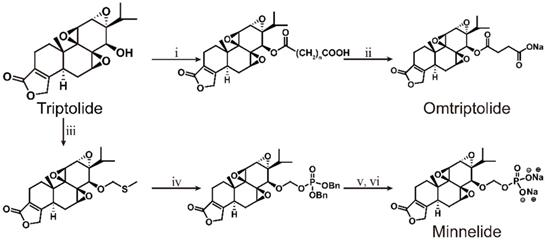
Although triptolide has strong pharmacological activity, its clinical application is severely restricted due to its poor solubility and bioavailability, and the serious toxicity and side effects it induces, and a narrow therapeutic window. In recent years, researchers have modified the structure of triptolide to increase its water solubility and reduce the toxicity and side effects it induces without affecting its activity. Currently, a variety of triptolide derivatives, such as omtriptolide and minnelide (Figure 6) have been investigated, and some have entered the clinical trial stage (Table 2).
6.1. Omtriptolide
As early as 1997, researchers designed a new semisynthetic water-soluble derivative, omtriptolide (PG490-88, f60008), by introducing a fatty acid structure into triptolide C-14 [140]. Omtriptolide shows immunosuppressive and antitumor activities (scheme 5), and it is currently approved for phase I clinical trials of prostate cancer in the United States [141]. Long-term research results show that PG490-88 exhibits cytotoxicity in tumor cell lines, including H23 (NSCLC), HT1080 (fibrosarcoma) and COLO 205 (colon cancer) cells [4]. Studies have shown that when PG490-88 is used alone, it can cause the regression of lung cancer and colon cancer xenograft tumors, and the synergistic effect of PG490-88 and CPT-11 can also cause tumor regression [142].
In recent years, it has been found that PG490-88 can reduce the disease progression of kidney disease in various animal models. PG490-88 and tacrolimus (Tac) work synergistically to inhibit T cell activation and reduce IFN-c production and NF-AT/NF-jB activity, thereby prolonging the survival time of transplanted kidneys in a monkey model [143]. Some scholars have found that PG490-88 can attenuate acute humoural rejection by inhibiting complement activation and T cell infiltration, thereby significantly prolonging the survival time dog models after kidney transplantation [144]. Moreover, PG490-88 may reduce the amount of p-ERK released in cisplatin-induced acute kidney injury (AKI), thereby protecting against AKI and acute tubular necrosis (ATN) [145]. The latest research confirmed that PG490-88 can inhibit the activation of the NF-κB and MAPK signaling pathways as well as the production of pro-inflammatory mediators and cytokines and ultimately plays a protective role in I/R-injured rats [146].
Related research on triptolide and its derivatives.
| Compound | Chemical structure | Aqueous solubility | Pharmacological activity | Refs |
|---|---|---|---|---|
| Triptolide (PG490, LDTT-2) | 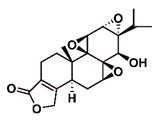 | Poor Solubility | Anti-cancer, anti-inflammatory and immunosuppression | [22, 23] [4] |
| Omtriptolide (PG490-88, F60008) | 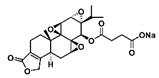 | Soluble | Anti-cancer, immunosuppression, inhibition of pro-inflammatory mediators and cytokines | [4] [146] |
| (5R)-5-Hydroxytriptolide (LLDT-8) | 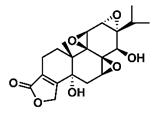 | Soluble | Immunosuppression and anti-inflammatory effects | [151, 155] |
| Minnelide | 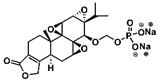 | Soluble | Extensive anti-cancer effects enhance the efficacy of chemotherapy drugs | [160, 168] |
Researchers conducted a phase I and pharmacological study of PG490-88 in patients with advanced solid tumors [141]. The adverse reactions were mainly fatigue, nausea, vomiting, diarrhoea, and constipation. The haematological side effects were mild grade 1 anaemia, but no liver or kidney toxicity was found. However, in two cases, the side effects were fatal. One patient died of neutrophilic sepsis, and another patient may have died of a complex clinical syndrome caused by cytokine release. Ultimately, researchers believe that the degree of PG490-88 conversion to triptolide in the human body is unpredictable; therefore, PG490-88 is not the best derivative of triptolide to use in the clinic. Phase I clinical trials were forced to be discontinued in 2009. According to the current experimental results, PG490-88 has a strong anticancer effect and reduced liver and kidney toxicity compared to triptolide, which provides a reference for the clinical application of triptolide.
Common triptolide derivatives and their research contents.
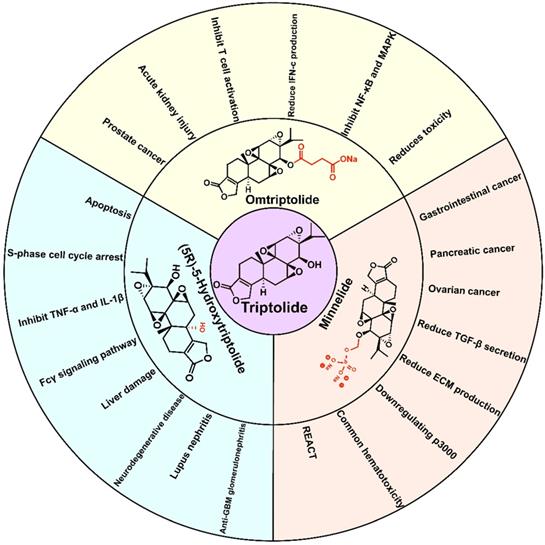
Total synthesis of Omtriptolide and Minnelide. ⅰ) HO2C(CH2)nCO2H, DCC/DMAP; ⅱ) n=2; ⅲ) Me2S, MeCN, BPO, 2 h; ⅳ) CH2Cl2, 4A MS, (BnO)2P(O)OH, NIS, THF; ⅴ) H2, Pb/C, THF; ⅵ) THF, Na2CO3.
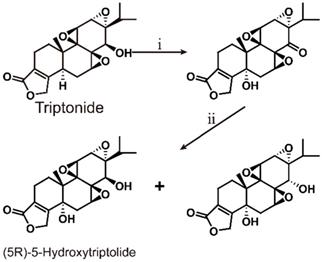
6.2. (5R)-5-Hydroxytriptolide
(5R)-5-Hydroxytriptolide (LLDT-8) is a new triptolide analogue with strong immunosuppressive and anti-inflammatory activity [147] (Scheme 6). Currently, LLDT-8 is being investigated as a low-toxicity immunosuppressive agent in Phase I clinical trials for the treatment of RA in China [148], and the results show that LLDT-8 can inhibit the production of MMP-13 and increase the expression of OPG/RANKL through the OPG/RANK/RANK ligand signaling pathway to weaken collagen-induced arthritis (CIA). This experiment used CIA to simulate RA [149]. Early studies have shown that LLDT-8 may be closely related to anti-inflammatory, antioxidant and cytokine effects and can protect against bleomycin-induced lung fibrosis in mice [150]. Recently, researchers constructed the first lncRNA-TF-mRNA coexpression network, which further explain changes in whole-genome lncRNA and mRNA expression before and after LLDT-8 treatment. The authors believe that lncRNAs may be biomarkers and targets for LLDT-8 drug development [151]. At the same time, LLDT-8 showed a strong anti-inflammatory effect, for example, by regulating the Fcγ signaling pathway to improve anti-GBM glomerulonephritis [152] and inhibiting the expression of renal chemokines to suppress infiltration of kidney immune cells, thereby improving lupus nephritis [153].
Total synthesis of (5R)-5-Hydroxytriptolide. ⅰ) SeO2, heat reflux 10 h; ⅱ) NaBH4, 2 h
LLDT-8 also has a certain therapeutic effect on neurological diseases. Some scholars have studied the anti-inflammatory and neuroprotective effects of LLDT-8 on cerebral ischaemia-reperfusion injury. The results show that it may inhibit the neuroinflammation mediated by microglia through the IκB/NF-κB cascade, play an anti-inflammatory effect, and protect against acute cerebral ischaemia-reperfusion injury [154]. Studies have shown that LLDT-8 can reduce PD-like behaviour and dopaminergic neurodegeneration and neuroinflammation of the nigrostriatal system, providing a new method and entry point for the treatment of PD [155]. LLDT-8 can effectively inhibit pro-inflammatory factors (TNF-α and IL-1β) to suppress the NF-κB signaling pathway and thereby reduce neuroinflammation, and it is used to treat neurodegenerative diseases [156]. In addition, LLDT-8 can also reduce serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels and reduce liver balloon cell formation and macrocystic steatosis, thereby inhibiting liver damage [157]. LLDT-8 can also regulate the expression levels of stearoyl-CoA desaturase 1 (SCD1) and hepatic peroxisome proliferator-activated receptor α (PPARα), significantly promoting lipid breakdown and inhibiting lipid synthesis.
Clinical trials confirmed that LLDT-8 has broad-spectrum antitumor activity, inducing S-phase cell cycle arrest and apoptosis. As a novel transcription inhibitor, LLDT-8 has a potential therapeutic effect on P-glycoprotein-mediated drug-resistant tumors [158]. Researchers used mouse spleen cells for LLDT-8 cytotoxicity experiments and found that although the inhibitory activity of LLDT-8 against ConA and LPS cell proliferation was reduced 20-fold and the inhibitory activity against allo-MLR was reduced 14-fold, the toxicity of LLDT-8 was reduced 122-fold compared with triptolide. In addition, data from an acute toxicity study showed that LLDT-8 exhibited a 10-fold reduction in toxicity in mice [159]. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) used for the analysis and characterization and benzylamine chemical derivatization to determine the content of LLDT-8 in human plasma is a more sensitive and reliable quantitative method for studying pharmacokinetics [148]. This method covers a wide linear dynamic range (0.030-100 ng/ml), and within this linear range, it shows better accuracy (RE<11.7) and precision (RSD<8.6). Considering current clinical research, LLDT-8 may be a more suitable clinical alternative drug for triptolide. In terms of pharmacological activity, LLDT-8 not only retains the strong immunosuppressive and anti-inflammatory activity of triptolide but also has broad-spectrum antitumor activity. In clinical applications, compared with triptolide, the toxicity of LLDT-8 is greatly reduced.
6.3. Minnelide
Minnelide is a water-soluble derivative formed by adding a phosphate group to triptolide, endowing it with a wide range of anticancer effects (Scheme 5). Currently, minnelide has entered Phase I clinical trials for gastrointestinal cancer and pancreatic cancer [160, 161]. Studies have shown that minnelide can eliminate colon cancer cells in vitro, reduce the growth of primary colon cancer, and metastasize colorectal cancer to the liver in vivo and may become a new strategy for the treatment of primary and metastatic colon cancer [162]. A University of Minnesota team found that minnelide reduces CD133+-derived tumor volume and the number of tumor-initiating cells (TICs) in the tumor [163]. This is the first report on the efficacy of minnelide in the syngeneic system of immune tolerance. Subsequently, the group conducted in-depth research and established an in vitro model for studying the characteristics of tumor stem cells and tumor-initiating cells and found that minnelide has good prospects in a preclinical evaluation [164]. The team also found that minnelide can deplete extracellular matrix components by depleting hyaluronic acid and collagen to improve drug delivery and survival [165].
In addition to pancreatic cancer and gastrointestinal cancer, minnelide can also inhibit the activity of NF-κB and prevent metastasis. It effectively reduces the tumor burden and metastasis potential of osteosarcoma, has the least impact on osteoblasts of the tested compounds, and may be developed into a very effective chemotherapy drug for osteosarcoma [166]. Studies have confirmed that minnelide can induce cell death of prostate cancer cells by downregulating AR and its variants [167]. Minnelide can effectively inhibit the proliferation of platinum-sensitive and drug-resistant ovarian cancer cell lines, thereby improving the efficacy of standard chemotherapy, such as carboplatin and paclitaxel [160, 168]. In addition, minnelide combined with an anti-DR5 monoclonal antibody provides a new therapeutic option against metastatic renal cell carcinoma [169].
In recent years, minnelide has mainly been investigated for the treatment of pancreatic cancer and has entered a phase Ⅱ clinical trial of advanced pancreatic cancer [170]. Minnelide is the fastest developed derivative of triptolide. Clinical trials show that the maximum patient-tolerated dose is 0.2 mg/kg. At this dose, minnelide can induce irreversible CAFs to acquire an inactive state phenotype and reduce TGF-β secretion and ECM production, thereby reducing the proliferation of TECs to cause tumor regression [171]. Minnelide can also inhibit the pro-survival signaling in pancreatic cancer cells by downregulating the expression of p3000 and reducing the transcriptional activity of the HIF-1α transcription complex. Furthermore, minnelide regulates downstream effects by reducing hypoxia and associated signaling [172].
The results of a phase I clinical trial in 27 patients with refractory gastrointestinal cancer (17 pancreas, 7 large intestine and 3 other gastrointestinal tract cancers) showed that the overall treatment effect was good except for haematotoxicity that common among the patients and usually was resolved within 2-3 days after drug withdrawal [173]. Minnelide was administered by intravenous infusion every 28 days on days 1-5, 8-12, and 15-19, with a dose range of 0.16-0.8 mg/m2. However, two patients may have suffered reversible acute cerebellar toxicity (REACT) in a phase I clinical trial of minnelide. The patient's imaging scans showed a slight increase in the PLAIR signal and a marked decrease in cerebellar cortical spread [174]. However, the imaging-based findings of another patient indicated a nearly opposite situation: They revealed possible post-reversible encephalopathy syndrome or acute toxic leukoencephalopathy. These results indicate that minnelide may cause potential reversible acute cerebellar toxicity. Therefore, more in-depth research and analysis are needed in the course of clinical trials. The latest research shows that c-myc transcription in AML cells can be inhibited in a range far lower than the equivalent human dose that patients can safely tolerate in phase I clinical trials, which leads to cell cycle arrest and apoptosis, thus significantly reducing the burden of leukaemia [175]. After entering the body, minnelide can be rapidly and completely converted into triptolide, which is beneficial for controlling the dosage of the drug, improving its safety and effectiveness.
7. Conclusions and prospects
Triptolide is the main active ingredient of the traditional Chinese medicinal herb T. wilfordii. It has a unique chemical structure and promising pharmacological activity. But there are still many challenges in the translation or triptolide from use in traditional to modern drugs, such as poor water solubility, a narrow treatment window, and strong toxicity and side effects. At this stage, the main solution is to modify the different parts of the triptolide structure, such as C-14 hydroxyl group, to obtain derivatives that have improved poor water solubility and fewer and less severe side effects.
In addition, the production of triptolide is also a major challenge. The traditional method for obtaining triptolide cannot meet commercial needs. With good results, research on the total synthesis of triptolide has led to researchers analyzing and optimizing synthetic routes of triptolide. At present, the research on the chemical synthesis of triptolide mainly focuses on the optimization and innovation of synthesis conditions, so as to improve the yield of triptolide. In addition, the use of biosynthetic methods to produce triptolide is the research focus of many groups. Although the biosynthetic pathway of triptolide has not been fully elucidated, the upstream biosynthetic pathway of terpenoids in T. wilfordii has been analyzed. Moreover, high-quality genome sequencing and annotation of T. wilfordii genes have been completed, laying the foundation for better identification of genes encoding key enzymes in the triptolide biosynthesis pathway.
Many reviews have been focused on triptolide. However, thus far, these reviews have been basically aimed at introducing the research progress of triptolide, such as its anticancer effects [176], derivative identification [170] and advances in its total chemical synthesis [119]. In this review, triptolide is described in detail from different perspectives such as pharmacological activity and biosynthesis, aiming to provide new ideas for researchers in different disciplines and promote the research progress of triptolide. Currently, the biosynthetic pathway of triptolide has been resolved sufficiently to identify dehydroabietic acid as a key intermediate, and the chemical synthesis of triptolide from dehydroabietic acid has been reported by scholars. This progress suggests that, in the near future research, researchers can combine biosynthesis with chemical synthesis to realize the industrial production of triptolide. Similarly, the combination of pharmacological activity research and synthetic pathway analysis can be used to study the pharmacological activities of intermediates similar to triptolide through synthetic pathways to screen analogues with the rich pharmacological activities of triptolide that induce fewer side effects, such as triptinin B and triptophenolide. For the research of triptolide derivatives, the common derivatization methods include hydroxylation or glycosylation, which may be realized by cytochrome P450 and glycosyltransferase. The analysis of biosynthetic pathways also includes dioxygenase and methyltransferase, which provide more possibilities for the study of triptolide derivatives. In the study of the pharmacological activities of triptolide derivatives and triptolide, many pathways and targets are the same, which can provide more ideas for improving the production of triptolide and its derivatives.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81773830), National Key R&D Program of China (2020YFA0908000), the Key Project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302-1806-03), the National Program for Special Support of Eminent Professionals, and National Natural Science Foundation of China (No. 81803650).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wang Q, Meng J, Dong A, Yu JZ, Zhang GX, Ma CG. The Pharmacological Effects and Mechanism of Tripterygium wilfordii Hook F in Central Nervous System Autoimmunity. J Altern Complement Med. 2016;22:496-502
2. Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769-74
3. Kupchan SM, Court WA, Dailey RG, Gilmore CJ, Bryan RF. Tumor inhibitors. LXXIV. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc. 1972;94:7194-5
4. Zhou ZL, Yang YX, Ding J, Li YC, Miao ZH. Triptolide: structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat Prod Rep. 2012;29:457-75
5. Luo XL, Shao Q, Qu HB, Cheng YY. Simple method for determination of five terpenoids from different parts of Tripterygium wilfordii and its preparations by HPLC coupled with evaporative light scattering detection. J Sep Sci. 2007;30:1284-91
6. Wang J, Wang A, Zeng H, Liu L, Jiang W, Zhu Y. et al. Effect of triptolide on T-cell receptor beta variable gene mRNA expression in rats with collagen-induced arthritis. Anat Rec (Hoboken). 2012;295:922-7
7. Chuang IC, Yang CM, Song TY, Yang NC, Hu ML. The anti-angiogenic action of 2-deoxyglucose involves attenuation of VEGFR2 signaling and MMP-2 expression in HUVECs. Life Sci. 2015;139:52-61
8. Su Z, Sun H, Ao M, Zhao C. Atomic Force Microscopy Study of the Anti-inflammatory Effects of Triptolide on Rheumatoid Arthritis Fibroblast-like Synoviocytes. Microsc Microanal. 2017;23:1002-12
9. Fan D, He X, Bian Y, Guo Q, Zheng K, Zhao Y. et al. Triptolide Modulates TREM-1 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis. Int J Mol Sci. 2016;17:498
10. Wang S, Liu Z, Wang J, Wang Y, Liu J, Ji X. et al. The triptolide-induced apoptosis of osteoclast precursor by degradation of cIAP2 and treatment of rheumatoid arthritis of TNF-transgenic mice. Phytother Res. 2019;33:342-9
11. Jiang Y, Zhong M, Long F, Yang R. Deciphering the Active Ingredients and Molecular Mechanisms of Tripterygium hypoglaucum (Levl.) Hutch against Rheumatoid Arthritis Based on Network Pharmacology. Evid Based Complement Alternat Med. 2020;2020:2361865
12. Song X, Zhang Y, Dai E, Wang L, Du H. Prediction of triptolide targets in rheumatoid arthritis using network pharmacology and molecular docking. Int Immunopharmacol. 2020;80:106179
13. Zhang L, Chang J, Zhao Y, Xu H, Wang T, Li Q. et al. Fabrication of a triptolide-loaded and poly-gamma-glutamic acid-based amphiphilic nanoparticle for the treatment of rheumatoid arthritis. Int J Nanomedicine. 2018;13:2051-64
14. Li P, Yang X, Yang Y, He H, Chou CK, Chen F. et al. Synergistic effect of all-trans-retinal and triptolide encapsulated in an inflammation-targeted nanoparticle on collagen-induced arthritis in mice. J Control Release. 2020;319:87-103
15. Liu Y, Jin J, Xu H, Wang C, Yang Y, Zhao Y. et al. Construction of a pH-responsive, ultralow-dose triptolide nanomedicine for safe rheumatoid arthritis therapy. Acta Biomater. 2020
16. Zhao X, Tang X, Yan Q, Song H, Li Z, Wang D. et al. Triptolide ameliorates lupus via the induction of miR-125a-5p mediating Treg upregulation. Int Immunopharmacol. 2019;71:14-21
17. Zhou Y, Hong Y, Huang H. Triptolide Attenuates Inflammatory Response in Membranous Glomerulo-Nephritis Rat via Downregulation of NF-kappaB Signaling Pathway. Kidney Blood Press Res. 2016;41:901-10
18. Li Y, Yu C, Zhu WM, Xie Y, Qi X, Li N. et al. Triptolide ameliorates IL-10-deficient mice colitis by mechanisms involving suppression of IL-6/STAT3 signaling pathway and down-regulation of IL-17. Mol Immunol. 2010;47:2467-74
19. Crews GM, Erickson L, Pan F, Fisniku O, Jang MS, Wynn C. et al. Down-regulation of TGF-beta and VCAM-1 is associated with successful treatment of chronic rejection in rats. Transplant Proc. 2005;37:1926-8
20. Hong Y, Zhou W, Li K, Sacks SH. Triptolide is a potent suppressant of C3, CD40 and B7h expression in activated human proximal tubular epithelial cells. Kidney Int. 2002;62:1291-300
21. Chun Chen, Shanmin Yang, Mei Zhang. et al. Triptolide mitigates radiation-induced pulmonary fibrosis via inhibition of axis of alveolar macrophages-NOXes-ROS-myofibroblasts. Cancer Biol Ther. 2016
22. Kim ST, Kim SY, Lee J, Kim K, Park SH, Park YS. et al. Triptolide as a novel agent in pancreatic cancer: the validation using patient derived pancreatic tumor cell line. BMC Cancer. 2018;18:1103
23. Zhang YQ, Shen Y, Liao MM, Mao X, Mi GJ, You C. et al. Galactosylated chitosan triptolide nanoparticles for overcoming hepatocellular carcinoma: Enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms. Nanomedicine. 2019;15:86-97
24. Wei YM, Wang YH, Xue HQ, Luan ZH, Liu BW, Ren JH. Triptolide, A Potential Autophagy Modulator. Chin J Integr Med. 2019;25:233-40
25. Li R, Zhang X, Tian X, Shen C, Zhang Q, Zhang Y. et al. Triptolide inhibits tumor growth by induction of cellular senescence. Oncol Rep. 2017;37:442-8
26. Hou Z-y, Tong X-p, Peng Y-b, Zhang B-k, Yan M. Broad targeting of triptolide to resistance and sensitization for cancer therapy. Biomed Pharmacother. 2018;104:771-80
27. Yang A, Qin S, Schulte BA, Ethier SP, Tew KD, Wang GY. MYC Inhibition Depletes Cancer Stem-like Cells in Triple-Negative Breast Cancer. Cancer Res. 2017;77:6641-50
28. Wang J, Zhang ZQ, Li FQ, Chen JN, Gong X, Cao BB. et al. Triptolide interrupts rRNA synthesis and induces the RPL23MDM2p53 pathway to repress lung cancer cells. Oncol Rep. 2020;43:1863-74
29. Reno TA, Kim JY, Raz DJ. Triptolide Inhibits Lung Cancer Cell Migration, Invasion, and Metastasis. Ann Thorac Surg. 2015;100:1817-24 discussion 24-5
30. Hamdi AM, Jiang ZZ, Guerram M, Yousef BA, Hassan HM, Ling JW. et al. Biochemical and computational evaluation of Triptolide-induced cytotoxicity against NSCLC. Biomed Pharmacother. 2018;103:1557-66
31. Nardi I, Reno T, Yun X, Sztain T, Wang J, Dai H. et al. Triptolide inhibits Wnt signaling in NSCLC through upregulation of multiple Wnt inhibitory factors via epigenetic modifications to Histone H3. Int J Cancer. 2018;143:2470-8
32. Zhu J, Wang H, Chen F, Lv H, Xu Z, Fu J. et al. Triptolide enhances chemotherapeutic efficacy of antitumor drugs in non-small-cell lung cancer cells by inhibiting Nrf2-ARE activity. Toxicol Appl Pharmacol. 2018;358:1-9
33. Zheng L, Jia J, Dai H, Wan L, Liu J, Hu L. et al. Triptolide-Assisted Phosphorylation of p53 Suppresses Inflammation-Induced NF-kappaB Survival Pathways in Cancer Cells. Mol Cell Biol. 2017 37
34. Liu J, Cheng H, Han L, Qiang Z, Zhang X, Gao W. et al. Synergistic combination therapy of lung cancer using paclitaxel- and triptolide-coloaded lipid-polymer hybrid nanoparticles. Drug Des Devel Ther. 2018;12:3199-209
35. Li SG, Shi QW, Yuan LY, Qin LP, Wang Y, Miao YQ. et al. C-Myc-dependent repression of two oncogenic miRNA clusters contributes to triptolide-induced cell death in hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2018;37:51
36. Sun YY, Xiao L, Wang D, Ji YC, Yang YP, Ma R. et al. Triptolide inhibits viability and induces apoptosis in liver cancer cells through activation of the tumor suppressor gene p53. Int J Oncol. 2017;50:847-52
37. Liu X, Zhao P, Wang X, Wang L, Zhu Y, Gao W. Triptolide Induces Glioma Cell Autophagy and Apoptosis via Upregulating the ROS/JNK and Downregulating the Akt/mTOR Signaling Pathways. Front Oncol. 2019;9:387
38. Zhang L, Yu JS. Triptolide reverses helper T cell inhibition and down-regulates IFN-gamma induced PD-L1 expression in glioma cell lines. J Neurooncol. 2019;143:429-36
39. Zhang H, Li H, Liu Z, Ge A, Guo E, Liu S. et al. Triptolide inhibits the proliferation and migration of medulloblastoma Daoy cells by upregulation of microRNA-138. J Cell Biochem. 2018;119:9866-77
40. Jiang J, Song X, Yang J, Lei K, Ni Y, Zhou F. et al. Triptolide Inhibits Proliferation and Migration of Human Neuroblastoma SH-SY5Y Cells by Upregulating MicroRNA-181a. Oncol Res. 2018;26:1235-43
41. Noel P, Hussein S, Ng S, Antal CE, Lin W, Rodela E. et al. Triptolide targets super-enhancer networks in pancreatic cancer cells and cancer-associated fibroblasts. Oncogenesis. 2020;9:100
42. Ma JX, Sun YL, Yu Y, Zhang J, Wu HY, Yu XF. Triptolide enhances the sensitivity of pancreatic cancer PANC-1 cells to gemcitabine by inhibiting TLR4/NF-kappaB signaling. Am J Transl Res. 2019;11:3750-60
43. Dai H, Jiang Y, Luo Y, Bie P, Chen Z. Triptolide enhances TRAIL sensitivity of pancreatic cancer cells by activating autophagy via downregulation of PUM1. Phytomedicine. 2019;62:152953
44. Ding B, Wahid MA, Wang Z, Xie C, Thakkar A, Prabhu S. et al. Triptolide and celastrol loaded silk fibroin nanoparticles show synergistic effect against human pancreatic cancer cells. Nanoscale. 2017;9:11739-53
45. Feng J, Xu M, Wang J, Zhou S, Liu Y, Liu S. et al. Sequential delivery of nanoformulated alpha-mangostin and triptolide overcomes permeation obstacles and improves therapeutic effects in pancreatic cancer. Biomaterials. 2020;241:119907
46. Yuan S, Wang L, Chen X, Fan B, Yuan Q, Zhang H. et al. Triptolide inhibits the migration and invasion of human prostate cancer cells via Caveolin-1/CD147/MMPs pathway. Biomed Pharmacother. 2016;84:1776-82
47. Yang CY, Lin CK, Lin GJ, Hsieh CC, Huang SH, Ma KH. et al. Triptolide represses oral cancer cell proliferation, invasion, migration, and angiogenesis in co-inoculation with U937 cells. Clin Oral Investig. 2017;21:419-27
48. Yanchun M, Yi W, Lu W, Yu Q, Jian Y, Pengzhou K. et al. Triptolide prevents proliferation and migration of Esophageal Squamous Cell Cancer via MAPK/ERK signaling pathway. Eur J Pharmacol. 2019;851:43-51
49. Acikgoz E, Tatar C, Oktem G. Triptolide inhibits CD133(+) /CD44(+) colon cancer stem cell growth and migration through triggering apoptosis and represses epithelial-mesenchymal transition via downregulating expressions of snail, slug, and twist. J Cell Biochem. 2020;121:3313-24
50. Jiang X, Cao G, Gao G, Wang W, Zhao J, Gao C. Triptolide decreases tumor-associated macrophages infiltration and M2 polarization to remodel colon cancer immune microenvironment via inhibiting tumor-derived CXCL12. J Cell Physiol. 2020
51. Yu D, Liu Y, Zhou Y, Ruiz-Rodado V, Larion M, Xu G. et al. Triptolide suppresses IDH1-mutated malignancy via Nrf2-driven glutathione metabolism. Proc Natl Acad Sci U S A. 2020;117:9964-72
52. Wang R, Ma X, Su S, Liu Y. Triptolide antagonized the cisplatin resistance in human ovarian cancer cell line A2780/CP70 via hsa-mir-6751. Future Med Chem. 2018;10:1947-55
53. Chan SF, Chen YY, Lin JJ, Liao CL, Ko YC, Tang NY. et al. Triptolide induced cell death through apoptosis and autophagy in murine leukemia WEHI-3 cells in vitro and promoting immune responses in WEHI-3 generated leukemia mice in vivo. Environ Toxicol. 2017;32:550-68
54. Li F, Zhao D, Yang S, Wang J, Liu Q, Jin X. et al. ITRAQ-Based Proteomics Analysis of Triptolide On Human A549 Lung Adenocarcinoma Cells. Cell Physiol Biochem. 2018;45:917-34
55. Wang M, Chen B, Chai L. Triptolide suppresses the proliferation and induces the apoptosis of nasopharyngeal carcinoma cells via the PI3K/Akt pathway. Oncol Lett. 2019;17:1372-8
56. Wang CY, Bai XY, Wang CH. Traditional Chinese medicine: a treasured natural resource of anticancer drug research and development. Am J Chin Med. 2014;42:543-59
57. Meng C, Zhu H, Song H, Wang Z, Huang G, Li D. et al. Targets and molecular mechanisms of triptolide in cancer therapy. Chin J Cancer Res. 2014;26:622-6
58. Hou ZY, Tong XP, Peng YB, Zhang BK, Yan M. Broad targeting of triptolide to resistance and sensitization for cancer therapy. Biomed Pharmacother. 2018;104:771-80
59. Yan P, Sun X. Triptolide: A new star for treating human malignancies. J Cancer Res Ther. 2018;14:S271-S5
60. Pefanis E, Wang J, Rothschild G, Lim J, Kazadi D, Sun J. et al. RNA exosome-regulated long non-coding RNA transcription controls super-enhancer activity. Cell. 2015;161:774-89
61. Qin G, Li P, Xue Z. Triptolide induces protective autophagy and apoptosis in human cervical cancer cells by downregulating Akt/mTOR activation. Oncol Lett. 2018;16:3929-34
62. Li C, Zhang B, Lv W, Lai C, Chen Z, Wang R. et al. Triptolide inhibits cell growth and GRP78 protein expression but induces cell apoptosis in original and radioresistant NPC cells. Oncotarget. 2016;7:49588-96
63. Xie CQ, Zhou P, Zuo J, Li X, Chen Y, Chen JW. Triptolide exerts pro-apoptotic and cell cycle arrest activity on drug-resistant human lung cancer A549/Taxol cells via modulation of MAPK and PI3K/Akt signaling pathways. Oncol Lett. 2016;12:3586-90
64. Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966-75
65. Kong J, Wang L, Ren L, Yan Y, Cheng Y, Huang Z. et al. Triptolide induces mitochondria-mediated apoptosis of Burkitt's lymphoma cell via deacetylation of GSK-3beta by increased SIRT3 expression. Toxicol Appl Pharmacol. 2018;342:1-13
66. Ziaei S, Halaby R. Immunosuppressive, anti-inflammatory and anti-cancer properties of triptolide: A mini review. Avicenna J Phytomed. 2016;6:149-64
67. Titov DV, Gilman B, He QL, Bhat S, Low WK, Dang Y. et al. XPB, a subunit of TFIIH, is a target of the natural product triptolide. Nat Chem Biol. 2011;7:182-8
68. He QL, Titov DV, Li J, Tan M, Ye Z, Zhao Y. et al. Covalent modification of a cysteine residue in the XPB subunit of the general transcription factor TFIIH through single epoxide cleavage of the transcription inhibitor triptolide. Angew Chem Int Ed Engl. 2015;54:1859-63
69. Manzo SG, Zhou ZL, Wang YQ, Marinello J, He JX, Li YC. et al. Natural product triptolide mediates cancer cell death by triggering CDK7-dependent degradation of RNA polymerase II. Cancer Res. 2012;72:5363-73
70. Yi JM, Huan XJ, Song SS, Zhou H, Wang YQ, Miao ZH. Triptolide Induces Cell Killing in Multidrug-Resistant Tumor Cells via CDK7/RPB1 Rather than XPB or p44. Mol Cancer Ther. 2016;15:1495-503
71. Giri B, Sethi V, Modi S, Garg B, Banerjee S, Saluja A. et al. "Heat shock protein 70 in pancreatic diseases: Friend or foe". J Surg Oncol. 2017;116:114-22
72. Li J, Hao J. Treatment of Neurodegenerative Diseases with Bioactive Components of Tripterygium wilfordii. Am J Chin Med. 2019;47:769-85
73. Huang YY, Zhang Q, Zhang JN, Zhang YN, Gu L, Yang HM. et al. Triptolide up-regulates metabotropic glutamate receptor 5 to inhibit microglia activation in the lipopolysaccharide-induced model of Parkinson's disease. Brain Behav Immun. 2018;71:93-107
74. Wang L, Zhang L, Hou Q, Zhu X, Chen Z, Liu Z. Triptolide attenuates proteinuria and podocyte apoptosis via inhibition of NF-kappaB/GADD45B. Sci Rep. 2018;8:10843
75. Zhai X, Wang L, Xu C, Hou Q, Zhang L, Li Z. et al. Triptolide preserves glomerular barrier function via the inhibition of p53-mediated increase of GADD45B. Arch Biochem Biophys. 2019;671:210-7
76. Han F, Xue M, Chang Y, Li X, Yang Y, Sun B. et al. Triptolide Suppresses Glomerular Mesangial Cell Proliferation in Diabetic Nephropathy Is Associated with Inhibition of PDK1/Akt/mTOR Pathway. Int J Biol Sci. 2017;13:1266-75
77. Xue M, Cheng Y, Han F, Chang Y, Yang Y, Li X. et al. Triptolide Attenuates Renal Tubular Epithelial-mesenchymal Transition Via the MiR-188-5p-mediated PI3K/AKT Pathway in Diabetic Kidney Disease. Int J Biol Sci. 2018;14:1545-57
78. Tu L, Su P, Zhang Z, Gao L, Wang J, Hu T. et al. Genome of Tripterygium wilfordii and identification of cytochrome P450 involved in triptolide biosynthesis. Nat Commun. 2020;11:971
79. Su P, Guan H, Zhao Y, Tong Y, Xu M, Zhang Y. et al. Identification and functional characterization of diterpene synthases for triptolide biosynthesis from Tripterygium wilfordii. Plant J. 2018;93:50-65
80. Lynen F. Biosynthetic pathways from acetate to natural products. Pure Appl Chem. 1967;14:137-67
81. Newman JD, Chappell J. Isoprenoid biosynthesis in plants: carbon partitioning within the cytoplasmic pathway. Crit Rev Biochem Mol Biol. 1999;34:95-106
82. Iván Ahumada, Albert Cairó, Andréa Hemmerlin, Víctor González, Irene Pateraki, Thomas J. Bach, et al. Characterisation of the gene family encoding acetoacetyl-CoA thiolase in Arabidopsis. Functional Plant Biology. 2008;35:p.1100-11
83. Liao P, Wang H, Hemmerlin A, Nagegowda D, Bach T, Wang M. et al. Past achievements, current status and future perspectives of studies on 3-hydroxy-3-methylglutaryl-CoA synthase (HMGS) in the mevalonate (MVA) pathway. Plant Cell Reports. 2014;33:1005-22
84. Pablo Leivar VMG, Susanna Castel, Richard N. Trelease, Carmen López-Iglesias, Montserrat Arró, et al. Subcellular Localization of Arabidopsis 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase. Plant Physiol. 2005;137:p.00000057-69
85. Simkin AJ, Guirimand G, Papon N, Courdavault V, Thabet I, Ginis O. et al. Peroxisomal localisation of the final steps of the mevalonic acid pathway in planta. Planta. 2011;234:903-14
86. Cordier H, Karst F, Berges T. Heterologous expression in Saccharomyces cerevisiae of an Arabidopsis thaliana cDNA encoding mevalonate diphosphate decarboxylase. Plant Mol Biol. 1999;39:953-67
87. Liu YJ, Zhao YJ, Su P, Zhang M, Tong YR, Hu TY. et al. The MVA pathway genes expressions and accumulation of celastrol in Tripterygium wilfordii suspension cells in response to methyl jasmonate treatment. J Asian Nat Prod Res. 2016;18:619-28
88. Liu YJ, Zhao YJ, Zhang M, Su P, Wang XJ, Zhang XN. et al. Cloning and characterisation of the gene encoding 3-hydroxy-3-methylglutaryl-CoA synthase in Tripterygium wilfordii. Molecules. 2014;19:19696-707
89. Lichtenthaler HK. THE 1-DEOXY-D-XYLULOSE-5-PHOSPHATE PATHWAY OF ISOPRENOID BIOSYNTHESIS IN PLANTS. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:47-65
90. Wright LP, Rohwer JM, Ghirardo A, Hammerbacher A, Ortiz-Alcaide M, Raguschke B. et al. Deoxyxylulose 5-phosphate synthase controls flux through the methylerythritol 4-phosphate pathway in Arabidopsis. Plant Physiol. 2014 165
91. Kuzuyama T, Takahashi S, Watanabe H, Seto H. Direct Formation of 2-C-Methyl-D-erythritol 4-Phosphate from 1-Deoxy-D-xylulose 5-Phosphate by 1-Deoxy-D-xylulose 5-Phosphate Reductoisomerase, a New Enzyme in the Non-Mevalonate Pathway to Isopentenyl Diphosphate. Tetrahedron Lett. 1998;39:4509-12
92. Kuzuyama T, Takagi M, Kaneda K, Dairi T, Seto H. Formation of 4-(cytidine 5'-diphospho)-2-C-methyl-d-erythritol from 2-C-methyl-d-erythritol 4-phosphate by 2-C-methyl-d-erythritol 4-phosphate cytidylyltransferase, a new enzyme in the nonmevalonate pathway. Tetrahedron Letters. 2000;41:703-6
93. Calisto BM, Perez-Gil J, Bergua M, Querol-Audi J, Fita I, Imperial S. Biosynthesis of isoprenoids in plants: structure of the 2C-methyl-D-erithrytol 2,4-cyclodiphosphate synthase from Arabidopsis thaliana. Comparison with the bacterial enzymes. Protein Sci. 2007;16:2082-8
94. Seemann M, Tse Sum Bui B, Wolff M, Miginiac-Maslow M, Rohmer M. Isoprenoid biosynthesis in plant chloroplasts via the MEP pathway: direct thylakoid/ferredoxin-dependent photoreduction of GcpE/IspG. FEBS Lett. 2006;580:1547-52
95. Botella-Pavia P, Besumbes O, Phillips MA, Carretero-Paulet L, Boronat A, Rodriguez-Concepcion M. Regulation of carotenoid biosynthesis in plants: evidence for a key role of hydroxymethylbutenyl diphosphate reductase in controlling the supply of plastidial isoprenoid precursors. Plant J. 2004;40:188-99
96. Phillips MA, D'Auria JC, Gershenzon J, Pichersky E. The Arabidopsis thaliana Type I Isopentenyl Diphosphate Isomerases Are Targeted to Multiple Subcellular Compartments and Have Overlapping Functions in Isoprenoid Biosynthesis. Plant Cell. 2008;20:677-96
97. Tong Y, Su P, Zhao Y, Zhang M, Wang X, Liu Y. et al. Molecular Cloning and Characterization of DXS and DXR Genes in the Terpenoid Biosynthetic Pathway of Tripterygium wilfordii. Int J Mol Sci. 2015;16:25516-35
98. Zhang Y, Zhao Y, Wang J, Hu T, Tong Y, Zhou J. et al. The expression of TwDXS in the MEP pathway specifically affects the accumulation of triptolide. Physiol Plant. 2020;169:40-8
99. Cheng Q, Tong Y, Wang Z, Su P, Gao W, Huang L. Molecular cloning and functional identification of a cDNA encoding 4-hydroxy-3-methylbut-2-enyl diphosphate reductase from Tripterygium wilfordii. Acta Pharm Sin B. 2017;7:208-14
100. Newman JD, Chappell J. Isoprenoid biosynthesis in plants: carbon partitioning within the cytoplasmic pathway. Crit Rev Biochem Mol Biol. 1999;34:95-106
101. Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Isoprenoid biosynthesis in bacteria: a novel pathway for the early steps leading to isopentenyl diphosphate. Biochem J. 1993;295( Pt 2):517-24
102. Vandermoten S, Haubruge E, Cusson M. New insights into short-chain prenyltransferases: structural features, evolutionary history and potential for selective inhibition. Cell Mol Life Sci. 2009;66:3685-95
103. Zhao YJ, Cheng QQ, Su P, Chen X, Wang XJ, Gao W. et al. Research progress relating to the role of cytochrome P450 in the biosynthesis of terpenoids in medicinal plants. Appl Microbiol Biotechnol. 2014;98:2371-83
104. Zhang M, Su P, Zhou YJ, Wang XJ, Zhao YJ, Liu YJ. et al. Identification of geranylgeranyl diphosphate synthase genes from Tripterygium wilfordii. Plant Cell Rep. 2015;34:2179-88
105. Su P, Gao L, Tong Y, Guan H, Liu S, Zhang Y. et al. Analysis of the role of geranylgeranyl diphosphate synthase 8 from Tripterygium wilfordii in diterpenoids biosynthesis. Plant Sci. 2019;285:184-92
106. Hansen NL, Heskes AM, Hamberger B, Olsen CE, Hallstrom BM, Andersen-Ranberg J. et al. The terpene synthase gene family in Tripterygium wilfordii harbors a labdane-type diterpene synthase among the monoterpene synthase TPS-b subfamily. Plant J. 2017;89:429-41
107. Su P, Guan H, Zhang Y, Wang X, Gao L, Zhao Y. et al. Probing the Single Key Amino Acid Responsible for the Novel Catalytic Function of ent-Kaurene Oxidase Supported by NADPH-Cytochrome P450 Reductases in Tripterygium wilfordii. Front Plant Sci. 2017;8:1756
108. Forman V, Callari R, Folly C, Heider H, Hamberger B. Production of Putative Diterpene Carboxylic Acid Intermediates of Triptolide in Yeast. Molecules. 2017 22
109. Zhu C, Miao G, Guo J, Huo Y, Zhang X, Xie J. et al. Establishment of Tripterygium wilfordii Hook. f. Hairy root culture and optimization of its culture conditions for the production of triptolide and wilforine. J Microbiol Biotechnol. 2014;24:823-34
110. Miao GP, Zhu CS, Yang YQ, Feng MX, Ma ZQ, Feng JT. et al. Elicitation and in situ adsorption enhanced secondary metabolites production of Tripterygium wilfordii Hook. f. adventitious root fragment liquid cultures in shake flask and a modified bubble column bioreactor. Bioprocess Biosyst Eng. 2014;37:641-50
111. Su P, Cheng Q, Wang X, Cheng X, Zhang M, Tong Y. et al. Characterization of eight terpenoids from tissue cultures of the Chinese herbal plant, Tripterygium wilfordii, by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Biomed Chromatogr. 2014;28:1183-92
112. Song Y, Chen S, Wang X, Zhang R, Tu L, Hu T. et al. A novel strategy to enhance terpenoids production using cambial meristematic cells of Tripterygium wilfordii Hook. f. Plant Methods. 2019;15:129
113. Zhou YJ, Gao W, Rong Q, Jin G, Chu H, Liu W. et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J Am Chem Soc. 2012;134:3234-41
114. Dai Z, Liu Y, Huang L, Zhang X. Production of miltiradiene by metabolically engineered Saccharomyces cerevisiae. Biotechnol Bioeng. 2012;109:2845-53
115. Hu T, Zhou J, Tong Y, Su P, Li X, Liu Y. et al. Engineering chimeric diterpene synthases and isoprenoid biosynthetic pathways enables high-level production of miltiradiene in yeast. Metab Eng. 2020;60:87-96
116. Buckanin RS, Chen SJ, Frieze DM, Sher FT, Berchtold GA. Total synthesis of triptolide and triptonide. J Am Chem Soc. 1980;102:1200-1
117. Van Tamelen EE, Demers JP, Taylor EG, Koller K. Total synthesis of l-triptonide and l-triptolide. J Am Chem Soc. 1980;102:5424-5
118. Yang D, Ye X, Xu M. Enantioselective total synthesis of (-)-triptolide, (-)-triptonide, (+)- triptophenolide, and (+)-triptoquinonide. J Org Chem. 2000;65:2208-08
119. Zhang X, Xiao Z, Xu H. A review of the total syntheses of triptolide. Beilstein J Org Chem. 2019;15:1984-95
120. Frank T, Sher Glenn, A Berchtold. Studies on the total synthesis of triptolide. 1. J Org Chem. 1977
121. Xu H, Tang H, Feng H, Li Y. Divergent total synthesis of triptolide, triptonide, tripdiolide, 16-hydroxytriptolide, and their analogues. J Org Chem. 2014;79:10110-22
122. Zhou B, Li X, Feng H, Li Y. Efficient synthesis of the key intermediate triptophenolide methyl ether for the synthesis of (-)-triptolide. Tetrahedron. 2010;66:5396-401
123. Fujii A, Hashiguchi S, Uematsu N, Ikariya T, Noyori R. Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation of Ketones Using a Formic Acid-Triethylamine Mixture. J. Am. Chem. Soc. 1996;118:2521-22
124. Cannillo A, Schwantje TR, Begin M, Barabe F, Barriault L. Gold-Catalyzed Photoredox C(sp(2)) Cyclization: Formal Synthesis of (+/-)-Triptolide. Org Lett. 2016;18:2592-5
125. Lai CK, Buckanin RS, Chen SJ, Zimmerman DF, Sher FT, Berchtold GA. Total synthesis of racemic triptolide and triptonide. J. Org. Chem. 1982;47:2364-9
126. Cao LJ, Hou ZY, Li HD, Zhang BK, Fang PF, Xiang DX. et al. The Ethanol Extract of Licorice (Glycyrrhiza uralensis) Protects against Triptolide-Induced Oxidative Stress through Activation of Nrf2. Evid Based Complement Alternat Med. 2017;2017:2752389
127. Fu Q, Huang X, Shu B, Xue M, Zhang P, Wang T. et al. Inhibition of mitochondrial respiratory chain is involved in triptolide-induced liver injury. Fitoterapia. 2011;82:1241-8
128. Hasnat M, Yuan Z, Naveed M, Khan A, Raza F, Xu D. et al. Drp1-associated mitochondrial dysfunction and mitochondrial autophagy: a novel mechanism in triptolide-induced hepatotoxicity. Cell Biol Toxicol. 2019;35:267-80
129. Zhao J, Xie C, Mu X, Krausz KW, Patel DP, Shi X. et al. Metabolic alterations in triptolide-induced acute hepatotoxicity. Biomed Chromatogr. 2018;32:e4299
130. Lu Y, Xie T, Zhang Y, Zhou F, Ruan J, Zhu W. et al. Triptolide Induces hepatotoxicity via inhibition of CYP450s in Rat liver microsomes. BMC Complement Altern Med. 2017;17:15
131. Fu L, Zhou L, Geng S, Li M, Lu W, Lu Y. et al. Catalpol coordinately regulates phase I and II detoxification enzymes of Triptolide through CAR and NRF2 pathways to reduce Triptolide-induced hepatotoxicity. Biomed Pharmacother. 2020 129
132. Zheng N, Wang T, Wei A, Chen W, Zhao C, Li H. et al. High-content analysis boosts identification of the initial cause of triptolide-induced hepatotoxicity. J Appl Toxicol. 2019;39:1337-47
133. Huang JF, Zhao Q, Dai MY, Xiao XR, Zhang T, Zhu WF. et al. Gut microbiota protects from triptolide-induced hepatotoxicity: Key role of propionate and its downstream signalling events. Pharmacol Res. 2020;155:104752
134. Shen Q, Wang J, Yuan Z, Jiang Z, Shu T, Xu D. et al. Key role of organic cation transporter 2 for the nephrotoxicity effect of triptolide in rheumatoid arthritis. Int Immunopharmacol. 2019;77:105959
135. Huang W, Liu C, Xie L, Wang Y, Xu Y, Li Y. Integrated network pharmacology and targeted metabolomics to reveal the mechanism of nephrotoxicity of triptolide. Toxicol Res (Camb). 2019;8:850-61
136. Wang SR, Chen X, Ling S, Ni RZ, Guo H, Xu JW. MicroRNA expression, targeting, release dynamics and early-warning biomarkers in acute cardiotoxicity induced by triptolide in rats. Biomed Pharmacother. 2019;111:1467-77
137. Xi Y, Wang W, Wang L, Pan J, Cheng Y, Shen F. et al. Triptolide induces p53-dependent cardiotoxicity through mitochondrial membrane permeabilization in cardiomyocytes. Toxicol Appl Pharmacol. 2018;355:269-85
138. Ma B, Qi H, Li J, Xu H, Chi B, Zhu J. et al. Triptolide disrupts fatty acids and peroxisome proliferator-activated receptor (PPAR) levels in male mice testes followed by testicular injury: A GC-MS based metabolomics study. Toxicology. 2015;336:84-95
139. Xiong S, Li Y, Xiang Y, Peng N, Shen C, Cai Y. et al. Dysregulation of lncRNA and circRNA Expression in Mouse Testes after Exposure to Triptolide. Curr Drug Metab. 2019;20:665-73
140. Qi Y, Mao Musser, John H. Immunosuppressive compounds and methods. CA.
141. Kitzen JJ, de Jonge MJ, Lamers CH, Eskens FA, van der Biessen D, van Doorn L. et al. Phase I dose-escalation study of F60008, a novel apoptosis inducer, in patients with advanced solid tumours. Eur J Cancer. 2009;45:1764-72
142. Fidler JM, Li K, Chung C, Wei K, Ross JA, Gao M. et al. PG490-88, a derivative of triptolide, causes tumor regression and sensitizes tumors to chemotherapy. Mol Cancer Ther. 2003;2:855-62
143. Chen G, Sun H, Arp J, Garcia B, Wang X, Wise Y. et al. A Synergistic Effect Between PG490-88 and Tacrolimus Prolongs Renal Allograft Survival in Monkeys. Am J Transplant. 2006;6:714-23
144. Wang X, Sun H, Chen G, Liu W, Wise Y, Yung C. et al. Immunosuppression with a combination of pg490-88 and a subtherapeutic dose of FK506 in a canine renal allograft model. Transplantation. 2005;79:1537-44
145. Kim HJ, Ravichandran K, Ozkok A, Wang Q, He Z, Jani A. et al. The water-soluble triptolide derivative PG490-88 protects against cisplatin-induced acute kidney injury. J Pharmacol Exp Ther. 2014;349:518-25
146. Pao HP, Liao WI, Wu SY, Hung KY, Huang KL, Chu SJ. PG490-88, a derivative of triptolide, suppresses ischemia/reperfusion-induced lung damage by maintaining tight junction barriers and targeting multiple signaling pathways. Int Immunopharmacol. 2019;68:17-29
147. Zhou R, Tang W, He PL, Yang YF, Li YC, Zuo JP. (5R)-5-hydroxytriptolide inhibits the immune response of human peripheral blood mononuclear cells. Int Immunopharmacol. 2009;9:63-9
148. Liu J, Chen X, Zhang Y, Miao H, Liu K, Li L. et al. Derivatization of (5R)-hydroxytriptolide from benzylamine to enhance mass spectrometric detection: application to a Phase I pharmacokinetic study in humans. Anal Chim Acta. 2011;689:69-76
149. Zeng JZ, Ma LF, Meng H, Yu HM, Zhang YK, Guo A. (5R)-5-hydroxytriptolide (LLDT-8) prevents collagen-induced arthritis through OPG/RANK/RANKL signaling in a rat model of rheumatoid arthritis. Exp Ther Med. 2016;12:3101-6
150. Ren YX, Zhou R, Tang W, Wang WH, Li YC, Yang YF. et al. (5R)-5-hydroxytriptolide (LLDT-8) protects against bleomycin-induced lung fibrosis in mice. Acta Pharmacol Sin. 2007;28:518-25
151. Guo S, Liu J, Jiang T, Lee D, Wang R, Zhou X. et al. (5R)-5-Hydroxytriptolide (LLDT-8) induces substantial epigenetic mediated immune response network changes in fibroblast-like synoviocytes from rheumatoid arthritis patients. Sci Rep. 2019;9:11155
152. Qi Q, Li H, Lin ZM, Yang XQ, Zhu FH, Liu YT. et al. (5R)-5-hydroxytriptolide ameliorates anti-glomerular basement membrane glomerulonephritis in NZW mice by regulating Fcgamma receptor signaling. Acta Pharmacol Sin. 2018;39:107-16
153. Zhang LY, Li H, Wu YW, Cheng L, Yan YX, Yang XQ. et al. (5R)-5-hydroxytriptolide ameliorates lupus nephritis in MRL/lpr mice by preventing infiltration of immune cells. Am J Physiol Renal Physiol. 2017;312:F769-F77
154. Chen Y, Zhang L, Ni J, Wang X, Cheng J, Li Y. et al. LLDT-8 protects against cerebral ischemia/reperfusion injury by suppressing post-stroke inflammation. J Pharmacol Sci. 2016;131:131-7
155. Su R, Sun M, Wang W, Zhang J, Zhang L, Zhen J. et al. A Novel Immunosuppressor, (5R)-5-Hydroxytriptolide, Alleviates Movement Disorder and Neuroinflammation in a 6-OHDA Hemiparkinsonian Rat Model. Aging Dis. 2017;8:31-43
156. Cui YQ, Zheng Y, Tan GL, Zhang DM, Wang JY, Wang XM. (5R)-5-hydroxytriptolide inhibits the inflammatory cascade reaction in astrocytes. Neural Regen Res. 2019;14:913-20
157. Dong Y, Lu H, Li Q, Qi X, Li Y, Zhang Z. et al. (5R)-5-hydroxytriptolide ameliorates liver lipid accumulation by suppressing lipid synthesis and promoting lipid oxidation in mice. Life Sci. 2019;232:116644
158. Wang L, Xu Y, Fu L, Li Y, Lou L. (5R)-5-hydroxytriptolide (LLDT-8), a novel immunosuppressant in clinical trials, exhibits potent antitumor activity via transcription inhibition. Cancer Lett. 2012;324:75-82
159. Zhou R, Zhang F, He PL, Zhou WL, Wu QL, Xu JY. et al. (5R)-5-hydroxytriptolide (LLDT-8), a novel triptolide analog mediates immunosuppressive effects in vitro and in vivo. Int Immunopharmacol. 2005;5:1895-903
160. Patil S, Lis LG, Schumacher RJ, Norris BJ, Morgan ML, Cuellar RA. et al. Phosphonooxymethyl Prodrug of Triptolide: Synthesis, Physicochemical Characterization, and Efficacy in Human Colon Adenocarcinoma and Ovarian Cancer Xenografts. J Med Chem. 2015;58:9334-44
161. Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S. et al. A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4:156ra39
162. Oliveira A, Beyer G, Chugh R, Skube SJ, Majumder K, Banerjee S. et al. Triptolide abrogates growth of colon cancer and induces cell cycle arrest by inhibiting transcriptional activation of E2F. Lab Invest. 2015;95:648-59
163. Banerjee S, Nomura A, Sangwan V, Chugh R, Dudeja V, Vickers SM. et al. CD133+ tumor initiating cells in a syngenic murine model of pancreatic cancer respond to Minnelide. Clin Cancer Res. 2014;20:2388-99
164. Nomura A, McGinn O, Dudeja V, Sangwan V, Saluja AK, Banerjee S. Minnelide effectively eliminates CD133(+) side population in pancreatic cancer. Mol Cancer. 2015;14:200
165. Banerjee S, Modi S, McGinn O, Zhao X, Dudeja V, Ramakrishnan S. et al. Impaired Synthesis of Stromal Components in Response to Minnelide Improves Vascular Function, Drug Delivery, and Survival in Pancreatic Cancer. Clin Cancer Res. 2016;22:415-25
166. Banerjee S, Thayanithy V, Sangwan V, Mackenzie TN, Saluja AK, Subramanian S. Minnelide reduces tumor burden in preclinical models of osteosarcoma. Cancer Lett. 2013;335:412-20
167. Isharwal S, Modi S, Arora N, Uhlrich C 3rd, Giri B, Barlass U. et al. Minnelide Inhibits Androgen Dependent, Castration Resistant Prostate Cancer Growth by Decreasing Expression of Androgen Receptor Full Length and Splice Variants. Prostate. 2017;77:584-96
168. Rivard C, Geller M, Schnettler E, Saluja M, Vogel RI, Saluja A. et al. Inhibition of epithelial ovarian cancer by Minnelide, a water-soluble pro-drug. Gynecol Oncol. 2014;135:318-24
169. Brincks EL, Kucaba TA, James BR, Murphy KA, Schwertfeger KL, Sangwan V. et al. Triptolide enhances the tumoricidal activity of TRAIL against renal cell carcinoma. FEBS J. 2015;282:4747-65
170. Noel P, Von Hoff DD, Saluja AK, Velagapudi M, Borazanci E, Han H. Triptolide and Its Derivatives as Cancer Therapies. Trends Pharmacol Sci. 2019;40:327-41
171. Dauer P, Zhao X, Gupta VK, Sharma N, Kesh K, Gnamlin P. et al. Inactivation of Cancer-Associated-Fibroblasts Disrupts Oncogenic Signaling in Pancreatic Cancer Cells and Promotes Its Regression. Cancer Res. 2018;78:1321-33
172. McGinn O, Gupta VK, Dauer P, Arora N, Sharma N, Nomura A. et al. Inhibition of hypoxic response decreases stemness and reduces tumorigenic signaling due to impaired assembly of HIF1 transcription complex in pancreatic cancer. Sci Rep. 2017;7:7872
173. Greeno E, Borazanci EH, Gockerman JP, Korn RL, Vonhoff DD. Phase I dose escalation and pharmokinetic study of a modified schedule of 14-o-phosphonooxymethyltriptolide. J Clin Oncol. 2016;34:TPS472-TPS
174. Khanipour Roshan S, Spano AD, McKinney AM, Nascene DR. Potentially reversible acute cerebellar toxicity associated with Minnelide. Neuroradiology. 2017;59:419-21
175. Giri B, Gupta VK, Yaffe B, Modi S, Roy P, Sethi V. et al. Pre-clinical evaluation of Minnelide as a therapy for acute myeloid leukemia. J Transl Med. 2019;17:163
176. Lv H, Jiang L, Zhu M, Li Y, Luo M, Jiang P. et al. The genus Tripterygium: A phytochemistry and pharmacological review. Fitoterapia. 2019;137:104190
177. Garg B, Giri B, Majumder K, Dudeja V, Banerjee S, Saluja A. Modulation of post-translational modifications in beta-catenin and LRP6 inhibits Wnt signaling pathway in pancreatic cancer. Cancer Lett. 2017;388:64-72
Author contact
![]() Corresponding authors: Wei Gao: Beijing Shijitan Hospital, Capital Medical University, Beijing, 100038, China (Email: weigaoedu.cn); Luqi Huang: State Key Laboratory Breeding Base of Dao-di Herbs, National Resource Center for Chinese Materia Medica, Chinese Academy of Chinese Medical Sciences, Beijing, 100700, China (Email: huangluqi01com).
Corresponding authors: Wei Gao: Beijing Shijitan Hospital, Capital Medical University, Beijing, 100038, China (Email: weigaoedu.cn); Luqi Huang: State Key Laboratory Breeding Base of Dao-di Herbs, National Resource Center for Chinese Materia Medica, Chinese Academy of Chinese Medical Sciences, Beijing, 100700, China (Email: huangluqi01com).
 Global reach, higher impact
Global reach, higher impact