13.3
Impact Factor
Theranostics 2021; 11(13):6573-6591. doi:10.7150/thno.55664 This issue Cite
Review
The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies
Department of Medical Sciences, Section of Experimental Medicine, School of Medicine, University of Ferrara. Ferrara, Italy.
*These authors contributed equally to this work.
Received 2020-11-9; Accepted 2021-3-15; Published 2021-4-30
Abstract
Mesenchymal stem cells (MSCs) have been identified in many adult tissues. MSCs can regenerate through cell division or differentiate into adipocytes, osteoblasts and chondrocytes. As a result, MSCs have become an important source of cells in tissue engineering and regenerative medicine for bone tissue and cartilage. Several epigenetic factors are believed to play a role in MSCs differentiation. Among these, microRNA (miRNA) regulation is involved in the fine modulation of gene expression during osteogenic/chondrogenic differentiation. It has been reported that miRNAs are involved in bone homeostasis by modulating osteoblast gene expression. In addition, countless evidence has demonstrated that miRNAs dysregulation is involved in the development of osteoporosis and bone fractures. The deregulation of miRNAs expression has also been associated with several malignancies including bone cancer. In this context, bone-associated circulating miRNAs may be useful biomarkers for determining the predisposition, onset and development of osteoporosis, as well as in clinical applications to improve the diagnosis, follow-up and treatment of cancer and metastases. Overall, this review will provide an overview of how miRNAs activities participate in osteogenic/chondrogenic differentiation, while addressing the role of miRNA regulatory effects on target genes. Finally, the role of miRNAs in pathologies and therapies will be presented.
Keywords: epigenetics, microRNAs, MSC differentiation, disease, tumour
Introduction
Mesenchymal stem cells (MSCs) are non-hematopoietic multipotent cells and, as a result of their potential promising properties, are widely studied in regenerative medicine [1]. MSCs can renew themselves through cell division, whereas they differentiate both into osteoblasts and chondrocytes after exposure to specific soluble factors in the microenvironment. In vitro, osteogenic differentiation of stem cells typically involves the use of dexamethasone, β-glycerolphosphate and ascorbic acid [2] while MSCs placed in aggregate in a medium containing specific component, such as dexamethasone, ascorbate-2-phosphate, selenious acid, sodium pyruvate and transforming growth factor β (TGF-β) will undergo chondrogenic differentiation [3].
Because several epigenetic factors play an important role in MSCs differentiation, a large number of studies have been conducted on the biological properties of MSCs and factors that might facilitate MSC chondrogenic/osteogenic differentiation as well as scaffolds/biomaterials for tissue engineering [4,5]. Indeed, biomaterials provide the biological structure for MSCs to stimulate chondrogenic [6] and osteogenic [7,8] differentiation. The addition of ions [9] has been proved to enhance the osteogenic potential of scaffolds. On the other hand, biological macromolecules such as growth factors [10] and microRNAs (miRNAs) can be associated with scaffolds in order to increase differentiation potential [11]. MiRNAs represent a family of small single-stranded, non-coding RNA molecules consisting of approximately 21-25 nucleotides (nt) which were discovered in nematode Caenorhabditis elegans and they were considered small temporal RNAs [12]. It has been predicted that miRNAs account for 1-5% of the human genome, regulate at least 30% of protein-coding genes [13] and are known to be generated through a multi-step process (Figure 1). Moreover, it is known that miRNAs play a pivotal role in musculoskeletal functions, such as cell differentiation, survivability and apoptosis. To date, the most well-known function of miRNAs is the ability to negatively regulate gene expression at post-transcriptional level by interfering with target messenger RNA (mRNA) translation [14]. The degree and nature of complementarity between the miRNA seed site and the 3′-UTR of its target mRNA determined the gene silencing mechanism: if complementarity is complete, the mRNA target will be degraded; if complementarity is partial, mRNA target translation is inhibited [14] causing a reduction of protein levels. Several computational algorithms have been developed in order to predict miRNA targets. These include miRWalk, Diana-Tools, miRanda, Microrna.org, mirDB, TargetMiner, TargetScan and PicTar. This software is able to predict miRNA targets based on bioinformatic tools, although, each target has to be experimentally validated using different techniques such as PCR and Western Blot after prediction.
MiRNAs are endogenous oligonucleotides, which can be released into the extracellular space and therefore are found in almost all body [15] and exhibit great potential as biomarkers for non-invasive cancer diagnosis and prognosis [16], as well as for therapy and surgical treatment responses [16]. Targeted drugs based on molecules which mimic the action of miRNAs, or molecules which contrast its action (known as antago-miR), have been developed to cure different diseases, including cancer [17].
In addition, mimic or antago-miR have been proposed not only for therapy monitoring, but also as targets [18] for innovative therapeutic approaches in regenerative medicine in association with stem cells [5] and scaffolds [19] in order to improve tissue regeneration. MiRNAs could positively or negatively regulate chondrogenic and osteogenic differentiation by targeting either negative or positive genes or transcription factors (TFs), respectively. However, some miRNAs exert both stimulatory and inhibitory effects during chondrogenic and osteogenic differentiation, as shown below.
Chondrogenic and osteogenic differentiation
Chondrogenesis is a key developmental process in endochondral ossification, which is a fundamental step during skeletal development. Endochondral ossification starts when undifferentiated mesenchymal cells aggregate to form mesenchymal condensation at the point of each future skeletal part by acquiring characteristics of non-hypertrophic chondrocytes. During chondrogenesis, chondrocytes differentiate into proliferating chondrocytes, pre-hypertrophic and then, hypertrophic chondrocytes. Lastly, hypertrophic chondrocytes undergo apoptosis and are replaced by bone. Chondrogenic and osteogenic differentiation of MSCs are complex developmental processes governed by different factors, such as signaling pathways, growth factors and TFs that are required in a precise spatio-temporal sequence to each proper chondrocyte and osteocyte formation.
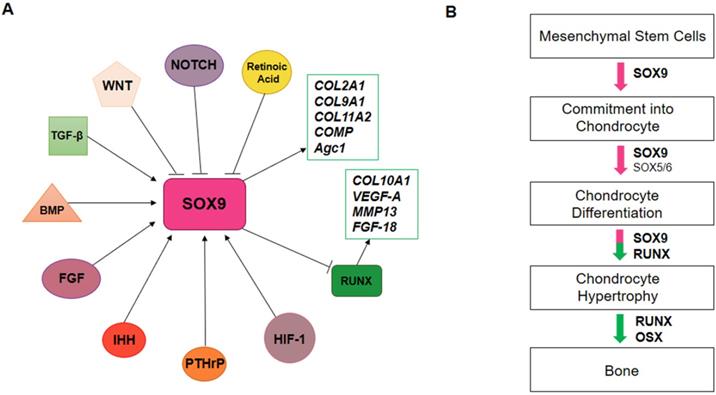
SRY-Box Transcription Factor 9 (SOX9), the master regulator of chondrogenesis, is highly expressed in mesenchymal osteo/chondro-progenitors, which are proliferating, and pre-hypertrophic chondrocytes, but declines in hypertrophic chondrocytes. SOX9 exerts its effects on chondrogenesis by directing the expression of chondrocyte-specific extracellular matrix genes, such as collagen type II alpha 1 chain (COL2A1), collagen type IX alpha 1 chain (COL9A1), collagen type XI alpha 2 chain (COL11A2), cartilage oligomeric protein (COMP) and aggrecan (Agc1 or ACAN) (Figure 2A). SOX9 regulates chondrocyte gene expression in collaboration with others transcriptional coactivators, SOX5 and SOX6. During chondrocyte hypertrophy, SOX9, SOX5 and SOX6 expression decreases. Furthermore, other transcription factors, such as Runt-related transcription factor 2 (RUNX2) and Runt-related transcription factor 3 (RUNX3), are activated to promote chondrocyte hypertrophy (Figure 2B). RUNX2 directly binds and activates genes like Indian hedgehog (Ihh), COL10A, matrix metalloproteinase 13 (MMP13) and vascular epithelial growth factor (VEGF-A).
Other chondrogenesis related genes are DEC1 and DEC2, two basic helix-loop-helix (bHLH) proteins, which regulate cell differentiation in different tissues including cartilage. Specifically, DEC1 is considered a transcriptional factor cAMP-induced that modulates chondrogenesis [20]. DEC2 is a negative regulator of MSC differentiation in chondrogenic lineage, whereas its over-expression downregulates the mRNA expression of chondrogenic markers [21].
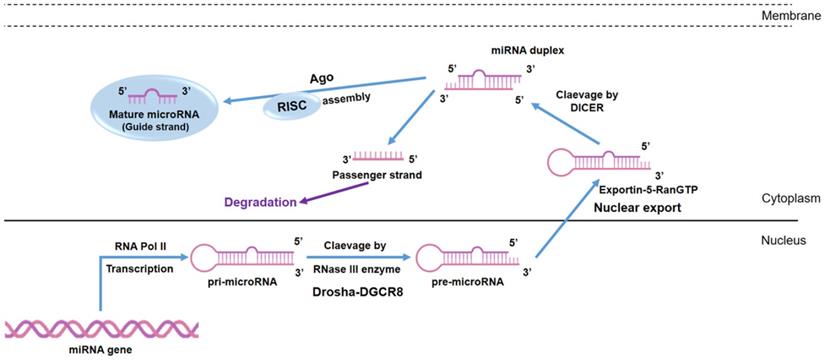
MiRNA biogenesis. MiRNAs are transcribed by RNA polymerases II (Pol II) in the form of a first precursor called primary miRNA (pri-miRNA). The pri-miRNA is converted into the precursor miRNA (pre-miRNA) via the cutting activity of the Drosha enzyme, a nuclear endoribonuclease III. Pre-miRNAs are exported into the cytoplasm as a result of the action of Ran-GTP and Exportin-5, a nuclear export factor. In the cytoplasm, Dicer clivates the pre-miRNA into a double stranded miRNA (miRNA duplex) of about 18-22 nt. The mature miRNA strand is incorporated into the RNA- induced silencing complex (RISC), which guides the miRNAs to the 3′UTR of its target.
SOX9 regulation during chondrogenesis. A) In chondrogenesis, SOX9 is negatively regulated by WNT, NOTCH and retinoic acid pathways. Otherwise, TGF-β, BMP, FGF, IHH, PTHrP, HIF-1 pathways positively regulate SOX9 expression. SOX9 induces COL2A1, COL9A1, COL11A2, COMP and Agc1 expression and inhibits RUNX expression, which in turn activates COL10a1, MMP13, VEGF-A and FGF-18 genes. B) Endochondral ossification is regulated by transcription factors. SOX9 promotes the commitment of mesenchymal stem cells into chondrocytes; SOX5/6/9 promotes chondrocytes differentiation; during chondrocyte hypertrophy, SOX5/6/9 expression decreases and RUNX and OSX expression is activated to promote bone formation.
TGF-β and bone morphogenic protein (BMP) signaling have a key role in chondrogenic and osteogenic processes. TGF-β/BMP pathways stimulate chondroblasts to proliferate and deposit cartilage-specific extracellular matrix molecules, such as Agc1, COL2A1 and glycosaminoglycans (GAG). Several miRNAs that post-transcriptionally module these signaling pathways by inducing or inhibiting chondrogenesis are reported in Table 1.
In osteogenesis, the TGF-β pathway is activated in the early stages, allowing transition from osteo-progenitor cells to immature osteoblasts to take place through osteogenic marker expression (Figure 3). In osteogenic differentiation, the homeodomain protein Distal-less Homeobox 5 (DLX5) is an activator of master TFs in osteogenesis: RUNX2 and the zinc finger transcription factor OSX. OSX, also known as Sp7, is an osteoblast-specific transcription factor that induces osteoblast differentiation and bone formation. OSX is a downstream RUNX2 gene, which is considered to be a fundamental osteogenic TF that regulates osteoblastic genes, such as osteopontin (OPN), osteocalcin (OCN), osteonectin (ON) and collagen type 1 (COL1). Some evidences have demonstrated that OSX is also regulated via RUNX2-independent mechanism.
MicroRNAs involved in chondrogenic differentiation, their direct target genes and cell type in which the role of miRNAs has been studied
| miRNA | Cell type | Target gene | Effect on chondrogenesis |
|---|---|---|---|
| miR-20a | ATDC5 | Atg7 | - |
| miR-20b | iPSC | EPAS1 | - |
| miR-29a | MSCs, synovial fibroblasts | FOXO3A, VEGF | - |
| miR-520d-5p | hMSCs | HDAC1 | + |
| miR-381 | ATDC5, SW1353 | HDAC4 | - |
| miR-1 | Primary chicken chondrocyte | HDAC4 | - |
| miR-221 | MSCs | FOXO3 | - |
| miR-27b | NP cells | MMP-13 | + |
| miR-9 | Chondrogenic progenitors, articular chondrocytes | MMP-13, PRTG | + |
| miR-124 | murine BM-MSCs | NFATC1 | - |
| miR-92a | Chondrogenic progenitors | Nog3 | + |
| miR-127-5p | Rat BM-MSCs | MMP-13 | + |
| miR-182-5p | BM-MSCs | PTHLH | - |
| miR-140-5p | hMSCs | RALA | + |
| miR-455-3p | ATDC5 | RUNX2 | + |
| miR-320 | ATDC5 | RUNX2, MMP13 | + |
| miR-30a | hMSCs | SOX9 | - |
| miR-30b | C3H10T1/2 | SOX9 | - |
| miR-574-3p | hMSCs | RXRα | + |
| miR-199a | C3H10T1/2 | SMAD1 | - |
| miR-155 | A549 | SMAD1 | + |
| miR-194 | MSCs | SOX9 or SOX5 | - |
| miR-145 | murine and human MSCs | SOX9 | - |
| miR-101 | Rat BM-MSCs | SOX9 | - |
| miR-1247 | human chondrocytes | SOX9 | - |
| miR-495 | MSCs | SOX9 or SOX5 | - |
| miR-140 | hMSCs, mouse cells | HDAC4, ADAMTS‐5 | - |
| miR-337 | human and rat chondrocyte | TGFBR2 | - |
| miR-193b | ATDC5, hASCs | TGFBR2, DDAH1 | - |
| miR-17-92 cluster | MPCs | TGF-β signaling pathway, SQSTM1/p62 | - |
| miR-210 | Rat Medial Meniscal Model | HOXA-1/9 | + |
| miR-107 | C28/I2 cell | PTEN | + |
| miR-424 | hiPSCs | VEGF, VEGFR2, FGFR1 | + |
Indeed, the homeobox gene MSX2, which is up-regulated by BMP2, induces OSX over-expression, thus favoring osteoblastic differentiation [22].
In addition to TGF‐β/BMP signaling cascades, Wingless/Int-1 (Wnt)/β-catenin signaling pathway is crucial for MSC osteogenic differentiation by guiding MSCs into pre-osteoblast cells. Twist Family BHLH Transcription Factor 1 (TWIST-1) gene is a target for the Wnt/β-catenin cascade, which is required for cranial bone lineage commitment. Interestingly, TWIST-1 exerts its function by maintaining Wnt responsiveness. TWIST-1 knockdown leads to cranial bone agenesis, as well as eye and palate malformation [23]. TGF-β/BMP and Wnt/β-catenin signaling pathways affect each other in regulating skeletal tissue formation in vivo and in vitro. Furthermore, several miRNAs, which are reported in Table 2, post-transcriptionally module these signaling pathways by inducing or inhibiting osteogenesis.
MicroRNAs involved in osteogenic differentiation, their direct target genes and cell type in which the role of miRNAs has been studied
| miRNA | Cell type | Target gene | Effect on osteogenesis |
|---|---|---|---|
| miR-98 | hMSCs | BMP-2 | - |
| miR-140‐5p | hMSCs | BMP-2 | - |
| miR-370 | MC3T3‐E1 | BMP-2 | - |
| miR-93-5p | hMSCs | BMP-2, SP7 | - |
| miR-195-5p | PDLSCs | BMPR1A | - |
| miR-100 | hMSCs | BMPR2 | - |
| miR‐106a‐5p | PDLSCs | BMP-2, E2F5 | - |
| miR-153 | hMSCs | BMPR2 | - |
| miR-132 | UC-MSCs | β-catenin | - |
| miR-29a | BM‐MSCs, MC3TC‐E1 | HDAC4 | + |
| miR-29b | BM‐MSCs, MC3TC‐E1, USSC | HDAC4 | + |
| MiR-376c-3p | BM-MSCs | IGF1R | - |
| miR-410-3p | hRIFs | MSX2 | - |
| miR-23a cluster | MC3T3‐E1 | Prdm16 | + |
| miR-140 | hPDLFs | RhoA | - |
| miR-23a | MSCs, MC3T3-E1 | RUNX2 | - |
| miR-23b | MSCs | RUNX2 | - |
| miR-30b | MSCs | RUNX2 | - |
| miR-30c | MSCs | RUNX2 | - |
| miR-133a-5p | MC3T3-E1 | RUNX2 | - |
| miR-135a-5p | C2C12 | RUNX2 | - |
| miR-137 | MSCs | RUNX2 | - |
| miR-137-3p | BM-MSCs | RUNX2 | - |
| miR-203 | MSCs, MC3T3‐E1 | RUNX2, MAPK/STAT pathways activation | -/+ |
| miR-204 | MSCs, MPCs, BM-MSCs | RUNX2 | - |
| miR-211 | MPCs, BM-MSCs | RUNX2 | - |
| miR-217 | MSCs | RUNX2 | - |
| miR-221 | MSCs | RUNX2 | - |
| miR-338 | MSCs | RUNX2 | - |
| miR-433 | C3H10T1/2 | RUNX2 | - |
| miR-505 | MC3T3-E1 | RUNX2 | - |
| miR-628-3p | MG63 | RUNX2 | - |
| miR-1305 | PDLSCs | RUNX2 | - |
| miR-30 family | MC3T3-E1, MSCs | RUNX2, SMAD1 | - |
| miR-338-3p | BM-MSCs, MC3T3-E1 | RUNX2, FGFR2 | - |
| miR-205 | MSCs, BM-MSCs | RUNX2, SATB2 | - |
| miR-222-3p | BM-MSCs | RUNX2, SMAD5 | - |
| miR-133a | MSCs, C2C12 | RUNX2, SMAD5 | - |
| miR-135a | MSCs, MC3T3-E1, ATDC5 | RUNX2, SMAD5 | - |
| miR-143 | MSCs | RUNX2, SP7 | - |
| miR-34c | MSCs, MC3T3‐E1 | RUNX2 | - |
| miR-375 | C2C12, hASCs | RUNX2, Yap1 | -/+ |
| miR-133 | C2C12 | RUNX2 | - |
| miR-130a-3p | hASCs | SIRT7 | + |
| miR-26b | USSC | HDAC4 | - |
| miR-203-3p | MSCs | SMAD1 | - |
| miR-26a | USSC, BM-MSCs, hASCs | SMAD1, GSK3b, HDAC4 | -/+ |
| miR-135 | C2C12 | SMAD1, SMAD5 | - |
| miR-146a | ADSCs | SMAD4 | - |
| miR-144-3p | C3H10T1/2 | SMAD4 | - |
| miR-17-5p | BM‐MSCs | SMAD7 | + |
| miR-21-5p | BM-MSCs | SMAD7 | + |
| miR-21 | BM-MSCs | SMAD7 | + |
| miR-21a | C3H10T1/2 | SMAD7 | + |
| miR-590-5p | C3H10T1/2, MG63, MC3T3-E1 | SMAD7 | + |
| miR-497-5p | SCAP | Smurf2 | + |
| miR-93 | C57BL/6 | SP7 | - |
| miR-637 | hMSCs | SP7 | - |
| miR-145 | C2C12, MC3T3-E1, BM-MSCs | SP7, CBFB | - |
| miR-214 | BM‐MSCs, MC3T3‐E1, C2C12, C57BL/6 | SP7, ATF4, β-catenin, FGFR1 | - |
| miR-378 | hMSCs | Wnt6, Wnt10 | - |
MiRNAs in chondrogenic differentiation
MiRNAs positively regulating chondrogenesis
Among miRNAs regulating chondrogenesis, miR-140 is the most-highly studied. MiR-140 targets Sp1 in order to maintain chondrocyte proliferation [24]. Histone deacetylase 4 (HDAC4) and ADAM Metallopeptidase with Thrombospondin Type 1 Motif 5 (ADAMTS‐5) have also been proposed as target genes for miR-140 [24]. MiR-140-3p and miR-140-5p are up-regulated in human MSCs (hMSCs) during chondrogenesis and target genes identified in terminal hypertrophic differentiation. Specifically, miR-140-5p targets the 3'UTC of Ras-related protein Ral-A (RALA), enhancing SOX9 and ultimately, ACAN [25]. MiR-520d-5p also promotes hMSC chondrogenesis and regulates chondrocyte metabolism by targeting Histone Deacetylase 1 (HDAC1) [26]. MiR-574-3p has been found to be early up-regulated during chondrocyte differentiation. The retinoid X receptor (RXRα) has been identified as a direct target for miR-574-3p in hMSCs, whereas it seems that this miRNA is also involved in a negative feedback loop required for chondrocyte lineage maintenance and MSC multipotency [27]. In rat bone marrow stromal cells (BM-MSCs), the expression of early chondrogenesis markers SOX9, COL2A1 and ACAN was stimulated after miR-127-5p transfection, while it decreased in late markers COL10A1 and RUNX2. Therefore, miR-127-5p upregulation seems to promote chondrogenesis, thus preventing hypertrophic differentiation [28].
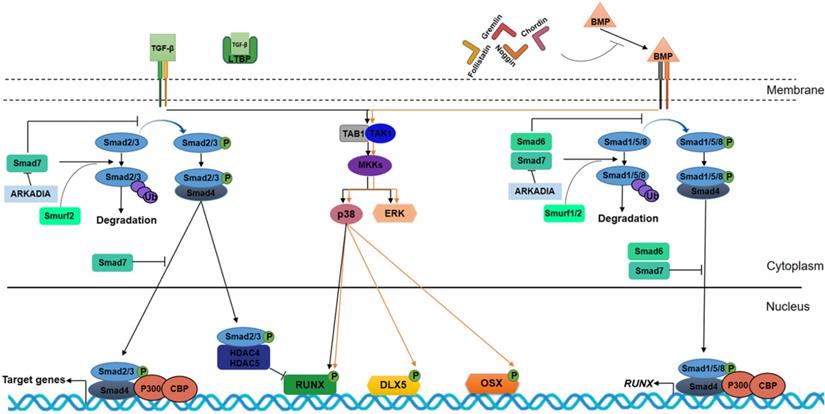
TGF-β and BMPs signaling pathways in osteogenesis. TGF-βs and BMPs bind to the extracellular domains of specific receptors and require Sma and Mad related (SMAD) proteins for signal transduction within the cells. TGF-βs and BMPs work through heterodimeric receptors consisting of type I and type II kinase receptors. TβRI/ALK5 is the type I receptor for TGF-βs, and BMPR1A/ALK3, BMPR1B/ALK6 and AcvR1/ALK2 are type I receptors for BMPs. TβRII/TGFBR2 and BMPR2 are the type II kinase receptor for TGF-βs and BMPs, respectively. Upon binding of the ligand to the receptor, the receptor forms homodimeric complexes, resulting in one subunit phosphorylating its partner subunit on the serine/threonine residues. This starts a cascade of events involving SMAD protein phosphorylation. The TGF-β pathway requires SMAD2 and SMAD3, whereas BMP signaling is dependent on SMAD1, 5 and 8. Phosphorylated receptor-regulated SMADs (R-SMAD) react with SMAD4 to create a heterocomplex. This complex, consisting of R-SMAD and common-mediator SMAD (Co-SMAD), enters the nucleus and controls transcription by binding to target gene promoters. Phosphorylated R-SMAD (R-SMAD-Pi) interacts with SMAD4 protein, then translocates into the cell nucleus where AMP response element binding protein (CREB)-binding protein (CBP) and P300 coactivators are recruited which regulate gene targets. SMAD2/3-Pi in the nucleus and SMAD4-unlinked recruit HDAC4 and HDAC5 by inhibiting RUNX2 and OSX expression. In the Non-Smad-dependent signaling pathway, TGF-β activates kinase 1 (TAK1) and TAK1-binding protein 1 (TAB1) to initiate the MKKs (MAPK pathway member-encoding genes kinases)-p38 MAPK or -Erk (extracellular signal regulated kinase) signaling cascades. Interaction between BMPs and their receptors result in comparable events triggered by TGF‐β. In BMP signaling, R-SMAD (SMAD1/5/8) bind SMAD4 to translocate into the nucleus, where they induce RUNX2 expression. Similarly, Non-Smad-dependent BMP-stimulated pathway induces DLX5, RUNX2, and OSX expression and leads to osteoblast and osteocyte differentiation. Latent TGF-beta binding proteins (LTBPs) bind TGF-β and prevent its interaction with TβRI and TβRII receptors; otherwise, Noggin, Chordin, Gremlin and Follistatin bind to BMP proteins and block their signaling cascades. In TGF- β signaling, SMAD7 inhibits SMAD2/3 translocation into the nucleus, and, alongside Smurf2 induces R-SMAD proteasome-mediated degradation. Similarly, in BMP signaling, SMAD6/7 inhibit SMAD1/5/8 translocation into the nucleus and, alongside Smurf1/2 induces SMAD1/5/8 and RUNX2 proteasome-mediated degradation. In turn, SMAD6 expression is regulated by RUNX2 in a negative feedback loop. ARKADIA protein positively regulates osteoblast differentiation by binding SMAD6/7 proteins.
MiRNAs negatively regulating chondrogenesis
MiR-124 directly targets and inhibits the Nuclear Factor of Activated T Cells 1 (NFATC1). The latter directly interacts with the SOX9 promoter [29]. During chondrogenesis, miR-124 is downregulated by promoter methylation. MiR-29a was previously identified as a chondrogenesis suppressor in MSCs [29]. MiR-29a targets the 3'UTR of transcription factor Forkhead Box Protein O3 (FOXO3A), which is up-regulated during chondrocyte differentiation. FOXO3A regulates SOX9, ACAN and COL2A1 expression during chondrogenesis [25]. MiR-30a regulates chondrogenic differentiation in hMSCs [30], while miR-30b regulates chondrogenic differentiation in mouse embryo-derived stem cells C3H10T1/2 [31].
In addition, decreased miR-221 induced an increase in chondrogenic markers, such as COL2A1, ACAN and SOX9 by targeting FOXO3. MiR-221 silencing promotes in vitro MSCs differentiation towards chondrocytes in the absence of TGFβ, enhancing in vivo cartilage repair. Antago-miR-221 treatment has been observed to restore FOXO3 in a context of tissue degeneration and inflammation [32]. MiR-182-5p is an inhibitor of chondrogenesis; it targets Parathyroid Hormone like Hormone (PTHLH) and its knockdown has been correlated to an increase in chondrogenic markers, such as SOX-9 and COL2A1 [33]. Another miRNAs inhibit chondrogenesis by directing targeting SOX9 is miR-101. SOX9 expression was negatively regulated by miR-101 in rat chondrocytes; it promoted chondrocyte late differentiation in rat BM-MSCs [34].
MiR-1247 targets cartilage transcription factor SOX9 and, in a negative feedback loop, SOX9 inhibits miR-1247 expression in human chondrocytes [35]. Upon miR-1247 over-expression, COL2A1 and miR-140 levels are significantly reduced. MiR-145 has also been reported to be downregulated during murine and human MSC chondrogenic differentiation. It directly targets the 3'UTR SOX9 [36]. Some miRNAs, such as miR-495 and miR-194, inhibit chondrocyte MSCs differentiation by targeting SOX9 or SOX5 [37].
TGF-beta signaling pathway regulation in chondrogenesis
TGFβ1, 2 and 3 are known as typical MSC chondrogenesis inducers. MicroRNA-337 is associated with chondrogenesis by regulating TGF-β type II receptor (TGFBR2) expression. MiR-337 negatively targets TGFBR2, which has an important role in cartilage development. Indeed, during the maturation phases of endochondral ossification the expression of miR-337 is substantially downregulated [38]. MiR-193b regulates early chondrogenesis by targeting TGFBR2 [39], while loss of miR-17-92 seems to reduce mesenchymal progenitor cell (MPC) proliferation by depressing TGF-β signaling [29].
BMP signaling cascade regulation in chondrogenesis
BMPs are members of the TGF-β family. MiR-199a adversely regulates early chondrocyte differentiation by directly targeting SMAD1 in the BMP signaling pathway. MiR-155 targets the 3′ UTR of multiple components in BMPs, including SMAD1 in epithelial cell line A549 [40]. MiR-92a has also been identified in the regulation of the BMP signaling pathway. Noggin3 (Nog3), a BMP antagonist, was identified as a direct target of miR-92a [38].
RUNX2 regulation in chondrogenesis
MiR-30 family members (miR-30a, -30b, -30c, and -30d) are able to target both RUNX2 and SMAD1, therefore preventing osteogenesis in mature chondrocytes [40]. MiR-455-3p also targets RUNX2 in ATDC5 cell lines and promotes early chondrocyte differentiation and inhibits chondrocyte maturation. It has been suggested that miR-455-3p increases COL2A1 promoter acetylation thereby increasing gene transcription and promoting chondrocyte differentiation [29]. MiR-320 targets RUNX2. Its expression was found to be elevated in ATDC5 cells, whereas it seems to be implicated in reducing cartilage hypertrophy and degeneration. MiR-320 also targets MMP13 and reduces its levels [29]. During growth plate development, miR-1 plays an important role in the regulation of chondrocyte differentiation by targeting HDAC4, which in turn regulates chondrocyte hypertrophy by inhibiting RUNX2 [41].
MMP-13 regulation in chondrogenesis
MMP-13 has been shown to be regulated by miRNAs including miR-9, miR-27b and miR-381. MiR-27b binds directly to the 3′ UTR of MMP-13 in nucleus pulposus (NP) cells [42]. MiR-381 has been identified as a regulator of chondrogenesis related genes. It was found to be up-regulated in late stages during in vitro chondrocyte differentiation. MiR-381 increases MMP-13 and RUNX2 expression and decreases COL2A1 expression in ATDC5 cells. It has been shown to target HDAC4 in SW1353 cells [29,43]. MiR-9 negatively regulates MMP-13 and protogenin (PRTG) expression. MiR-9 abrogation results in increased PRTG levels, inhibited cell proliferation and survival in chondrogenic progenitors and articular chondrocytes [41].
VEGF regulation in chondrogenesis
Some miRNAs positively regulating chondrogenesis through VEGF are miR-424 which is able to repress VEGF, vascular endothelial growth factor receptor 2 (VEGFR2) and fibroblast growth factor receptor 1 (FGFR1) in early chondrogenesis [44]. MiR-15 and -16 regulate VEGF expression and were downregulated during hypoxia [45]. MiR-107 targets phosphatase and tensin homolog (PTEN), modulating chondrocyte proliferation, apoptosis and extracellular matrix synthesis. Upregulation of miR-107 inhibits angiogenesis and VEGF expression and promotes ACAN and COL2A1 expression, attenuating MMP-13 and MMP-9 expression in C28/I2 cells [46]. MiR-210 promoted COL2A1 production from meniscus cells through VEGF and FGF2 upregulation in synovial cells by targeting different genes including Homeobox A 1/9 (HOXA-1/9) [41]. Finally, miR‐320 negatively regulates VEGF expression through ERK 1/2 and regulates both ADAMTS5 and HOXA10, which in turn regulate MMP13 through RUNX2 [47]. Studies involving hMSCs into chondrocytes found let-7b as a putative miRNA regulator of VEGF [48].
Another miRNA which negatively regulates chondrogenesis was miR-29a which targeted VEGF mRNA through binding to its 3′-UTR [49]. VEGF and anti-angiogenic miR-20a, miR-106a-5p, and miR-20b were also studied. MiR-20a is a critical negative regulator of chondrogenic differentiation inhibiting autophagy by targeting autophagy Related 7 (Atg7) [50]. Both, miR-17-5p and miR-20a promote chicken cell proliferation by targeting Mitogen-Activated Protein Kinase Kinase Kinase 2 (MAP3K2) and in consequence upregulating c-Myc. MiR-20b targets the early chondrogenic-related gene Endothelial PAS Domain Protein 1 (EPAS1), which is involved in embryonic organ development, by negatively regulating chondrogenic differentiation [44]. The miR-17-92 cluster and its paralogs, including miR-106a-92 and miR-106b-25, are highly expressed in myeloid progenitors and target Sequestosome 1 (SQSTM1/p62) [50]. Clusters miR-106b-25 and miR-17-92 were involved in TGF-β signaling modulation [41]. MiR-193b downregulates factors such as COL2A1, ACAN and SOX9, while it targets Dimethylarginine Dimethylaminohydrolase 1 (DDAH1), which in turn results in decreased VEGF secretion in vivo [41].
MiRNAs in osteogenic differentiation
RUNX2 regulation in osteogenesis
A panel of 11 miRNAs that target RUNX2 expressed in a lineage-related pattern in MSCs types, i.e., miR-23a, miR-30c, miR-34c, miR-133a, miR-135a, miR-137, miR-204, miR-205, miR-217, miR-218 and miR-338 has been reported. All these RUNX2‐targeting miRNAs (except miR‐218) significantly inhibit osteoblast differentiation [51]. In addition, other miRNAs that are related to the RUNX2 gene during MSCs differentiation to pre-osteoblasts, such as miR-23b, miR-30b, miR-143, miR-203 and miR-221, have also been identified [52]. For instance, a recent study has shown that miR-30 down-regulated RUNX2 by interacting with the 3′-UTR of RUNX2 in adipose-derived MSCs (hASCs) [53]. Li et al., reported that miR-133 inhibit RUNX2-mediated osteogenesis [54]. The over-expression of miR-23a in mouse osteoblast-like cells (MC3T3-E1) inhibits osteogenic differentiation by inhibiting RUNX2 [51]. In contrast, miR-23a cluster has been indicated as promoting osteogenic differentiation by modulating TGF-β signaling pathway by targeting PR domain-containing 16 (Prdm16) in MC3T3‐E1 cells [55]. MiR-133a is reported as being an important regulator in osteogenesis, as its expression is down-regulated in BMP-induced osteogenesis and can target and suppress RUNX2 expression in order to inhibit osteoblast differentiation [54]. A recent study has demonstrated inhibition of osteoblast differentiation marker expression, ECM mineralization and ALP activity, by miR-133a-5p in MC3T3-E1 cells [56]. MiR-135a strongly inhibits RUNX2 protein expression in both MC3T3-E1 and ATDC5 cells [51]. A recent study has reported that miR‑135a‑5p expression was significantly decreased during osteogenic differentiation in vitro in mouse myoblast cell line (C2C12), whereas it was significantly increased in post-menopausal females with osteoporosis [57]. Recently, Kong et al., have reported that miR-137-3p directly targets RUNX2 and its silencing promotes both osteogenesis and angiogenesis in vitro and in vivo [58].
In a recent study, it has been reported that miR‐203 can induce the upregulation of RUNX2 and OCN. The authors have suggested that miR‐203, which is up-regulated during osteogenic differentiation, could promote proliferation, migration and osteogenic differentiation of MC3T3‐E1 cells. Furthermore, it has been proposed that miR‐203 might affect MC3T3‐E1 cells by upregulating MSX2, along with MAPK/STAT pathway activation [59]. An in vitro study has demonstrated that miR-410-3p suppresses osteogenic-like differentiation by directly targeting MSX2 in human renal interstitial fibroblasts (hRIFs) [60].
Huang et al., reported that two homologous miRNAs, miR-204 and miR-211, suppress RUNX2 expression in MPCs and BM-MSCs promoting adipogenesis [61]. Hu et al., reported that miR-205 expression was down-regulated in a time-dependent manner during BM-MSC osteo-induction. Furthermore, these authors found that mir-205 could regulate protein expression of special AT-rich sequence-binding protein 2 (SATB2) and RUNX2 [62]. An in vitro analysis revealed that the over-expression of miR-338-3p can inhibit the expression of osteoblast differentiation markers such as OSX, thus reducing osteoblast differentiation. Bioinformatic analysis and dual luciferase assays confirmed that miR-338-3p can repress gene expression by targeting RUNX2 and FGFR2 [63]. It has also been reported that miR‐218 decreases RUNX2 expression in undifferentiated human-derived dental stem cells (hDSCs) [64]. Suppression of miR-222-3p activity has been reported as promoting osteogenic differentiation of human BM-MSCs by regulating the SMAD5-RUNX2 signaling axis [65]. It has been demonstrated that miR-375 expression levels decrease in a C2C12 cell model of osteogenic differentiation. Over-expression of miR-375 inhibited the activity of pivotal osteoblast markers, such as ALP, OCN and collagen, type I, α 1 (COL1A1) [66]. However, it has been demonstrated that miR‐375 over-expression enhances hASCs osteogenesis both in vitro and in vivo by directly targeting DEPTOR (mTOR inhibitor) and Yes‐associated protein1 (YAP1) in these cells [67].The circadian rhythm helps coordinate bone formation and resorption in the skeleton. MiR-433 was previously shown to target RUNX2 in C3H10T1/2 cells [68] and seems to help maintain the circadian rhythm in osteoblasts by regulating sensitivity to glucocorticoid receptor signaling [69]. MiR-505 expression was down-regulated during osteogenic differentiation in MC3T3-E1 cells [70]. Recently, a study has indicated that miR-628-3p was up-regulated in patients with atrophic non-union and exerted an inhibitory effect on osteogenesis through the suppression of its target gene, RUNX2. In vitro, miR-628-3p mimics attenuated the osteogenic differentiation of MG63 cells [71]. Interestingly, it has been reported that nicotine inhibits periodontal ligament-derived stem cells (PDLSCs) proliferation, migration, and osteogenic differentiation by increasing miR‐1305 levels. Restoration of miR-1305 relieves the inhibitory effect of nicotine on PDLSCs proliferation, migration and osteogenic differentiation by upregulating RUNX2 levels [72].
Under hypoxia, TWIST-1 is induced and acts as a transcription repressor of RUNX2 by binding to the E-box located on the RUNX2 promoter, thus, suppressing osteogenesis in hMSCs [73]. In addition to regulating protein-coding genes, it has recently become evident that TWIST-1 can also regulate miRNAs [74]. MiR-376c-3p targets IGF1R inhibiting BMSC osteogenic differentiation whereas it has been identified as a TWIST-1-regulated miRNA, which plays an important role in the osteogenesis of bone precursor cells and can mediate TWIST-1 inhibition of osteogenesis. Furthermore, overexpression of miR-376c-3p in TWIST-1 haploinsufficient calvarial cells can decrease the aberrant osteogenesis of these cells, which contributes to increased calvarial bone volume and premature fusion of coronal sutures [75].
Sp7 regulation in osteogenesis
Expression of the Sp7, a zinc finger transcription factor and critical regulator of osteoblast mineralization, was found to be inversely correlated to miR-93 in primary mouse osteoblasts from the calvaria of C57BL/6 mice [76]. By stimulating and/or inhibiting miR-93-5p expression in human BM-MSCs, Zhang et al., confirmed that osteogenic differentiation-related indictors, including OSX, were decreased by miR-93-5p [77]. MiR-145, a Sp7 regulator, suppresses the osteogenic differentiation of C2C12 and MC3T3-E1 cells [78]. It has been reported that miR‐145 and miR‐34c cooperatively inhibit osteoblast differentiation in MC3T3‐E1 cells [79]. Liu et al., [80] reported that miR‐143 and miR‐145 regulate odontoblast differentiation by binding the 3′‐UTR of Sp7. It has been reported that miR-132 expression also decreases during osteogenic differentiation of umbilical cord mesenchymal stem cells (UC-MSCs). MiR-132 upregulation inhibited the osteogenic differentiation of UC-MSCs by attenuating the activity of the Wnt/β-catenin signaling pathway targeting β-catenin and downregulating OSX levels [81]. MiR-214 expression decreases gradually during osteoblast differentiation and plays an important role in different stages of bone formation and resorption by targeting Sp7, Activating Transcription Factor 4 (ATF4), β-catenin and FGFR1 [82]. A study reported that moderate exercise could inhibit miR-214 expression in bone [83]. MiR-637 expression in hMSCs is indispensable for maintaining the balance of adipocytes and osteoblasts [84].
BMP regulation in osteogenesis
Bone morphogenetic proteins BMP and BMPRs are known as important factors for skeletal development, regeneration and homeostasis [85].
Several miRNAs negatively regulate BMP-induced osteoblastogenesis. For instance, mir-93-5p, mir-98 and miR-140-5p inhibit osteogenic differentiation in human MSCs by direct targeting BMP-2 [77,86,87]. In human periodontal ligament fibroblasts (hPDLFs) miR-140 is able to inhibit osteodifferentiation. Indeed, the authors have suggested that miR-140 suppresses osteogenic differentiation of hPDLFs by targeting RhoA, an important member of the small GTPase of RHO family that participates in key processes of osteogenic differentiation [88].
In PDLSCs, miR-106a-5p targets BMP-2 and E2F Transcription Factor 5 (E2F5) inhibiting the osteogenic differentiation [89]. It has been demonstrated that miR-370 attenuates the osteogenic differentiation of BMP2-stimulated MC3T3-E1 cells by targeting BMP-2 and erythroblastosis virus E26 oncogene homolog 1 (Ets1) [90]. Recently, a study was carried out using bioinformatics tools which revealed BMP-2 to be a potential target gene for miR-370-3p in hASCs [91]. It has also been reported that mir-195-5p inhibits osteogenesis in human PDLSCs by targeting BMP receptor type I (BMPR1A) [92]. Furthermore, it has been shown that miR-100 and mir-153 attenuate osteogenic differentiation of hMSCs by targeting BMPR2 [93,94].
SMAD regulation in osteogenesis
MiR-144-3p inhibits the proliferation of C3H10T1/2 cells by arresting cells at the G0/G1 phase. Results from bioinformatics analysis, luciferase assays and western blotting have demonstrated that miR-144-3p directly targets SMAD4 [95]. It has been reported that miR-146a negatively regulates the osteogenesis and bone regeneration from hASCs both in vitro and in vivo [96]. A single study reported that miR-203-3p targets the 3′-UTR of SMAD1 mRNA; interestingly, miR-203-3p was identified as being up-regulated in the diabetic group and when compared to normal rats [97].
MiR-26a is required for skeletal muscle differentiation/regeneration in mice and it is up-regulated during osteogenic differentiation in unrestricted somatic stem cells (USSC) and shares target genes which inhibit osteogenesis with miR-29b. These miRNAs accelerate osteogenic differentiation and are likely to be mediated by osteo-inhibitory proteins such as CDK6 and HDAC4 [98].
Interestingly, Su et al., identified miR-26a as a positive regulator in BM-MSC osteogenic differentiation, but as a negative regulator in hASCs osteogenic differentiation [99]. It has been reported that the expression levels of some miR-30 family members, such as miR-30a, -30b, -30c, and -30d, are significantly down-regulated during osteoblast differentiation; for instance, miR-30 mediates osteogenesis inhibition by targeting SMAD1 and RUNX2 in MC3T3-E1 [40]. In early osteogenesis, BMP-2 signaling appears to downregulate miR-133 and miR-135 which suppress two essential TFs for osteogenesis, RUNX2 and SMAD5, forming a transcriptional complex [100].
Finally, a recent study has reported that miR-497-5p increases in human stem cells from apical papilla (SCAP), osteo/odontogenic differentiation. Furthermore, bioinformatic analysis and dual luciferase reporter assay have identified SMAD specific E3 ubiquitin protein ligase 2 (Smurf2) as a negative osteogenesis. This gene is a target of miR-497-5p, demonstrating that miR-497-5p acts as a positive regulator of osteo/odontogenic differentiation in SCAP [101].
SMAD7 regulation in osteogenesis
The literature shows that SMAD7 is one of the target genes for miR-21a and SMAD7 has an antagonistic role in TGF-β1 signaling [102]. Indeed, miR-21 promotes BM-MSCs bone formation and this process is regulated, in part, by the SMAD7-SMAD1/5/8-RUNX2 pathway [102]. Sanjeev et al., indicated that treatment with phytol, an acyclic unsaturated diterpene alcohol and a secondary metabolite derived from aromatic plants, promotes osteoblast differentiation in C3H10T1/2 cells via RUNX2 due to down-regulation of SMAD7 by miR-21a [103]. In addition, pulsed electromagnetic fields (PEMFs) could mediate its effects on bone metabolism by activating the TGF-β signaling pathway and stimulating miR-21-5p expression in human BM-MSCs [104]. It has been reported that miR-17-5p, which is located in the miR-17/92 cluster and has important roles in various cancers, regulated osteoblastic differentiation and cell proliferation by directly targeting SMAD7 in human BM-MSCs [105].
MiR-590-5p has been reported to stimulate osteoblast differentiation of C3H10T1/2 and MG63 cells by indirectly protecting and stabilizing the RUNX2 protein by targeting SMAD7 [106].
HDAC4 regulation in osteogenesis
Over-expression of miR-29a and miR-29b can block HDAC4 expression and enhance osteoblast differentiation in primary BM-MSCs and MC3TC-E1 cells [107,108]. Delivery of osteogenesis-promoting miRNAs is a promising approach for enhancing bone regeneration [109]. For this reason, Liu et al., have developed an R9-LK15/miR-29b nano-complex that has high transfection efficiency and promotes osteogenic differentiation [109].
Wnt/β-catenin signaling pathway
Wnt family is formed of 19 secreted glycoproteins that are involved in various biological processes, such as regulation of MSCs fate and includes two distinct canonical (β-catenin dependent) and non-canonical branches. The effects of Wnt signaling pathway on osteoblastogenesis has been shown via overexpression and knockdown of Wnt pathway ligands and components [110].
Many members of the sirtuin family have been confirmed as being related to Wnt signaling. Recently, it has been indicated that the sirtuin family is closely related to osteogenic differentiation [111]. In hASCs, the over-expression of miR-130a-3p induces a significant increase in RUNX2 mRNA targeting SIRT7 [112].
Wnt6 and Wnt10, which act as MSCs fate regulators, were identified as targets of miR-378. The use of miR-378-mimics could suppress osteogenesis in hMSCs, whereas anti-miR-378 allows for osteogenic differentiation. Thus, miR-378 inhibits osteogenesis and bone formation by inactivating Wnt/β- catenin signaling [113].
MiRNAs in bone fractures and bone diseases
Fracture healing is a proliferative physiological process whereby the body facilitates bone fracture repairs [114]. In the first phase of this process, MSCs are recruited to the fracture site and differentiated into fibrocytes, chondrocytes or osteoblasts. Subsequently, in the following phase, ECM and hard callus are generated, alongside angiogenesis and revascularization. At last, during the late phase, the callus is continuously remodeled to respond to the biomechanical and biological demands of the new bone [115]. MiRNAs play a critical role in many physiological processes, as reported above. Moreover, they have recently emerged as key regulators in the complex process of fracture healing. Li et al. [116] have highlighted high miR-214-5p expression in the plasma of patients with hand or intra-articular calcaneal fractures and demonstrated the importance of miR-214-5p down-regulation which resulted in the enhancement of osteoblastic cell viability and resistance to apoptosis [116]. SMAD6 is known to be a critical feedback inhibitory governor of BMP/SMAD signaling. In silico prediction has indicated that miR-186 is a regulatory miRNA of SMAD6 as far as fracture healing is concerned [117]. In addition, miR-186 could activate the BMP signaling pathway to promote fracture healing by inhibiting SMAD6 in a mouse model of femoral fracture [117]. Lee and colleagues have shown the meaningful therapeutic effect of miR-29b-3p in femoral fracture healing; this in vivo study showed that a single injection of miR-29b-3p, delivered via a microbubble-ultrasound system, 14 days post fracture improved healing outcome [118]. The presence of 134 miRNA was detected in plasma from 4 patients with trochanteric fractures compared with 4 healthy controls [119]. Moreover, the levels of six miRNAs (miR-16, miR-19b-1, miR-25, miR-92a, miR-101, and miR-129-5p) were also shown to be dysregulated. Shi et al., [120] investigated whether miR-218 can have an impact to bone regeneration. Specifically, when injected at the fracture site of mouse femoral transverse fractures, miR‐218 overexpressing cells significantly increased bone volume at 2 and 4 weeks post fracture compared to the control group [120].
In recent years, there has been an increase in the prevalence of bone diseases due to population aging. Bone diseases affect millions of people worldwide. However, these pathologies are more common among the elderly [121]. Age-related bone and osteoarticular diseases include different pathologies such as metabolic bone diseases, osteoporosis (OP) and osteoarthritis [121]. Among these, OP, a chronic skeletal disorder characterised by increasing of bone fragility and susceptibility to fracture, is the most common bone disease in humans and represents one of the greatest public health problems [122]. OP affects about 200 million people all over the world; it affects both sexes and all races. Fractures are the most serious consequence associated with OP, causing significant burdens in terms of health care costs, morbidity and mortality annually [121]. Currently, the gold standard for diagnosing OP is bone mineral density (BMD) analysis which is measured by means of dual X-ray absorptiometry (DXA). BMD measurements of the hip and spine can be used to determine/confirm the diagnosis of OP and predict future fracture risks and monitor patients [122]. Diagnostic approaches to this pathology have limited sensitivity and specificity, also as far as monitoring disease progression or treatment is concerned. Therefore, new biomarkers are needed to improve the management of OP patients.
Despite the critical importance of miRNAs in regulating various cell functions, their role in OP has not yet been investigated in depth in well-characterized human bone. However, identifying specific miRNAs associated with OP could provide additional information, mainly by integrating multiple signals, which, when amalgamated imaging and biochemical markers, could improve a standard diagnostic pathway and increase prognostic potential [121].
In recent years, miRNAs have been widely studied as biomarkers in clinical practice thanks to their key modulatory role in multiple biological functions and their association with numerous physiological and pathological conditions [123]. As reported above, altered expression levels of miRNAs were found to be associated with bone pathologies and tumours in several studies. Although miRNAs have been identified as potential biomarkers, their evaluation for clinical application requires the standardization of all aspects of miRNA analysis from discovery to validation. Standardization of pre-analytical, analytical, and post-analytical protocols would allow all the variables that can introduce bias in the detection of biomarkers to be monitored [124].
However, there are some drawbacks for miRNAs as biomarkers, such as lack of standardized protocols for the management of all the subject-related factors (age, gender, lifestyle, diseases) and sample-associated variables (matrix/source, sample collection and handling), that may affect the concentration/expression level of the miRNA and its measurement, respectively [125]. An important source of variability in the analytical phase of miRNA evaluation is the quantification platform used (PCR, next generation sequencing and microarray). Results obtained using different technologies show significant differences in evaluating miRNA levels. Therefore, it is fundamental for analytical protocols and the platform used to be standardized [123]. When considering the post-analytical phase, the most challenging problem is represented by the absence of a standardized methodology for the reference gene selection alongside the normalization strategy used for miRNA quantification [124].
Though there are still several problems yet to be solved in the miRNA validation process, the use of miRNAs as biomarkers in clinical application and research has shown different advantages. Specifically, miRNAs can be isolated and evaluated in human biofluids (e.g., urine, plasma and serum) with non-invasive procedures [123]. Moreover, miRNA detection and quantification are based on PCR methods that are characterized by reproducible results with elevated specificity and sensitivity. Lastly, miRNAs in human biofluids, can be found to be both bind to proteins or enclose in exosomes, ectosomes or in lipoproteins, thus showing elevated stability.
Interestingly, TAmiRNA, a European firm, leader in miRNA-based diagnostics, has recently commercialized a product named Osteo-miR panel, using a set of 19 miRNAs, that is able to identify the risk of first fracture in post-menopausal osteoporosis and type-2 diabetes in women [126].
MiRNAs as circulating biomarkers in OP and osteoporotic fractures
Several studies have reported that circulating miRNAs, which have been analysed and validated in serum or plasma as well as in whole blood, could be used as prognostic or diagnostic markers for discriminating OP patients from non-OP subjects.
Circulating levels of miR-133a and miR-21 were analysed in 120 plasma samples from Chinese postmenopausal women. Their level resulted, respectively, as increased and decreased in plasma from OP and osteopenia patients compared to healthy subjects and both are associated with BMD [127]. Li et al, analysed miR-133a expression levels in serum samples from ten postmenopausal Chinese women affected by OP compared to the healthy control group. In this study miR-133a was significantly up-regulated in postmenopausal osteoporotic women compared to the control group, and negatively correlated with lumbar spine BMD, according to previous studies [127,128]. In addition, this study highlights that miRNA-133a is involved in regulating postmenopausal OP by promoting osteoclast differentiation [128].
MiR-21 down-regulation has been identified in BM-MSCs isolated from women with postmenopausal OP [127]. Nine miRNAs, namely miR-21, miR-23a, miR-24, miR-93, miR-100, miR-122a, miR-124a, miR-125b and miR-148a were shown to be significantly increased in serum from 30 patients with OP, when compared to 30 non-osteoporotic controls [129]. In the same study, five (miR-21, miR-23a, miR-24, miR-25, miR-100 and miR-125b) of these nine validated miRNAs also resulted as up-regulated in the bone tissue of osteoporotic patients [129]. An additional paper validated the increased serum levels of miR-148a, miR-125b, miR-124a, miR-122a, miR-100, miR-93, miR-24, miR-23a and miR-21 in another independent cohort of fractured postmenopausal OP women [130]. MiR-30b-5p, miR-103-3p, miR-142-3p and miR-328-3p were found to be down-regulated in postmenopausal OP after animal experiments and serum validation analysis of miRNA from OP patients. All of the aforementioned showed positive correlations with BMD [131]. Five miRNAs, including of miR-590-5p, miR-194-5p, miR-151a-3p, miR-151b and miR-130b-3p were increased in whole blood samples collected from postmenopausal OP Chinese women when compared to the osteopenia group [132]. Out of these, miR-194-5p circulating levels were strongly increased and negatively correlated with BMD. In addition, miR-194-5p was functionally associated with eight OP-related pathways suggesting that this miRNA may affect TGF-β and Wnt signalling pathways, which were shown to play critical roles in the pathology of postmenopausal OP [132].
The analysis of miRNAs isolated from 74 serum samples derived from postmenopausal women, divided into OP and control groups, showed that miR-148a-3p expression levels were significantly increased in the OP patient group compared to the controls [133]. MiR-148a could have different roles in OP pathogenesis. Specifically, it promotes the differentiation of osteoclast targeting the gene V-mafmusculo aponeurotic fibrosarcoma oncogene homolog B (MAFB), which is a transcription factor negatively regulating RANKL-induced differentiation of osteoclasts [133].
Other potential circulating markers for postmenopausal OP identified in serum are miR-23b-3p and miR-140-3p, which are increased in OP patients compared to control groups and are associated with low bone mineral density [134]. Interestingly, target genes of both miRNAs have been reported as being related to osteoblast differentiation in vivo or in vitro studies [134]. Similarly, in another study increased levels of serum miR-483-5p were found in OP women vs. non-OP controls [135]. Additionally, Insulin-like growth factor-2 (IGF2) was found to be directly targeted by miRNA-483-5p, suggesting that this miRNA is involved in the pathogenesis of OP by promoting osteoclast differentiation [135].
A recent study, conducted on a cohort of postmenopausal OP women and their control group, has reported increased levels of miR-338 cluster, including miR-3065-5p and miR-338-3p. In vitro and in vivo studies have suggested that RUNX2 and SOX4 could be two critical functional targets of the miR-338 cluster by mediating its regulation during bone formation [136]. Lastly, abnormal miR-135b-5p expression in the bone tissue of patients with OP has suggested that this miRNA could be involved in OP occurrence and development by inhibiting osteogenic differentiation and osteoblast proliferation by targeting RUNX2 [114].
Vertebral fractures characterize OP. Postmenopausal women with and without vertebral fractures were included in a single-site, case-control, observational, cross-sectional study at a University hospital [137]. The study identified seven significantly up-regulated miRNAs (miR-375, miR-532-3p, miR-19b-3p, miR-152-3p, miR-23a-3p, miR-335-5p and miR-21-5p) in patients with vertebral fractures and low BMD compared to low BMD and healthy individuals, regardless of OP treatment.
MiRNAs and bone tumours
MiRNAs have been drawing attention as novel biomarkers for the diagnosis, prognosis and treatment of bone tumours, mainly osteosarcoma (OS), chondrosarcoma (CS) and Ewing's sarcoma (ES). OS is the most common bone tumour [138], while ES and CS arise from bone/soft and cartilage tissues, respectively [138]. Unlike other tumours, which are diagnosed in the elderly [139-142], OS and ES affect children, adolescents and young adults [143]. CS is instead considered an adulthood cancer [138]. Bone tumour onset/progression depends on the dysregulation of different factors, including miRNAs and targets.
The miR-34 family (miR-34a, -b, and -c) plays a role in regulating OS essential targets, including Notch, p53, Bcl-2, PI3K/Akt, c-Myc, Bim and Runx2 [144]. The members of this miR family present tumour suppressive characteristics, which are downregulated in OS [144]. The miRNA cluster made up ofmiR-127-3p, -154, -299-5p, -329, -337-3p, -376a/c, -377, -382, -409, -410, -432, -493, -495, -453, -654-5p and -758, which is located within the chromosome 14q32 locus [145], appears to be very significant. Indeed, these miRNAs are downregulated in OS [145], while their main target is the onco-protein c-Myc. Recent studies have confirmed that miR-143 is downregulated, while miR-21, -221 and -106a are up-regulated in OS tissues. Their differential expression is also associated with tumour staging and grading, as well as lung metastasis [146]. Specifically, miR-21 and -221 play a role in OS pathogenesis as oncogenic miRNAs (onco-miRs) [147]. MiR-21 is involved in the control of TGF-β1 [147], which regulates cell growth, differentiation and apoptosis. PTEN and PDC4 are two additional targets for miR-21. This interaction leads to OS cell growth, migration, invasion and metastasis [148]. Since miR-21 inhibition in patients significantly delays OS progression, this miR is considered to be a prognostic marker [148]. MiR-221 over-expression induces cisplatin resistance and reduces apoptosis in OS cell lines via PTEN inhibition, while its silencing overturns these effects [149]. The increased expression of miR-221 has also been associated with OS tumour staging, metastatic stating, as well as chemotherapy response [150]. For these reasons, miR-221 can be employed as a marker in OS staging, metastasis and prognosis [150]. Other potential OS prognostic markers include miR-15b, -378, -568, -574, -494 and -601. These miRNAs are involved in chemotherapy responses in OS patients [151]. The expression of several 14q32-associated miRNAs, including miR-139-5p, -299, -323, -379, -382, -411, -493, -539 and -758, has also been linked to OS recurrence-free survival [152]. Additional miRNAs, i.e.miR-134, -382 and -544 have been inversely correlated to aggressive OS behavior, increased metastatic potential and accelerated time to death [152]. Circulating miRNAs which were found to be dysregulated in serum could represent OS diagnostic/prognostic tools. The plasma/serum levels of miRNA-34b have been detected as lower in OS patients and related to metastasis status [153]. MiR-21 and -148a, have been found to be dysregulated in OS patients sera and/or plasma [154,155] and could therefore be used for diagnostic purposes. Circulating miRNAs could also improve OS type identification. For instance, osteoblastic OS type is associable with miR-21 and -320a increase and miR-143 and -199a-3p decrease in sera [156]. In addition, miR-21 content tested high in sera from late-stage OS patients. The presence of some specific miRNAs has been associated with increased risk of both local recurrence and metastasis and worse survival rates in patients with osteosarcoma. In patients who have OS, miRNAs profiles in those with pathologic fractures are different from patients without pathologic fractures. MiR-656-3p, miR-493-5p and miR-381-3p were up-regulated in patients with pathologic fractures, whereas miR-363, miR-85-5p and miR-20b-5p were downregulated.
MiRNAs dysregulation is a critical process in CS onset/progression. Several miRNAs, including miR-30a [157] and -494 [158], regulate several SOX gene family members (SOX4, -5 and -9) which play crucial roles in CS [159]. One of these miRNAs, miR-494, has also been linked to poor CS survival/prognosis, underlying that it may be used as a prognostic marker for therapy [158]. An anti-tumour effect has been reported for miR-23b, which is down-expressed in CS and targets an important factor of CS pathogenesis, i.e., Src kinase [160]. Interestingly, treating CS cells with (i) naringin, which is a compound belonging to the flavonoid family, and/or (ii) paeonol, other than suppressing cell migration/invasion, also changes miRNA expression [161,162]. Indeed, naringin induces miR-126 over-expression, followed by cell migration and invasion inhibition via VCAM-1 down-regulation [162], while paeonol increases miR-141 levels, which in turn inhibit cell motility by PKC and c-Src kinase activities [161]. Previous studies, which focused on chemokine CCL5 and VEGF-dependent angiogenesis, underlined the importance of miRNA dysregulation in CS. Indeed, CCL5 has been reported as promoting VEGF-dependent angiogenesis and negatively regulating-199a in CS cells [163]. Moreover, another study indicated miR-181a as a hypoxia-regulated miRNA which controls VEGF expression in CS cells [164]. Therefore, this miRNA could be considered as a potential therapeutic tool for CS angiogenesis monitoring [164]. Additionally, miR-181a overexpression causes the development of ossification in the posterior longitudinal ligament (OPLL) by targeting PBX1 [165]. Other miRNAs, which are involved in CS onset/development are miR-125b, which tested as down-regulated in both CS tissues/cell lines [166], whereas the miR-143/145 cluster presents tumour suppressor features [167]. The former, when overexpressed, inhibits cell proliferation and enhances cell sensitivity to doxorubicin (a chemotherapy agent) by targeting the oncoprotein ErbB2 [166]. The latter directly inhibits FSCN1, which is a malignancy-promoting protein associated with CS progression [167]. Lastly, additional miRNAs whose dysregulation has been found to be involved in CS onset/development include hub-miRNAs, such as miR-622, -4539, -145, -25 and -96, which are dysregulated in CS tissues/cells [168].
ES pathogenesis is linked to miRNAs dysregulation. The EWSR1/FLI1 fusion protein, which is the main oncogenic factor in ES, negatively regulates miR-193bexpression. This miRNA also targets the oncoprotein ErbB4 to inhibit anchorage-independent growth [169]. In addition, a set of additional 91 miRNAs, including miR-145, -205, -190 and -35a-5p fall under EWSR1/FLI1 regulation [170]. Among these, miR-35a-5p controls cell proliferation/invasiveness in ES cells by targeting CD99, which is an ES-related oncoprotein[170]. Other miRNAs have been identified as dysregulated in ES. A group of 58 miRNAs have been identified as dysregulated in ES [171]. Among these, miR-21, -181a/b, -29a/b, -497, -195, -let-7a, -34a and -1915 share BCL-2 as the same target [171]. Another study has reported the altered expression of miR-21, - 31, -106b, -145, -150, 371-5p, -557 and -598 in ES animal models. These miRNAs have been predicted to regulate a number of ES-related genes, including IGF1, EWSR1, FLI1 and their fusion gene [172]. Several miRNAs which have been identified as tumour suppressors in ES include miR-30d, -107, -124, -138 and -199b-5p [173]. Moreover, miR-138 plays a role as a tumour suppressor in osteosarcoma by targeting DEC2 [174]. In addition, miRNAs belonging to the miR-17~92a, -106b~25 and -106a~363clusters are dysregulated in ES [175]. Among these, the miR-17~92 cluster controls TGFB/BMP pathways, whose down-regulations are crucial for ES pathogenesis [175]. Previous works have suggested a potentially regulatory role for miR-21 in ES pathogenesis by targeting Activated Leukocyte Cell Adhesion Molecule (ALCAM/CD166), whose expression is characteristic of tumourigenicity [176]. Another piece of work has identified miR-199a-3p as a key driver which is able to reverse ES malignancy by inhibiting cell migration through the abrogation of CD99, which is a membrane protein expressed in ES [177]. MiR-301 has also been reported to acts as an onco-miR in ES cells, promoting cell cycle progression and proliferation by PTEN inhibition [178]. Lastly, miRNAs also play a role in ES metastasis. MiR-124over-expression leads to ES cell invasion in vitro and metastasis in vivo [179], whereas miR-138 inhibits the ES metastatic potential by Focal adhesion kinase (FAK) suppression [180].
The abovementioned studies suggest that a number of miRNAs are dysregulated in OS, CS and ES and thus representing potentially promising tools for the diagnosis, prognosis and treatment of these bone tumours. MiRNAs could also be employed as novel therapeutic targets for bone tumour therapy.
MiRNA-based therapies and delivery systems
MiRNA-based therapies could be applied for both cancer treatments and regenerative medicine. The most challenging issue in the application of miRNA-based therapies is the development of safe and efficient delivery systems. To this purpose, the use of miRNA-based scaffolds could allow spatio-temporal control and avoid biological and mechanical barriers that inhibit successful miRNA delivery [181]. In regenerative medicine, scaffolds act principally as templates for cell attachment and tissue repair. To date, a variety of scaffolds (e.g., collagen scaffolds, solid porous scaffolds and hydrogel scaffolds) have been developed to locally deliver miRNAs to cells at the site of damage [182]. Therefore, the use of scaffold-based miRNAs to deliver therapeutics could be a better method to locally release miRNAs while prolonging the delivery time-frames [183]. MiRNA-based therapy applies both cell-free and cell-mediated scaffolds, also termed in situ or ex situ delivery, respectively. In cell-free scaffolds, miRNAs are directly loaded into the scaffold structure which are implanted in the defect site where miRNAs then interact with the resident and homing cells to regenerate such defects [181]. Contrariwise, in cell-mediated scaffolds, cells are transfected with miRNAs in vitro, loaded onto scaffolds, and finally implanted into the damage site [181]. Ultimately, a series of issues still need to be resolved before miRNA scaffold-based therapies can be used, including developing a more efficient scaffold-vector delivery system, improving biological stability, increasing specific targeting capacity, and prolonging presentation in addition to reducing off-target effects [182]. Several reports for scaffold-based miRNA delivery fields have focused on the application of miRNA 26a in inducing bone regeneration both in cell-mediated and cell-free scaffolding [184-187]. While, in the cartilage regeneration field, the most interesting study describing scaffold-based miRNA delivery has reported on a new strategy based on hydrogel-mediated delivery of an antimiR-221 to induce cartilage repair [188].
Conclusion
MiRNAs play a pivotal role in cellular function, including MSC survivability and differentiation. Several studies have been performed to better characterise miRNAs and their unique regulatory functions. This review has highlighted that these molecules can exert different effects on chondrogenic and osteogenic differentiation. As discussed above, some miRNAs can exhibit contrasting effects on chondrogenesis and osteogenesis while others can exert both stimulatory and inhibitory effects. MiRNAs are also involved in the pathogenesis of diseases related to cartilage and bone tissue. Indeed, some miRNAs, such as miR-21, have been found to be dysregulated in OP, ES and OS patients. Serum miRNA, miR-140-3p, alongside miR-23b-3p, could be considered potential biomarkers for OP and OP fractures in post-menopausal women. Several studies have demonstrated miR-148a dysregulation in bone fractures, OP and OS patients. Thus, it represents a diagnostic marker for such pathologies. MiR-494, which is related to poor prognosis in this pathology, could be a promising diagnostic marker for CS. Therefore, miRNAs could be employed as novel therapeutic targets for bone and cartilage disease/tumour therapy. The identification/validation of these miRNAs and their targets, in association with further research into new safe, efficient delivery systems for miRNAs or their regulation tools, could encourage the transfer of miRNAs into clinical practice.
Future perspectives
The role of miRNAs as regulators of MSC chondrogenic and osteogenic differentiation and their involvement in cartilage and bone disease has been largely described in this review. The studies cited herein have described the most well-known function of miRNAs, which is to negatively regulate gene expression by interfering with mRNA. One strategy to improve the chondrogenic and osteogenic potential of MSCs could be to either expose cells to mimic/antago-miRs that enhance or inhibit the effect of these miRNAs, respectively. MiRNA-based therapy applies both cell-free and cell-mediated scaffolds, which is also termed in situ or ex situ delivery, respectively. In cell-free scaffolds, miRNAs are directly loaded into the scaffold structure and are implanted into the defective site where miRNAs then interact with the resident and homing cells to regenerate damaged tissue. Contrariwise, in cell-mediated scaffolds, cells are transfected with miRNAs in vitro, loaded onto scaffolds, and finally implanted into the damaged site. In vivo, interaction between transplanted MSCs, neighboring cells, and humoral factors secreted from the neighboring cells is believed to influence the proliferation and differentiation of MSCs via miRNAs. In this context, human gingival fibroblasts (HGF), a type of periodontal cell, are believed to influence osteogenesis in hMSCs. Indeed, HGF secrete Insulin-like growth factor (IGF)-1 which regulates miRNA expression. Specifically, miR-101-3p and the transcription factor Ets variant 1 (ETV1) are key factors which are regulated by IGF-1 [189]. In the same way, it has been reported that humoral factors from human periodontal ligament cells (HPL cells) co-cultured with hMSCs suppress osteogenesis in hMSCs. The suppressive effect in these cells appears to be mediated by miR-299-5p and SOX11 [190]. However, further studies are needed to elucidate transplanted MSC site-specific differentiation.
Recently, an unconventional aspect of miRNA is emerging within the framework of complex regulatory pathogenesis networks. Specifically, some evidence highlights the role of miRNAs in controlling the expression of other miRNAs in a mechanism known as miRNA-miRNA interaction. MiRNA regulation by another miRNA mainly depends on nucleotide complementary sequencing. Interaction may occur between miRNA and pri-miRNA or mature miRNA, or indirectly by targeting TFs or miRNA regulators. MiRNA-miRNA crosstalk has been reported as being involved in the onset of different diseases, such as cardiac pathologies, ovarian cancer and head and neck squamous cell carcinoma (HNSCC). MiR-21 has been found to be up-regulated in many solid human malignancies, whereas it participates in several miRNA-miRNA interactions. Specifically, miR-21 interacts with miR-499 and miR-145 leading to HNSCC invasion and colorectal cancer, respectively [191], while interaction between miR-21 and miR-122 induces hepatocellular carcinoma [192]. These discoveries, in combination with those reported in this review, indicate that crosstalk miRNA-miRNA deserves to be explored in order to understand the complex hidden networks that cause cartilage and bone diseases. Another miRNAs mechanism of function, which deserves to be looked into with future research, is the crosstalk between miRNAs and other non-coding RNA molecules such as long non-coding RNAs (lncRNAs). It has been reported that interplay between lncRNAs and miRNAs is involved in MSCs osteogenic differentiation [193]. Consequently, new studies into crosstalk between lncRNAs-miRNAs in cartilage and bone diseases would be relevant.
Although much work still needs to be done, the development of efficient miRNA-based therapies would appear to offer significant promise, particularly in personalized medicine.
Acknowledgements
We thank Professor Georgia Emma Gili for revising the English text of our manuscript.
Funding
The works of the authors were supported, in part, by Regione Emilia-Romagna FESR POR project NIPROGEN and Ministero della Università e Ricerca project PRIN 2017.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mazzoni E, D'Agostino A, Iaquinta MR. et al. Hydroxylapatite-collagen hybrid scaffold induces human adipose-derived mesenchymal stem cells to osteogenic differentiation in vitro and bone regrowth in patients. Stem Cells Transl Med. 2020;9:377-88
2. Manfrini M, Di Bona C, Canella A. et al. Mesenchymal stem cells from patients to assay bone graft substitutes. J Cell Physiol. 2013;228:1229-37
3. Solchaga LA, Penick KJ, Welter JF. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol Biol Clifton NJ. 2011;698:253-78
4. Almalki SG, Agrawal DK. Key Transcription Factors in the Differentiation of Mesenchymal Stem Cells. Differ Res Biol Divers. 2016;92:41-51
5. Iaquinta MR, Mazzoni E, Bononi I. et al. Adult Stem Cells for Bone Regeneration and Repair. Front Cell Dev Biol. 2019;7:268
6. Li S, Tallia F, Mohammed AA, Stevens MM, Jones JR. Scaffold channel size influences stem cell differentiation pathway in 3-D printed silica hybrid scaffolds for cartilage regeneration. Biomater Sci. 2020;8:4458-66
7. Mazzoni E, D'Agostino A, Iaquinta MR. et al. Hydroxylapatite-collagen hybrid scaffold induces human adipose-derived mesenchymal stem cells to osteogenic differentiation in vitro and bone regrowth in patients. Stem Cells Transl Med. 2019;9:377-88
8. Mazzoni E, D'Agostino A, Manfrini M. et al. Human adipose stem cells induced to osteogenic differentiation by an innovative collagen/hydroxylapatite hybrid scaffold. FASEB J Off Publ Fed Am Soc Exp Biol. 2017;31:4555-65
9. Montesi M, Panseri S, Dapporto M, Tampieri A, Sprio S. Sr-substituted bone cements direct mesenchymal stem cells, osteoblasts and osteoclasts fate. PloS One. 2017;12:e0172100
10. Gan D, Liu M, Xu T, Wang K, Tan H, Lu X. Chitosan/biphasic calcium phosphate scaffolds functionalized with BMP-2-encapsulated nanoparticles and RGD for bone regeneration. J Biomed Mater Res A. 2018;106:2613-24
11. Balagangadharan K, Viji Chandran S, Arumugam B, Saravanan S, Devanand Venkatasubbu G, Selvamurugan N. Chitosan/nano-hydroxyapatite/nano-zirconium dioxide scaffolds with miR-590-5p for bone regeneration. Int J Biol Macromol. 2018;111:953-8
12. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843-54
13. Macfarlane L-A, Murphy PR. MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics. 2010;11:537-61
14. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-97
15. Huang S-W, Ali N, Zhong L, Shi J. MicroRNAs as biomarkers for human glioblastoma: progress and potential. Acta Pharmacol Sin. 2018;39:1405-13
16. Filipów S, Łaczmański Ł. Blood Circulating miRNAs as Cancer Biomarkers for Diagnosis and Surgical Treatment Response. Front Genet. 2019;10:169
17. Takahashi R-U, Prieto-Vila M, Kohama I, Ochiya T. Development of miRNA-based therapeutic approaches for cancer patients. Cancer Sci. 2019;110:1140-7
18. Arriaga MA, Ding M, Gutierrez AS, Chew SA. The Application of microRNAs in Biomaterial Scaffold-Based Therapies for Bone Tissue Engineering. Biotechnol J. 2019;14:1900084
19. Iaquinta MR, Mazzoni E, Manfrini M. et al. Innovative Biomaterials for Bone Regrowth. Int J Mol Sci. 2019 20
20. Shen M, Kawamoto T, Yan W. et al. Molecular characterization of the novel basic helix-loop-helix protein DEC1 expressed in differentiated human embryo chondrocytes. Biochem Biophys Res Commun. 1997;236:294-8
21. Sasamoto T, Fujimoto K, Kanawa M. et al. DEC2 is a negative regulator for the proliferation and differentiation of chondrocyte lineage-committed mesenchymal stem cells. Int J Mol Med. 2016;38:876-84
22. Matsubara T, Kida K, Yamaguchi A. et al. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem. 2008;283:29119-25
23. Goodnough LH, Chang AT, Treloar C, Yang J, Scacheri PC, Atit RP. Twist1 mediates repression of chondrogenesis by β-catenin to promote cranial bone progenitor specification. Dev Camb Engl. 2012;139:4428-38
24. Yang J, Qin S, Yi C. et al. MiR-140 is co-expressed with Wwp2-C transcript and activated by Sox9 to target Sp1 in maintaining the chondrocyte proliferation. FEBS Lett. 2011;585:2992-7
25. Budd E, Waddell S, de Andrés MC, Oreffo ROC. The Potential of microRNAs for Stem Cell-based Therapy for Degenerative Skeletal Diseases. Curr Mol Biol Rep. 2017;3:263-75
26. Lu J, Zhou Z, Sun B. et al. MiR-520d-5p modulates chondrogenesis and chondrocyte metabolism through targeting HDAC1. Aging. 2020;12:18545-60
27. Guérit D, Philipot D, Chuchana P. et al. Sox9-regulated miRNA-574-3p inhibits chondrogenic differentiation of mesenchymal stem cells. PloS One. 2013;8:e62582
28. Xue Z, Meng Y, Ge J. miR-127-5p promotes chondrogenic differentiation in rat bone marrow mesenchymal stem cells. Exp Ther Med. 2017;14:1481-6
29. Papaioannou G, Kozlova A, Kobayashi T. miRNA Regulation of Chondrogenesis. Curr Mol Biol Rep. 2018;4:208-17
30. Zhang H, Wang Y, Yang G, Yu H, Zhou Z, Tang M. MicroRNA-30a regulates chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells through targeting Sox9. Exp Ther Med. 2019;18:4689-97
31. Wa Q, He P, Huang S. et al. miR-30b regulates chondrogenic differentiation of mouse embryo-derived stem cells by targeting SOX9. Exp Ther Med. 2017;14:6131-7
32. Penolazzi L, Lambertini E, Bergamin LS. et al. MicroRNA-221 silencing attenuates the degenerated phenotype of intervertebral disc cells. Aging. 2018;10:2001-15
33. Bai M, Yin H, Zhao J, Li Y, Wu Y. miR-182-5p overexpression inhibits chondrogenesis by down-regulating PTHLH. Cell Biol Int. 2019;43:222-32
34. Dai L, Zhang X, Hu X, Zhou C, Ao Y. Silencing of microRNA-101 prevents IL-1β-induced extracellular matrix degradation in chondrocytes. Arthritis Res Ther. 2012;14:R268
35. Martinez-Sanchez A, Murphy CL. miR-1247 functions by targeting cartilage transcription factor SOX9. J Biol Chem. 2013;288:30802-14
36. Yang B, Guo H, Zhang Y, Chen L, Ying D, Dong S. MicroRNA-145 Regulates Chondrogenic Differentiation of Mesenchymal Stem Cells by Targeting Sox9. Linden R, Ed. PLoS ONE. 2011;6:e21679
37. Allas L, Boumédiene K, Baugé C. Epigenetic dynamic during endochondral ossification and articular cartilage development. Bone. 2019;120:523-32
38. Wu C, Tian B, Qu X. et al. MicroRNAs play a role in chondrogenesis and osteoarthritis (Review). Int J Mol Med. 2014;34:13-23
39. Hou C, Yang Z, Kang Y. et al. MiR-193b regulates early chondrogenesis by inhibiting the TGF-beta2 signaling pathway. FEBS Lett. 2015;589:1040-7
40. Wu T, Zhou H, Hong Y, Li J, Jiang X, Huang H. miR-30 family members negatively regulate osteoblast differentiation. J Biol Chem. 2012;287:7503-11
41. Li YP, Wei XC, Li PC. et al. The Role of miRNAs in Cartilage Homeostasis. Curr Genomics. 2015;16:393-404
42. Li H, Cui Q, Dong Z, Zhang J, Li H, Zhao L. Downregulation of miR-27b is Involved in Loss of Type II Collagen by Directly Targeting Matrix Metalloproteinase 13 (MMP13) in Human Intervertebral Disc Degeneration. Spine. 2016;41:E116-123
43. Hou C, Meng F, Zhang Z. et al. The Role of MicroRNA-381 in Chondrogenesis and Interleukin-1-β Induced Chondrocyte Responses. Cell Physiol Biochem. 2015;36:1753-66
44. Stelcer E, Kulcenty K, Rucinski M. et al. The Role of MicroRNAs in Early Chondrogenesis of Human Induced Pluripotent Stem Cells (hiPSCs). Int J Mol Sci. 2019;20:4371
45. Suárez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104:442-54
46. Tian F, Wang J, Zhang Z, Yang J. miR-107 modulates chondrocyte proliferation, apoptosis, and extracellular matrix synthesis by targeting PTEN. Int J Clin Exp Pathol. 2019;12:488-97
47. Ukai T, Sato M, Akutsu H, Umezawa A, Mochida J. MicroRNA-199a-3p, microRNA-193b, and microRNA-320c are correlated to aging and regulate human cartilage metabolism: MiRNA-199a-3p,-193b,-320c REGULATE CARTILAGE METABOLISM. J Orthop Res. 2012;30:1915-22
48. Hua Z, Lv Q, Ye W. et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PloS One. 2006;1:e116
49. Ko J-Y, Lee MS, Lian W-S. et al. MicroRNA-29a Counteracts Synovitis in Knee Osteoarthritis Pathogenesis by Targeting VEGF. Sci Rep. 2017;7:3584
50. Xu R, Wei Y, Yin X, Shi B, Li J. Author Correction: miR-20a suppresses chondrogenic differentiation of ATDC5 cells by regulating Atg7. Sci Rep. 2020;10:1179
51. Zhang Y, Xie R -l, Croce CM. et al. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci. 2011;108:9863-8
52. Seenprachawong K, Nuchnoi P, Nantasenamat C, Prachayasittikul V, Supokawej A. Computational identification of miRNAs that modulate the differentiation of mesenchymal stem cells to osteoblasts. PeerJ. 2016;4:e1976
53. Yi J, Liu D, Xiao J. LncRNA MALAT1 sponges miR-30 to promote osteoblast differentiation of adipose-derived mesenchymal stem cells by promotion of Runx2 expression. Cell Tissue Res. 2019;376:113-21
54. Li Z, Hassan MQ, Volinia S. et al. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci. 2008;105:13906-11
55. Zeng H-C, Bae Y, Dawson BC. et al. MicroRNA miR-23a cluster promotes osteocyte differentiation by regulating TGF-β signalling in osteoblasts. Nat Commun. 2017;8:15000
56. Zhang W, Wu Y, Shiozaki Y. et al. miRNA-133a-5p Inhibits the Expression of Osteoblast Differentiation-Associated Markers by Targeting the 3′ UTR of RUNX2. DNA Cell Biol. 2018;37:199-209
57. Shi X, Zhang Z. MicroRNA-135a-5p is involved in osteoporosis progression through regulation of osteogenic differentiation by targeting RUNX2. Exp Ther Med. 2019;18:2393-400
58. Kong L, Zuo R, Wang M. et al. Silencing MicroRNA-137-3p, which Targets RUNX2 and CXCL12 Prevents Steroid-induced Osteonecrosis of the Femoral Head by Facilitating Osteogenesis and Angiogenesis. Int J Biol Sci. 2020;16:655-70
59. Liu H, Chen B, Li Y. microRNA-203 promotes proliferation, differentiation, and migration of osteoblasts by upregulation of Msh homeobox 2. J Cell Physiol. 2019;234:17639-48
60. Cui Y, Zeng F, Zhu Z. et al. Suppression of osteogenic-like differentiation in human renal interstitial fibroblasts by miRNA-410-3p through MSX2. Transl Androl Urol. 2020;9:2082-93
61. Huang J, Zhao L, Xing L, Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357-64
62. Hu N, Feng C, Jiang Y, Miao Q, Liu H. Regulative Effect of Mir-205 on Osteogenic Differentiation of Bone Mesenchymal Stem Cells (BMSCs): Possible Role of SATB2/Runx2 and ERK/MAPK Pathway. Int J Mol Sci. 2015;16:10491-506
63. Liu H, Sun Q, Wan C, Li L, Zhang L, Chen Z. MicroRNA-338-3p Regulates Osteogenic Differentiation of Mouse Bone Marrow Stromal Stem Cells by Targeting Runx2 and Fgfr2: MicroRNA-338-3p REGULATES OSTEOGENIC DIFFERENTIATION. J Cell Physiol. 2014;229:1494-502
64. Gay I, Cavender A, Peto D. et al. Differentiation of human dental stem cells reveals a role for microRNA-218. J Periodontal Res. 2014;49:110-20
65. Yan J, Guo D, Yang S, Sun H, Wu B, Zhou D. Inhibition of miR-222-3p activity promoted osteogenic differentiation of hBMSCs by regulating Smad5-RUNX2 signal axis. Biochem Biophys Res Commun. 2016;470:498-503
66. Du F, Wu H, Zhou Z, Liu Y. microRNA-375 inhibits osteogenic differentiation by targeting runt-related transcription factor 2. Exp Ther Med. 2015;10:207-12
67. Chen S, Zheng Y, Zhang S, Jia L, Zhou Y. Promotion Effects of miR-375 on the Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Stem Cell Rep. 2017;8:773-86
68. Kim E-J, Kang I-H, Lee JW, Jang W-G, Koh J-T. MiR-433 mediates ERRγ-suppressed osteoblast differentiation via direct targeting to Runx2 mRNA in C3H10T1/2 cells. Life Sci. 2013;92:562-8
69. Smith SS, Dole NS, Franceschetti T, Hrdlicka HC, Delany AM. MicroRNA-433 Dampens Glucocorticoid Receptor Signaling, Impacting Circadian Rhythm and Osteoblastic Gene Expression. J Biol Chem. 2016;291:21717-28
70. Li W, Chen Z, Cai C, Li G, Wang X, Shi Z. MicroRNA-505 is involved in the regulation of osteogenic differentiation of MC3T3-E1 cells partially by targeting RUNX2. J Orthop Surg. 2020;15:143
71. Chen H, Ji X, She F, Gao Y, Tang P. miR-628-3p regulates osteoblast differentiation by targeting RUNX2: Possible role in atrophic non-union. Int J Mol Med. 2017;39:279-86
72. Chen Z, Liu H-L. Restoration of miR-1305 relieves the inhibitory effect of nicotine on periodontal ligament-derived stem cell proliferation, migration, and osteogenic differentiation. J Oral Pathol Med. 2017;46:313-20
73. Yang D-C, Yang M-H, Tsai C-C, Huang T-F, Chen Y-H, Hung S-C. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PloS One. 2011;6:e23965
74. Khanbabaei H, Teimoori A, Mohammadi M. The interplay between microRNAs and Twist1 transcription factor: a systematic review. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2016;37:7007-19
75. Camp E, Pribadi C, Anderson PJ, Zannettino ACW, Gronthos S. miRNA-376c-3p Mediates TWIST-1 Inhibition of Bone Marrow-Derived Stromal Cell Osteogenesis and Can Reduce Aberrant Bone Formation of TWIST-1 Haploinsufficient Calvarial Cells. Stem Cells Dev. 2018;27:1621-33
76. Yang L, Cheng P, Chen C. et al. miR-93/Sp7 function loop mediates osteoblast mineralization. J Bone Miner Res. 2012;27:1598-606
77. Zhang Y, Wei Q-S, Ding W-B. et al. Increased microRNA-93-5p inhibits osteogenic differentiation by targeting bone morphogenetic protein-2. Kim J-E, Ed. PLOS ONE. 2017;12:e0182678
78. Jia J, Tian Q, Ling S, Liu Y, Yang S, Shao Z. miR-145 suppresses osteogenic differentiation by targeting Sp7. FEBS Lett. 2013;587:3027-31
79. Fukuda T, Ochi H, Sunamura S. et al. MicroRNA-145 regulates osteoblastic differentiation by targeting the transcription factor Cbfb. FEBS Lett. 2015;589:3302-8
80. Liu M, Sun W, Liu Y, Dong X. The role of lncRNA MALAT1 in bone metastasis in patients with non-small cell lung cancer. Oncol Rep. 2016;36:1679-85
81. Xue Z-L, Meng Y-L, Ge J-H. Upregulation of miR-132 attenuates osteoblast differentiation of UC-MSCs. Eur Rev Med Pharmacol Sci. 2018;22:1580-7
82. Shi K, Lu J, Zhao Y. et al. MicroRNA-214 suppresses osteogenic differentiation of C2C12 myoblast cells by targeting Osterix. Bone. 2013;55:487-94
83. Yuan Y, Guo J, Zhang L. et al. MiR-214 Attenuates the Osteogenic Effects of Mechanical Loading on Osteoblasts. Int J Sports Med. 2019;40:931-40
84. Zhang J, Fu W, He M. et al. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Bronner-Fraser M, Ed. Mol Biol Cell. 2011;22:3955-61
85. Moghaddam T, Neshati Z. Role of microRNAs in osteogenesis of stem cells. J Cell Biochem. 2019;120:14136-55
86. Hwang S, Park S-K, Lee HY. et al. miR-140-5p suppresses BMP2-mediated osteogenesis in undifferentiated human mesenchymal stem cells. FEBS Lett. 2014;588:2957-63
87. Zhang G-P, Zhang J, Zhu C-H. et al. MicroRNA-98 regulates osteogenic differentiation of human bone mesenchymal stromal cells by targeting BMP2. J Cell Mol Med. 2017;21:254-64
88. Cui Z, Wang X-N, Lu Y. et al. miR-140 inhibits osteogenic differentiation of human periodontal ligament fibroblasts through ras homolog gene family, member A -transcriptional co-activator with PDZ-binding motif pathway. Kaohsiung J Med Sci. 2021;37:38-46
89. Jia B, Qiu X, Chen J. et al. A feed-forward regulatory network lncPCAT1/miR-106a-5p/E2F5 regulates the osteogenic differentiation of periodontal ligament stem cells. J Cell Physiol. 2019;234:19523-38
90. Itoh T, Ando M, Tsukamasa Y, Akao Y. Expression of BMP-2 and Ets1 in BMP-2-stimulated mouse pre-osteoblast differentiation is regulated by microRNA-370. FEBS Lett. 2012;586:1693-701
91. Yu L, Cen X, Xia K. et al. microRNA expression profiles and the potential competing endogenous RNA networks in NELL-1-induced human adipose-derived stem cell osteogenic differentiation. J Cell Biochem. 2020 jcb.29695
92. Chang M, Lin H, Fu H, Wang B, Han G, Fan M. MicroRNA-195-5p Regulates Osteogenic Differentiation of Periodontal Ligament Cells Under Mechanical Loading. J Cell Physiol. 2017;232:3762-74
93. Zeng Y, Qu X, Li H. et al. MicroRNA-100 regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells by targeting BMPR2. FEBS Lett. 2012;586:2375-81
94. Cao Y, Lv Q, Lv C. MicroRNA-153 suppresses the osteogenic differentiation of human mesenchymal stem cells by targeting bone morphogenetic protein receptor type II. Int J Mol Med. 2015;36:760-6
95. Huang C, Geng J, Wei X, Zhang R, Jiang S. MiR-144-3p regulates osteogenic differentiation and proliferation of murine mesenchymal stem cells by specifically targeting Smad4. FEBS Lett. 2016;590:795-807
96. Xie Q, Wei W, Ruan J. et al. Effects of miR-146a on the osteogenesis of adipose-derived mesenchymal stem cells and bone regeneration. Sci Rep. 2017;7:42840
97. Tang Y, Zheng L, Zhou J. et al. miR-203-3p participates in the suppression of diabetes-associated osteogenesis in the jaw bone through targeting Smad1. Int J Mol Med. 2018;41:1595-607
98. Trompeter H-I, Dreesen J, Hermann E. et al. MicroRNAs miR-26a, miR-26b, and miR-29b accelerate osteogenic differentiation of unrestricted somatic stem cells from human cord blood. BMC Genomics. 2013;14:111
99. Su X, Liao L, Shuai Y. et al. MiR-26a functions oppositely in osteogenic differentiation of BMSCs and ADSCs depending on distinct activation and roles of Wnt and BMP signaling pathway. Cell Death Dis. 2015;6:e1851-e1851
100. Fakhry M. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells. 2013;5:136
101. Liu J, Wang X, Song M. et al. MiR-497-5p Regulates Osteo/Odontogenic Differentiation of Stem Cells From Apical Papilla via the Smad Signaling Pathway by Targeting Smurf2. Front Genet. 2020;11:582366
102. Li X, Guo L, Liu Y. et al. MicroRNA-21 promotes osteogenesis of bone marrow mesenchymal stem cells via the Smad7-Smad1/5/8-Runx2 pathway. Biochem Biophys Res Commun. 2017;493:928-33
103. Sanjeev G, Sidharthan DS, Pranavkrishna S. et al. An osteoinductive effect of phytol on mouse mesenchymal stem cells (C3H10T1/2) towards osteoblasts. Bioorg Med Chem Lett. 2020;30:127137
104. Selvamurugan N, He Z, Rifkin D, Dabovic B, Partridge NC. Pulsed Electromagnetic Field Regulates MicroRNA 21 Expression to Activate TGF-β Signaling in Human Bone Marrow Stromal Cells to Enhance Osteoblast Differentiation. Stem Cells Int. 2017;2017:2450327
105. Jia J, Feng X, Xu W. et al. MiR-17-5p modulates osteoblastic differentiation and cell proliferation by targeting SMAD7 in non-traumatic osteonecrosis. Exp Mol Med. 2014;46:e107-e107
106. Vishal M, Vimalraj S, Ajeetha R. et al. MicroRNA-590-5p Stabilizes Runx2 by Targeting Smad7 During Osteoblast Differentiation: MICRORNA-590-5P STABILIZES RUNX2 BY TARGETING SMAD7. J Cell Physiol. 2017;232:371-80
107. Ko J-Y, Chuang P-C, Ke H-J, Chen Y-S, Sun Y-C, Wang F-S. MicroRNA-29a mitigates glucocorticoid induction of bone loss and fatty marrow by rescuing Runx2 acetylation. Bone. 2015;81:80-8
108. Li Z, Hassan MQ, Jafferji M. et al. Correction: Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem. 2019;294:10018-10018
109. Liu Q, Lin Z, Liu Y, Du J, Lin H, Wang J. Delivery of miRNA-29b Using R9-LK15, a Novel Cell-Penetrating Peptide, Promotes Osteogenic Differentiation of Bone Mesenchymal Stem Cells. BioMed Res Int. 2019;2019:1-12
110. Aslani S, Abhari A, Sakhinia E, Sanajou D, Rajabi H, Rahimzadeh S. Interplay between microRNAs and Wnt, transforming growth factor-β, and bone morphogenic protein signaling pathways promote osteoblastic differentiation of mesenchymal stem cells. J Cell Physiol. 2019;234:8082-93
111. Sun H, Wu Y, Fu D, Liu Y, Huang C. SIRT6 regulates osteogenic differentiation of rat bone marrow mesenchymal stem cells partially via suppressing the nuclear factor-κB signaling pathway. Stem Cells Dayt Ohio. 2014;32:1943-55
112. Yang S, Guo S, Tong S, Sun X. Exosomal miR-130a-3p regulates osteogenic differentiation of Human Adipose-Derived stem cells through mediating SIRT7/Wnt/β-catenin axis. Cell Prolif. 2020;53:e12890
113. Feng L, Zhang J-F, Shi L. et al. MicroRNA-378 Suppressed Osteogenesis of MSCs and Impaired Bone Formation via Inactivating Wnt/β-Catenin Signaling. Mol Ther Nucleic Acids. 2020;21:1017-28
114. Chen B, Yang W, Zhao H. et al. Abnormal expression of miR-135b-5p in bone tissue of patients with osteoporosis and its role and mechanism in osteoporosis progression. Exp Ther Med. 2020;19:1042-50
115. Cheung WH, Miclau T, Chow SK-H, Yang FF, Alt V. Fracture healing in osteoporotic bone. Injury. 2016;47(Suppl 2):S21-26
116. Li QS, Meng FY, Zhao YH, Jin CL, Tian J, Yi XJ. Inhibition of microRNA-214-5p promotes cell survival and extracellular matrix formation by targeting collagen type IV alpha 1 in osteoblastic MC3T3-E1 cells. Bone Jt Res. 2017;6:464-71
117. Wang C, Zheng G-F, Xu X-F. MicroRNA-186 improves fracture healing through activating the bone morphogenetic protein signalling pathway by inhibiting SMAD6 in a mouse model of femoral fracture: An animal study. Bone Jt Res. 2019;8:550-62
118. Lee WY, Li N, Lin S, Wang B, Lan HY, Li G. miRNA-29b improves bone healing in mouse fracture model. Mol Cell Endocrinol. 2016;430:97-107
119. Murata K, Ito H, Yoshitomi H. et al. Inhibition of miR-92a enhances fracture healing via promoting angiogenesis in a model of stabilized fracture in young mice. J Bone Miner Res Off J Am Soc Bone Miner Res. 2014;29:316-26
120. Shi L, Feng L, Liu Y. et al. MicroRNA-218 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells and Accelerates Bone Fracture Healing. Calcif Tissue Int. 2018;103:227-36
121. Bottani M, Banfi G, Lombardi G. The Clinical Potential of Circulating miRNAs as Biomarkers: Present and Future Applications for Diagnosis and Prognosis of Age-Associated Bone Diseases. Biomolecules. 2020;10:589
122. Sözen T, Özışık L, Başaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46-56
123. Hackl M, Heilmeier U, Weilner S, Grillari J. Circulating microRNAs as novel biomarkers for bone diseases - Complex signatures for multifactorial diseases? Mol Cell Endocrinol. 2016;432:83-95
124. Faraldi M, Gomarasca M, Banfi G, Lombardi G. Free Circulating miRNAs Measurement in Clinical Settings: The Still Unsolved Issue of the Normalization. Adv Clin Chem. 2018;87:113-39
125. Faraldi M, Sansoni V, Perego S. et al. Study of the preanalytical variables affecting the measurement of clinically relevant free-circulating microRNAs: focus on sample matrix, platelet depletion, and storage conditions. Biochem Medica. 2020;30:010703
126. Ladang A, Beaudart C, Locquet M, Reginster J-Y, Bruyère O, Cavalier E. Evaluation of a Panel of MicroRNAs that Predicts Fragility Fracture Risk: A Pilot Study. Calcif Tissue Int. 2020;106:239-47
127. Li H, Wang Z, Fu Q, Zhang J. Plasma miRNA levels correlate with sensitivity to bone mineral density in postmenopausal osteoporosis patients. Biomarkers. 2014;19:553-6
128. Li Z, Zhang W, Huang Y. MiRNA-133a is involved in the regulation of postmenopausal osteoporosis through promoting osteoclast differentiation. Acta Biochim Biophys Sin. 2018;50:273-80
129. Seeliger C, Karpinski K, Haug AT. et al. Five Freely Circulating miRNAs and Bone Tissue miRNAs Are Associated With Osteoporotic Fractures: miRNAs ASSOCIATED WITH OSTEOPOROTIC FRACTURES. J Bone Miner Res. 2014;29:1718-28
130. Kelch S, Balmayor ER, Seeliger C, Vester H, Kirschke JS, van Griensven M. miRNAs in bone tissue correlate to bone mineral density and circulating miRNAs are gender independent in osteoporotic patients. Sci Rep. 2017;7:15861
131. Chen J, Li K, Pang Q. et al. Identification of suitable reference gene and biomarkers of serum miRNAs for osteoporosis. Sci Rep. 2016;6:36347
132. Meng J, Zhang D, Pan N. et al. Identification of miR-194-5p as a potential biomarker for postmenopausal osteoporosis. PeerJ. 2015;3:e971
133. Bedene A, Mencej Bedrač S, Ješe L. et al. MiR-148a the epigenetic regulator of bone homeostasis is increased in plasma of osteoporotic postmenopausal women. Wien Klin Wochenschr. 2016;128:519-26
134. Ramírez-Salazar EG, Carrillo-Patiño S, Hidalgo-Bravo A. et al. Serum miRNAs miR-140-3p and miR-23b-3p as potential biomarkers for osteoporosis and osteoporotic fracture in postmenopausal Mexican-Mestizo women. Gene. 2018;679:19-27
135. Li K, Chen S, Cai P. et al. MiRNA-483-5p is involved in the pathogenesis of osteoporosis by promoting osteoclast differentiation. Mol Cell Probes. 2020;49:101479
136. Lin C, Yu S, Jin R. et al. Circulating miR-338 Cluster activities on osteoblast differentiation: Potential Diagnostic and Therapeutic Targets for Postmenopausal Osteoporosis. Theranostics. 2019;9:3780-97
137. Zarecki P, Hackl M, Grillari J, Debono M, Eastell R. Serum microRNAs as novel biomarkers for osteoporotic vertebral fractures. Bone. 2020;130:115105
138. Cortini M, Baldini N, Avnet S. New Advances in the Study of Bone Tumors: A Lesson From the 3D Environment. Front Physiol. 2019;10:814
139. Preti M, Rotondo JC, Holzinger D. et al. Role of human papillomavirus infection in the etiology of vulvar cancer in Italian women. Infect Agent Cancer. 2020;15:e2020
140. Tognon M, Luppi M, Corallini A. et al. Immunologic evidence of a strong association between non-Hodgkin lymphoma and simian virus 40. Cancer. 2015;121:2618-26
141. Mazzoni E, Martini F, Corallini A. et al. Serologic investigation of undifferentiated nasopharyngeal carcinoma and simian virus 40 infection. Head Neck. 2016;38:232-6
142. Rotondo JC, Mazzoni E, Bononi I, Tognon MG, Martini F. Association Between Simian Virus 40 and Human Tumors. Front Oncol. 2019 9
143. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83-103
144. Misso G, Di Martino MT, De Rosa G. et al. Mir-34: A new weapon against cancer? Mol Ther - Nucleic Acids. 2014;3:194
145. Maire G, Martin JW, Yoshimoto M, Chilton-MacNeill S, Zielenska M, Squire JA. Analysis of miRNA-gene expression-genomic profiles reveals complex mechanisms of microRNA deregulation in osteosarcoma. Cancer Genet. 2011;204:138-46
146. H Z, P Y, J W. Clinical significance of tumor miR-21, miR-221, miR-143, and miR-106a as biomarkers in patients with osteosarcoma. Int J Biol Markers. 2019;34:184
147. Nakka M, Allen-Rhoades W, Li Y. et al. Biomarker significance of plasma and tumor miR-21, miR-221, and miR-106a in osteosarcoma. Oncotarget. 2017;8:96738-52
148. Wang J, Liu S, Shi J. The Role of miRNA in the Diagnosis, Prognosis, and Treatment of Osteosarcoma. Cancer Biother Radiopharm. 2019;00:1-9
149. Zhao G, Cai C, Yang T. et al. MicroRNA-221 Induces Cell Survival and Cisplatin Resistance through PI3K/Akt Pathway in Human Osteosarcoma. PLoS ONE. 2013;8:e53906
150. Gong N, Gong M. MiRNA-221 from tissue may predict the prognosis of patients with osteosarcoma. Med U S. 2018;97:e11100
151. Viera GM, Salomao KB, de Sousa GR. et al. miRNA signatures in childhood sarcomas and their clinical implications. Clin Transl Oncol. 2019;21:1583-623
152. Sarver AL, Thayanithy V, Scott MC. et al. MicroRNAs at the human 14q32 locus have prognostic significance in osteosarcoma. Orphanet J Rare Dis. 2013;8:7
153. Tian Q, Jia J, Ling S, Liu Y, Yang S, Shao Z. A causal role for circulating miR-34b in osteosarcoma. Eur J Surg Oncol. 2014;40:67-72
154. Yuan J, Chen L, Chen X, Sun W, Zhou X. Identification of serum microRNA-21 as a biomarker for chemosensitivity and prognosis in human osteosarcoma. J Int Med Res. 2012;40:2090-7
155. Ma W, Zhang X, Chai J, Chen P, Ren P, Gong M. Circulating miR-148a is a significant diagnostic and prognostic biomarker for patients with osteosarcoma. Tumor Biol. 2014;35:12467-72
156. Ouyang L, Liu P, Yang S, Ye S, Xu W, Liu X. A three-plasma miRNA signature serves as novel biomarkers for osteosarcoma. Med Oncol. 2013;30:340
157. Lu N, Lin T, Wang L. et al. Association of SOX4 regulated by tumor suppressor miR-30a with poor prognosis in low-grade chondrosarcoma. Tumor Biol. 2015;36:3843-52
158. Li J, Wang L, Liu Z. et al. MicroRNA-494 inhibits cell proliferation and invasion of chondrosarcoma cells in vivo and in vitro by directly targeting SOX9. Oncotarget. 2015;6:26216-9
159. Pu F, Chen F, Shao Z. MicroRNAs as biomarkers in the diagnosis and treatment of chondrosarcoma. Tumor Biol. 2016;37:15433-6
160. Huang K, Chen J, Yang MS, Tang YJ, Pan F. Inhibition of Src by microRNA-23b increases the cisplatin sensitivity of chondrosarcoma cells. Cancer Biomark. 2017;18:231-9
161. Horng CT, Shieh PC, Tan TW, Yang WH, Tang CH. Paeonol suppresses chondrosarcoma metastasis through up-regulation of miR-141 by modulating PKCδ and c-Src signaling pathway. Int J Mol Sci. 2014;15:11760-72
162. Tan TW, Chou YE, Yang WH, Hsu CJ, Fong YC, Tang CH. Naringin suppress chondrosarcoma migration through inhibition vascular adhesion molecule-1 expression by modulating miR-126. Int Immunopharmacol. 2014;22:107-14
163. Liu GT, Huang YL, Tzeng HE, Tsai CH, Wang SW, Tang CH. CCL5 promotes vascular endothelial growth factor expression and induces angiogenesis by down-regulating miR-199a in human chondrosarcoma cells. Cancer Lett. 2015;20:939-58
164. Sun X, Wei L, Chen Q, Terek RM. MicroRNA Regulates Vascular Endothelial Growth Factor Expression in Chondrosarcoma Cells. Clin Orthop. 2014;473:907-13
165. Liu N, Zhang Z, Li L. et al. MicroRNA-181 regulates the development of Ossification of Posterior longitudinal ligament via Epigenetic Modulation by targeting PBX1. Theranostics. 2020;10:7492-509
166. Tang XY, Zheng W, Ding M. et al. MiR-125b acts as a tumor suppressor in chondrosarcoma cells by the sensitization to doxorubicin through direct targeting the ErbB2-regulated glucose metabolism. Drug Des Devel Ther. 2016;10:571-83
167. Urdinez J, Boro A, Mazumdar A. et al. The miR-143/145 Cluster, a Novel Diagnostic Biomarker in Chondrosarcoma, Acts as a Tumor Suppressor and Directly Inhibits Fascin-1. J Bone Miner Res Off J Am Soc Bone Miner Res. 2020;35:1077-91
168. Sui J, Liu Q, Zhang H, Kong Y. Deep integrative analysis of microRNA-mRNA regulatory networks for biomarker and target discovery in chondrosarcoma. J Cell Biochem. 2019;120:9631-8
169. Moore C, Parrish JK, Jedlicka P. MiR-193b, downregulated in Ewing Sarcoma, targets the ErbB4 oncogene to inhibit anchorage-independent growth. PLoS ONE. 2017;12:e0178028
170. Franzetti GA, Laud-Duval K, Bellanger D, Stern MH, Sastre-Garau X, Delattre O. MiR-30a-5p connects EWS-FLI1 and CD99, two major therapeutic targets in Ewing tumor. Oncogene. 2013;32:3915-21
171. Parafioriti A, Bason C, Armiraglio E. et al. Ewing's sarcoma: An analysis of miRNA expression profiles and target genes in paraffin-embedded primary tumor tissue. Int J Mol Sci. 2016;17:656
172. Mosakhani N, Guled M, Leen G. et al. An integrated analysis of miRNA and gene copy numbers in xenografts of Ewing's sarcoma. J Exp Clin Cancer Res. 2012;31:24
173. Li W, Li Y, Guo J, Pan H, Zhang Y, Wang X. Overexpression of miR-199b-5p inhibits Ewing's sarcoma cell lines by targeting CCNL1. Mol Med Rep. 2015;12:3359-64
174. Jiang B, Mu W, Wang J. et al. MicroRNA-138 functions as a tumor suppressor in osteosarcoma by targeting differentiated embryonic chondrocyte gene 2. J Exp Clin Cancer Res CR. 2016;35:69
175. Dylla L, Moore C, Jedlicka P. MicroRNAs in Ewing sarcoma. Front Oncol. 2013;3:65
176. Liu Y, Chen G, Liu H. et al. Integrated bioinformatics analysis of miRNA expression in Ewing sarcoma and potential regulatory effects of miR-21 via targeting ALCAM/CD166. Artif Cells Nanomedicine Biotechnol. 2019;47:2114-22
177. De Feo A, Sciandra M, Ferracin M. et al. Exosomes from CD99-deprived Ewing sarcoma cells reverse tumor malignancy by inhibiting cell migration and promoting neural differentiation. Cell Death Dis. 2019;17:471
178. Kawano M, Tanaka K, Itonaga I, Iwasaki T, Tsumura H. MicroRNA-301a promotes cell proliferation via PTEN targeting in Ewing's sarcoma cells. Int J Oncol. 2016;48:1531-40
179. Li Y, Shao G, Zhang M. et al. MiR-124 represses the mesenchymal features and suppresses metastasis in Ewing sarcoma. Oncotarget. 2017;8:10274-86
180. Tanaka K, Kawano M, Itonaga I. et al. Tumor suppressive microRNA-138 inhibits metastatic potential via the targeting of focal adhesion kinase in Ewing's sarcoma cells. Int J Oncol. 2016;48:1135-44
181. Leng Q, Chen L, Lv Y. RNA-based scaffolds for bone regeneration: application and mechanisms of mRNA, miRNA and siRNA. Theranostics. 2020;10:3190-205
182. Curtin CM, Castaño IM, O'Brien FJ. Scaffold-Based microRNA Therapies in Regenerative Medicine and Cancer. Adv Healthc Mater. 2018 7
183. Sriram M, Sainitya R, Kalyanaraman V, Dhivya S, Selvamurugan N. Biomaterials mediated microRNA delivery for bone tissue engineering. Int J Biol Macromol. 2015;74:404-12
184. Li Y, Fan L, Liu S. et al. The promotion of bone regeneration through positive regulation of angiogenic-osteogenic coupling using microRNA-26a. Biomaterials. 2013;34:5048-58
185. Zhang X, Li Y, Chen YE, Chen J, Ma PX. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat Commun. 2016;7:10376
186. Yan J, Lu X, Zhu X. et al. Effects of miR-26a on Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by a Mesoporous Silica Nanoparticle - PEI - Peptide System. Int J Nanomedicine. 2020;15:497-511
187. Paquet J, Moya A, Bensidhoum M, Petite H. Engineered cell-free scaffold with two-stage delivery of miRNA-26a for bone repair. Ann Transl Med. 2016;4:204
188. Lolli A, Sivasubramaniyan K, Vainieri ML. et al. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J Control Release Off J Control Release Soc. 2019;309:220-30
189. Kaneda-Ikeda E, Iwata T, Mizuno N. et al. Regulation of osteogenesis via miR-101-3p in mesenchymal stem cells by human gingival fibroblasts. J Bone Miner Metab. 2020;38:442-55
190. Kaneda-Ikeda E, Iwata T, Mizuno N. et al. Periodontal ligament cells regulate osteogenesis via miR-299-5p in mesenchymal stem cells. Differ Res Biol Divers. 2020;112:47-57
191. Hill M, Tran N. Global miRNA to miRNA Interactions: Impacts for miR-21. Trends Cell Biol. 2021;31:3-5
192. Hill M, Tran N. MicroRNAs Regulating MicroRNAs in Cancer. Trends Cancer. 2018;4:465-8
193. Lanzillotti C, De Mattei M, Mazziotta C. et al. Long non-coding RNAs and microRNAs interplay in osteogenic differentiation of mesenchymal stem cells. Front Cell Dev Biol. 2021
Author contact
![]() Corresponding author: Prof. Fernanda Martini, E-mail: mrfit.
Corresponding author: Prof. Fernanda Martini, E-mail: mrfit.
 Global reach, higher impact
Global reach, higher impact