13.3
Impact Factor
Theranostics 2021; 11(13):6293-6314. doi:10.7150/thno.57177 This issue Cite
Review
Perspectives on metals-based radioimmunotherapy (RIT): moving forward
1. Cancer Biology Graduate Program, Wayne State University School of Medicine, Detroit, MI 48201.
2. Department of Oncology, Karmanos Cancer Institute, Detroit, MI 48201.
3. Molecular Imaging Branch, Radiation Oncology Branch, National Cancer Institute, Bethesda, MD 20814.
Abstract
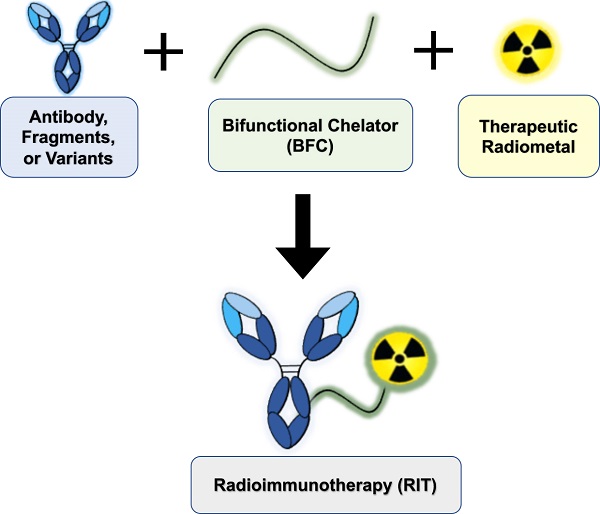
Radioimmunotherapy (RIT) is FDA-approved for the clinical management of liquid malignancies, however, its use for solid malignancies remains a challenge. The putative benefit of RIT lies in selective targeting of antigens expressed on the tumor surface using monoclonal antibodies, to systemically deliver cytotoxic radionuclides. The past several decades yielded dramatic improvements in the quality, quantity, recent commercial availability of alpha-, beta- and Auger Electron-emitting therapeutic radiometals. Investigators have created new or improved existing bifunctional chelators. These bifunctional chelators bind radiometals and can be coupled to antigen-specific antibodies. In this review, we discuss approaches to develop radiometal-based RITs, including the selection of radiometals, chelators and antibody platforms (i.e. full-length, F(ab')2, Fab, minibodies, diabodies, scFv-Fc and nanobodies). We cite examples of the performance of RIT in the clinic, describe challenges to its implementation, and offer insights to address gaps toward translation.
Keywords: radioimmunotherapy, radiopharmaceuticals, targeted radiotherapy, theranostics, oncology, cancer
 Global reach, higher impact
Global reach, higher impact