13.3
Impact Factor
Theranostics 2021; 11(13):6251-6277. doi:10.7150/thno.57689 This issue Cite
Review
Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS
1. Department of Nuclear Medicine, School of Medicine, Kyungpook National University, Daegu, Republic of Korea.
2. Department of Nuclear Medicine, Kyungpook National University Hospital, Daegu, Republic of Korea.
3. BK21 FOUR KNU Convergence Educational Program of Biomedical Sciences for Creative Future Talents, Department of Biomedical Science, School of Medicine, Kyungpook National University, Daegu, Republic of Korea.
Abstract
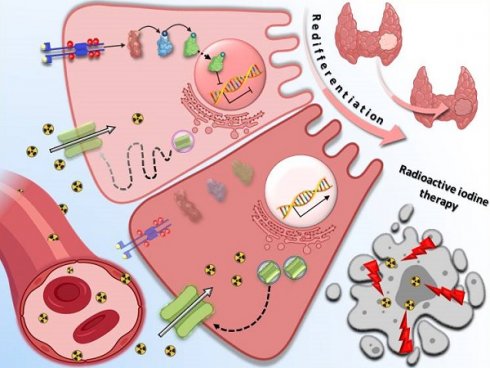
The advanced, metastatic differentiated thyroid cancers (DTCs) have a poor prognosis mainly owing to radioactive iodine (RAI) refractoriness caused by decreased expression of sodium iodide symporter (NIS), diminished targeting of NIS to the cell membrane, or both, thereby decreasing the efficacy of RAI therapy. Genetic aberrations (such as BRAF, RAS, and RET/PTC rearrangements) have been reported to be prominently responsible for the onset, progression, and dedifferentiation of DTCs, mainly through the activation of mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/AKT signaling pathways. Eventually, these alterations result in a lack of NIS and disabling of RAI uptake, leading to the development of resistance to RAI therapy. Over the past decade, promising approaches with various targets have been reported to restore NIS expression and RAI uptake in preclinical studies. In this review, we summarized comprehensive molecular mechanisms underlying the dedifferentiation in RAI-refractory DTCs and reviews strategies for restoring RAI avidity by tackling the mechanisms.
Keywords: radioactive iodine refractory thyroid cancer, redifferentiation, signaling pathways, sodium iodide symporter, membrane targeting
 Global reach, higher impact
Global reach, higher impact