13.3
Impact Factor
Theranostics 2021; 11(12):5675-5685. doi:10.7150/thno.46436 This issue Cite
Review
Application of mesenchymal stem cell therapy for aging frailty: from mechanisms to therapeutics
1. Department of Geriatrics, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, 200123, China.
2. Department of General medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, 200123, China.
3. Institute for Regenerative Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, 200123, China.
Received 2020-3-28; Accepted 2021-3-15; Published 2021-3-31
Abstract
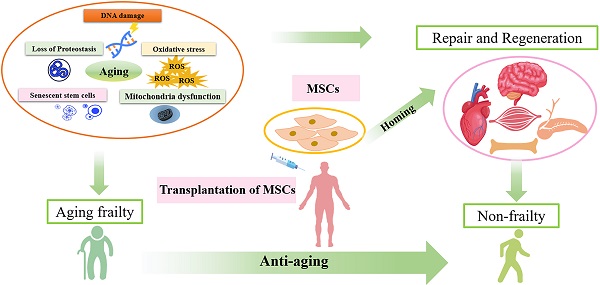
Aging frailty is a complex geriatric syndrome that becomes more prevalent with advancing age. It constitutes a major health problem due to frequent adverse outcomes. Frailty is characterized by disruption of physiological homeostasis and progressive decline of health status. Multiple factors contribute to development of frailty with advancing age, including genome instability, DNA damage, epigenetic alternations, stem cell exhaustion, among others. These interrelated factors comprehensively result in loss of tissue homeostasis and diminished reserve capacity in frailty. Therefore, the aged organism gradually represents symptoms of frailty with decline in physiological functions of organs. Notably, the brain, cardiovascular system, skeletal muscle, and endocrine system are intrinsically interrelated to frailty. The patients with frailty may display the diminished reserves capacity of organ systems. Due to the complex pathophysiology, no specific treatments have been approved for prevention of this syndrome. At such, effective strategies for intervening in pathogenic process to improve health status of frail patients are highly needed. Recent progress in cell-based therapy has greatly contributed to the amelioration of degenerative diseases related to age. Mesenchymal stem cells (MSCs) can exert regenerative effects and possess anti-inflammatory properties. Transplantation of MSCs represents as a promising therapeutic strategy to address the pathophysiologic problems of frail syndrome. Currently, MSC therapy have undergone the phase I and II trials in human subjects that have endorsed the safety and efficacy of MSCs for aging frailty. However, despite these positive results, caution is still needed with regard to potential to form tumors, and further large-scale studies are warranted to confirm the therapeutic efficacy of MSC therapy.
Keywords: mesenchymal stem cells, aging frailty, aging, regenerative medicine, stem cell therapy
Introduction
The global population is aging rapidly due to an increase in life expectancy [1], so too has the increasing prevalence of aging frailty [2]. Frailty is an age-associated geriatric syndrome, defined as a state of increased physiological vulnerability to stressors due to multiple system dysregulation and reduced functional reserves [3]. Aging frailty is associated with functional limitations in daily living, which conferred the greater risk of poor health outcomes in the older population, such as mortality, disability, hospitalization and falls [4-6], alongside the increased healthcare costs which presents a major public health problem worldwide [7, 8]. Despite decades of research that have led to a growing understanding of biological alterations of frailty, the approved medical therapy that can effectively attenuate or reverse aging frailty is still not available [9]. To date, clinicians have attempted several interventions to improve and modify frailty status, including physical exercises (e.g., strengthening exercises), nutrition (e.g., protein and Vitamin D), and multidisciplinary interventions [9, 10], but the efficacy of these interventions for protecting the frail patients against adverse outcomes is still controversial [11, 12]. Since frailty is one of the biggest threats to successful aging, a specific intervention that is expected to be effective to improve frailty status is highly needed. Currently, cell-based therapy is emerging as an innovative approach for several degenerative diseases. Mesenchymal stem cells (MSCs) represent as the ideal seeding cells for tissue engineering and regenerative medicine [13, 14]. To date, MSCs has become a promising candidate for intervening aging frailty. In this review, we mainly focused on the pathobiological process of aging frailty and summarized the roles and mechanisms of MSCs as the novel biologic agents used in the treatment of aging frailty. We also discussed the current status of MSCs utilized in clinical research as well as the challenge for successful clinical applications of MSC therapy.
Overview of Aging Frailty and its Pathophysiology
Aging frailty is a complex geriatric syndrome with multifactorial pathogenesis and declines in physiological reserves. Frail syndrome can lead to the reduced homeostatic capability to withstand stressors and increased vulnerabilities to environments, which account for the high risk of adverse events [2, 15, 16]. The overall prevalence of aging frailty in community worldwide is estimated to be between 5% and 20% [17-19]. The prevalence of frailty increases with age and women are more likely to be frail than men [18]. Aging frailty can be identified by two main models: physical frail phenotype and cumulative deficit index [2, 15]. According to the phenotypic model, frailty can be identified by the presence of at least three components: unintentional weight loss; self-reported exhaustion; weakness; slow walking speed and low physical performance [2]. It is characterized by diminished strength, endurance and reduced physiologic function, which increase an individual's vulnerability for developing increased dependency or death [20]. On the other hand, the deficit model describes frailty in terms of the accumulation of individual impairments that include comorbid diseases, symptoms, signs and disabilities, collectively referred to as deficits [15]. While these two instruments are different for evaluating frailty, both have received empirical validation.
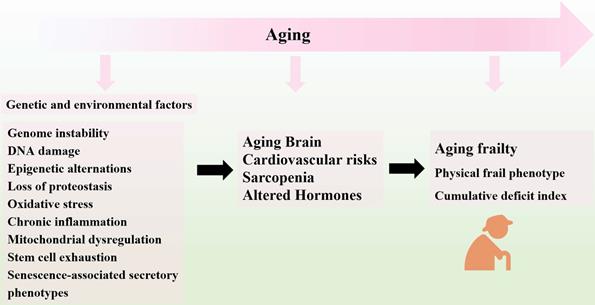
With the process of aging, frailty may be caused by multiple causes and contributors, including genetic and environmental factors [21-24]. To be more specific, genome instability [25], DNA damage [26], epigenetic alternations [27], loss of proteostasis [28], oxidative stress [29], chronic inflammation [30], mitochondrial dysregulation [31], and stem cell exhaustion [24, 32] are involved in the progression of aging frailty. These hallmarks are interconnected and ultimately lead to cellular senescence. The senescent cells increase in multiple tissues with aging [33], and secrete a host of inflammatory cytokines, chemokines, growth factors and matrix remodeling proteases, collectively known as the senescence-associated secretory phenotypes (SASP), which lead to the chronic inflammation and age-related tissue deterioration [34, 35]. Moreover, senescence reduces the regenerative potential of stem cells pools and leads to endogenous stem cells exhaustion. The resident stem cells, including MSCs, HSCs (hematopoietic stem cells), neural stem cells (NSCs) and satellite cells undergo senescence during aging process, showing age-related decline in repopulation capacity and differentiation potential with reduced lifespan [36-39]. The reduced abilities of stem cells fail to maintain their proliferation capacity and differentiation potential [40]. Accordingly, the capacity to regenerate damaged tissues decline of regeneration upon damage decline, which results in the imbalance of tissue homeostasis after injury or stress [34, 41]. The sum of these integrative hallmarks produces the clinical phenotypes of the elderly with aging frailty, as seen in physiological loss of reserve and reduced organ function [42]. The disfunctions of brain, heart, muscle, and endocrine system are linked to aging and impaired homeostasis, which are believed to be involved in the development of frailty [16]. The multiple types of aging-related damages may constitute the major culprits of phenotypes of frailty, as the integrative consequence of stem cell exhaustion, diminished homeostasis, and organ repair [43]. In this regard, regenerative medicine and cellular therapy has been long proposed and examined clinically. As a promising candidate for tissue regeneration, MSCs have gathered great attention in the field of regenerative medicine. Transplantation of MSCs may serve as an innovative therapeutic approach for preventing and even reversing development of aging frailty [44, 45].
Basic Characteristics of MSCs
MSCs are the non-hematopoietic stem cells which exhibit spindle-shaped structure and plastic-adherent properties [46]. Originally isolated from bone marrow in 1968 [47], MSCs were successively found to exist in various tissues and can be easily harvested from multiple tissues, including adipose tissue, marrow spaces of long bone, skeletal muscle, synovial fluids, umbilical cord blood, placenta, and dental pulp [48-51]. As the multipotent progenitors, MSCs have displayed the ability to give rise to several different phenotypes, including osteocytes, chondrocytes, adipocytes, fibroblast, and many others [52, 53]. However, MSCs exhibit heterogeneous features among their subpopulations regarding to their proliferation rate and secreted cytokines [54, 55]. In addition, the discrepancy of isolation and cultivation procedures between different laboratories also drives the development of standardized criteria for identifying unique populations of MSCs. In 2006, the International Society for Cellular Therapy (ISCT) has proposed the minimum criteria to define human MSCs [46]. According to ISCT, MSCs must be plastic-adherent and positive for specific surface makers, namely, CD73, CD90 and CD105 but be negative for CD14, CD19, CD34, CD45 and HLA-DR. More importantly, MSCs must be capable of differentiating into multilineage cell types in vitro. MSCs can migrate automatically toward injury areas and spontaneously differentiate into desired tissues to perform regenerative functions, which are described as tropism [48, 56]. The therapeutic effects of MSCs, including their anti-inflammatory and immunomodulatory abilities, are exerted via secretion of several cytokines and soluble factors and signaling pathway activation. MSCs had the low expression of MHC/HLA class I but do not express MHC/HLA class II, which can protect them from host immune detection. The biological property of immune evasion prolongs their persistence in the host and enhances their therapeutic effects [57]. To date, MSCs have been considered as one of the most promising stem cell types for cell therapy. MSCs are associated with unique capability of self-renewal and extensive potential of differentiation, which have generated great interest in the fields of regenerative medicine [58]. Multiple lines evidence have documented that the transplantation of MSCs can be utilized as a suitable therapeutic approach in the treatments of some intractable diseases, including traumatic brain injury [59] and spinal cord injury [60], cardiovascular diseases [61], stroke [62] and liver diseases [63]. The specific characteristics, along with the therapeutic benefits of MSCs support the potential use of MSCs in future therapies for aging frailty.
MSC Therapy for the Attenuation of Aging Frailty
Aging Brain
Frailty is a state of increased vulnerability to stressor events due to multimorbidity and multiple impairments in different systems. Aging brain or frail brain would lead to central nervous system impairments with cognitive decline, which play a crucial role in the development of physical frailty [64, 65]. More importantly, the deterioration of brain is associated with gait impairments, which is considered as an important contributor to frailty [66].
Neuroprotective Effects of MSCs
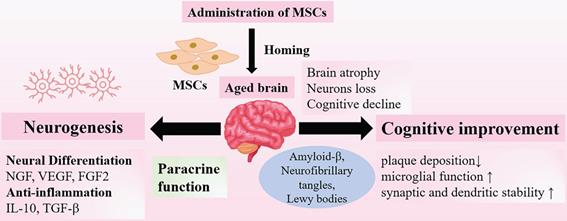
In aging process, almost all the brains undergo characteristic changes, including brain atrophy, loss of neurons and synapse connections. These age-related changes are responsible for the decline in neuronal activity and synaptic dysfunction that linked to neurodegeneration [67]. The effects of transplanted MSCs have been documented in vivo and vitro experiments in several studies, which have shown that MSCs could promote neurogenesis and improve neurological state [68, 69]. Intravenous infused MSCs can cross the blood-brain barrier (BBB), which is an essential prerequisite for proper efficacy [70-72]. Then intravenous injected MSCs can migrate to the injured regions and differentiate into neuron-like-cells via secreting various neurotrophic factors, such as nerve growth factor (NGF), vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF2). These secretomes are released from non-genetically modified MSCs, playing a significant role in inducing neuronal differentiation and increasing survival rates after injury [73, 74]. Likewise, administration of MSCs via intracerebral and intrathecal routes also showed positive results of neuronal regeneration promoted by MSCs in animal models [75, 76]. Moreover, microglia and astrocytes in aging brain become senescent and express the senescence-associated secretory phenotype; several inflammatory cytokines are secreted to maintain state of low-grade inflammation that play a significant role in natural aging and neurodegeneration [77]. MSCs possess anti-inflammatory properties adding to their neuroprotective effects. A great number of studies have showed that transplanted MSCs could reduce the levels of pro-inflammatory cytokines [78], or promote macrophages to polarize into the anti-inflammatory M2 phenotype [79]. The anti-inflammatory effects are conducted through secreting multiple cytokines, including IL-10 and transforming growth factor-β (TGF-β) [80]. At such, the anti-inflammatory microenvironments induced by transplanted MSCs help promote neurogenesis and prevent neural degeneration [78, 81]. Ameliorating cognitive decline may be a promising approach to prevent brain frailty. There are several altered proteins in the aged brains. The presence of amyloid-β, neurofibrillary tangles, Lewy bodies, the causative factors of neurodegenerative diseases, such as AD, may contribute to deterioration of brain [66, 82, 83]. Inspiringly, MSCs administration has been documented to reduce plaque deposition, restore microglial function and increase synaptic and dendritic stability in animal models of AD [68, 69]. To date, substantive preclinical studies are underway to provide positive results, and MSC-based therapy carries promise to reverse the deterioration of brain, which has become a potential therapeutic approach for the amelioration of aging frailty (Figure 2).
Overview of Aging Frailty and its Pathophysiology. In the process of aging, several genetic and environmental factors gradually result in the loss of tissue homeostasis and organ dysfunction, ultimately leading to the progression of frailty in the aged organism.
Neuroprotective Effects of MSCs on Aging Brain. Administration of MSCs have shown therapeutic potential for the treatment of age- related brain dysfunction. The neuroprotective effects of MSCs include promoting neurogenesis, neural differentiation and anti-inflammation, and these effects are mainly associated with paracrine functions. In addition, MSCs could improve cognitive functions through reducing plaque deposition and enhancing synaptic stability. Abbr: NGF: nerve growth factor; VEGF: vascular endothelial growth factor; FGF2: fibroblast growth factor 2; IL-2: interleukin-2; TGF-β: transforming growth factor-β.
Cardiovascular Risk
The cardiovascular diseases and aging frailty often coexist. Growing evidence has showed that cardiovascular diseases including myocardial infarction, atrial fibrillation and chronic heart failure are associated with the increased high incidence of aging frailty [84-86]. Cardiovascular diseases could give rise to physical disability and frailty through impaired muscle function [85, 87]. The interplay between cardiovascular diseases and frailty may provide a novel therapeutic strategy in the interventions of frailty.
Cardioprotective Effects of MSCs
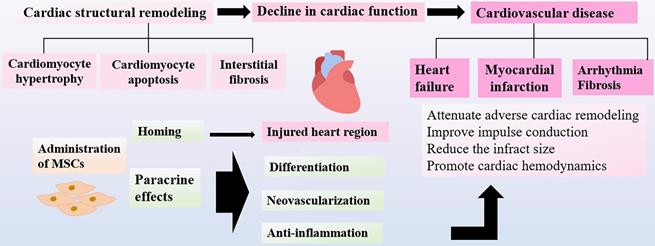
In the aging process, aging is associated with the gradual loss of biological functions, resulting in the increased cardiac vulnerability to cardiovascular dysfunction. The cardiac senescence is reflected by decreased cardiac performance and progressive cardiac structural remodeling. The various phenotypic changes in functions and structures of heart, including cardiomyocyte hypertrophy and apoptosis [88], interstitial fibrosis [89], comprehensively account for the decreased cardiac function, which may eventually lead to the progression of cardiovascular diseases in the aging populations. Several preclinical studies have demonstrated that MSCs could exert cardio-protective effects and promote cardiac functions through different mechanisms. MSCs could migrate to the injured zone and differentiate into endothelial cells and cardiomyocyte-like cells to promote neovascularization and cardiac functions, which can effectively offer repair in the sites of damaged myocardium. It has been found that MSCs exert many therapeutic functions through paracrine effects [90, 91]. MSCs can produce multiple cytokines and angiogenic factors released directly in soluble form or in extracellular vesicles and exosomes, playing a role in improving cardiac functions after damage [92]. The left ventricular ejection fraction (LVEF) is a significant parameter for evaluation of cardiac function, which would become deteriorated subsequently after ischemic events. It has been shown that LVEF can be successfully preserved in the MSCs treated group as compared to the control group in the animal model with ischemic myocardium [93]. The positive results have been further confirmed in clinical trials that transplantation of MSCs could significantly attenuate adverse ventricular remodeling and improve LVEF in patients with heart failure [94, 95]. Furthermore, many other studies have demonstrated MSC therapy can be capable of reducing the infract size and promoting cardiac hemodynamics in mice with ischemic myocardium [96]. Current evidence shows that MSCs could persist for 4 weeks after transplantation, predominantly in the border zone of infarcted myocardium, whereas few MSCs were detected in the normal cardiac tissues [97].
It has been well recognized that fibroblast could replace cardiomyocytes after injury, which cause myocardial remodeling and fibrotic scarring. The anti-fibrotic molecule, TNF-α-induced protein 6 (TNAIP6) is secreted by MSCs to decrease the damage to the heart and fibrosis. MSCs suppress the excessive inflammatory responses caused by cardiomyocyte cells injury and subsequent fibrosis [98]. In addition, MSCs attenuate arrhythmia by improving impulse conduction in the model of myocardial infarction [99]. Taken together, this novel approach of MSCs transplantation can ameliorate cardiovascular symptoms via several mechanisms, including angiogenesis, repair of the injured tissue, and reduction of infarct size as well as regulation of cardiac structural remodeling, which has a great potential to be applied in the regenerative medicine to improve the treatment of aging frailty [100] (Figure 3).
Sarcopenia
Sarcopenia is an age-related disease with the progressive loss of muscle mass and strength [101, 102]. The declines in skeletal mass and function pose significant risks for adverse outcomes including mortality, disability and falls among older adults [103-105]. The identification of sarcopenia is based on the co-occurrence of low muscle mass as well as slow gait speed or weak handgrip strength as measures of low muscle function [106]. Sarcopenia has been considered as an important component of frailty syndrome and the pathway through which the frail condition can be intervened or reversed [107].
Protective Effects of MSCs on Muscles
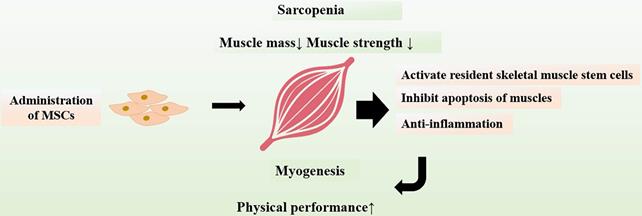
The interventions that can alleviate sarcopenia may be an important approach to improve or reverse frailty status. It has been showed that MSCs could attenuate sarcopenia via increasing skeletal muscle weight and myofiber cross-sectional area in animal models of sarcopenia [108]. The physical performance including muscle strength as well as endurance were significantly enhanced. MSCs also inhibit apoptosis of muscles and suppress expressions of chronic inflammatory cytokines, which may explain the improvement of skeletal muscle strength and function after transplantation of MSCs. In addition, MSCs have capability to activate resident skeletal muscle stem cells, which lead to myogenesis and differentiation of muscle tissues [109]. The positive results provide novel insights into sarcopenia intervention, suggesting a potential role for MSC therapy in aging frailty (Figure 4).
Altered Hormones
Advance in age leads to the disruption of endocrine system and imbalance of metabolic homeostasis, which may result in the breakdown of adaptation process in response to stresses [110]. The alternations in hormonal networks and abnormal hormonal excesses or deficits during aging can be translated in clinical scenarios that promote the pathogenesis of frailty and diseases [111]. As age-related disruption of the endocrine system is considered as a fundamental event in the pathogenesis of frailty, the efficacious strategies that can promote metabolism are needed.
Therapeutic Effects of MSCs on Hormones
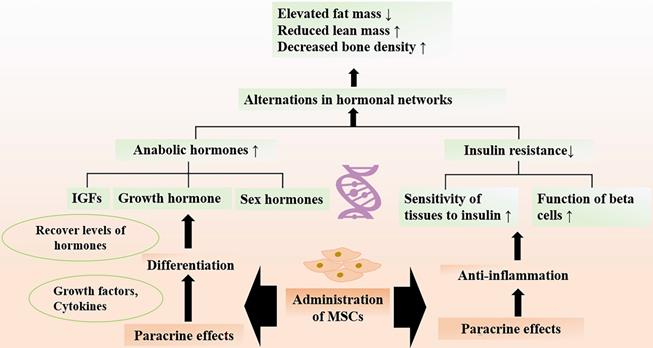
Accumulating evidence shows that adverse ageing profiles and frailty are related to the alternations in hormonal networks [110-112]. Age-related frailty is a common problem in older adults, as a result of the imbalance between the anabolic and catabolic hormones. The circulating anabolic hormones, including insulinlike growth factor (IGFs), growth hormone, and sex hormones, are important in maintaining healthy body compositions and organ functions. However, there is an overall decline in the amounts of hormones with age. For instance, the decreased levels of testosterone could lead to hypogonadism and reduced muscle mass. Researchers have documented that MSCs transplantation could recover the levels of testosterone back to normal through paracrine functions [113]. Notably, growth hormone and IGF1 also decrease with aging, the insufficient hormones result in body composition parameters with elevated fat mass and reduced lean mass [110, 114]. MSCs exerting beneficial paracrine effects are well recognized. It has been found that MSCs are capable of secreting multiple growth factors and cytokines, promoting regeneration of Leydig cells and many surrounding cells [113, 115]. In addition, MSCs can develop and differentiate into Leydig cells in the adult testis [116].
Cardioprotective Effects of MSCs. With advancing age, heart often develop decreased cardiac performance. The progressive cardiac structural remodeling results in low cardiac function and cardiovascular diseases that may contribute to aging frailty. After administration, MSCs home to the injured regions, where MSCs differentiate into endothelial cells and cardiomyocyte-like cells to promote neovascularization and cardiac functions. MSCs can suppress the excessive inflammatory responses and subsequent fibrosis via paracrine functions. MSC therapy show positive results by improving the prognosis of cardiovascular diseases.
Protective Effects of MSCs on Muscles. Sarcopenia is a major contributor to frailty in the elderly. Transplanted MSCs can exert protective effects on muscles, including inhibition of muscles apoptosis and regulation of chronic inflammatory as well as activation of resident skeletal muscle stem cells. Administration of MSCs can promote myogenesis and improve physical performance.
In addition to the deficiency of hormones, decreased sensitivity of tissues to actions of hormone take place in the elderly. Notably, insulin resistance develops with age, which is a state of poor sensitivity of peripheral tissues to insulin [117]. Insulin resistance may lead to metabolic disorders and accelerate decline in muscle strength and function that give rise to frailty [118, 119]. The roles of aging endocrine system in the development of frailty and as a target for interventions of frailty are investigated. The chronic inflammation is an important determiner of insulin resistance [120], so the protective role of MSCs in improving insulin sensitivity via suppressing the inflammatory activity has been focused. Preclinical study showed that MSCs after transplantation could significantly promote the response of target organs to insulin [121]. The therapeutic effect of MSCs may be attributed to regulation of immune process and systemic inflammation [122]. Numerous data have reported that MSC-based therapy can attenuate insulin resistance and improve beta cell function via inhibiting the production of inflammatory cytokines (e.g., IL-1β, Il-18, TNF-α) [123]. MSCs play a pivotal role in reducing the number of CD3+ and CD4+ T lymphocytes, which initiate the inflammatory process in the organism [122]. Given the therapeutic potential of MSCs on delineating the age-related alterations of hormones, MSC-based therapy may be a very promising candidate for promoting quality of life in the elderly population (Figure 5).
Clinical Transplantation of MSCs in Patients with Aging Frailty
While current evidence sheds a promising light for the stem cell-based therapy, data related to frailty is still limited in clinical settings [124, 125]. Aging FRailTy via IntravenoUS Delivery (CRATUS) went through the phase I and II stages. The phase I trial was a nonrandomized, dose-escalation study, which has reported the beneficial effects after transplantation of BM-derived MSCs in patients with aging frailty [124]. In that study, a total of 15 eligible patients were enrolled to receive the intravenous infusion of MSCs with the dose: 20-million, 100-million, 200-million, respectively (5 patients in each group). Inspiringly, all patients in the treatment groups had increased 6-minute walk distance at 3 months and 6 months. The levels of inflammatory cytokine, TNF-α decreased at 6 months. Among the three groups, 100-millon cell-dose group showed the best performance in the improvement of 6-minute walk distance, cognitive status and physical function. With regard to the safety of MSCs administration, no treatment-emergent serious adverse events occurred within 1-month post infusion. All patients could tolerate the doses of MSCs infused well. One death was reported at 258 days after infusion in the 200-million group which was determined to be irrelevant to MSCs transplantation. This study above-mentioned was succeeded by the randomized, double-blinded, and placebo-controlled, stage II of CRATUS study [125]. In the consecutive study, a total of 30 patients with aging frailty were randomized into 100-million, 200-million, and placebo groups. The results showed that immunologic improvement was seen in both the treatment groups. Notably, patients in the 100-million group performed better than that in the 200 million with improved 6-minute walk distance, short physical performance, forced expiratory volume in 1 second and decreased serum TNF-α levels from baseline to 6 months. More importantly, this study documented that intravenous administration of MSCs was safe, which did not incur any treatment-related serious adverse events for 12 months post infusion. Intriguingly, the consecutive two trials confirm that 100-million cells represent the superior dose level compared to 200-millon cells, yet the mechanism underlying the inverse dose relationship cannot be sufficiently explained [125]. A plausible explanation may be associated with deleterious effects of higher doses on cell retention, survival, or performance. Despite the positive findings, these two trials are preliminary and require larger RCTs to yield more convincing conclusions.
Therapeutic Effects of MSCs on Abnormal Hormones. Age-related alternation in hormonal networks include the decline in levels of circulating anabolic hormones and insulin resistance, which are associated with development of frailty. Administration of MSCs can increase the levels of anabolic hormones through paracrine functions and improve insulin sensitivity by regulating immune response. MSC therapy attenuate the age-related structural and functional changes of muscle and bone, thereby promoting the quality of life among the older adults.
In recent years, failure of MSCs to improve clinical outcome have been frequently encountered [126, 127], partially due to variability in culture methodologies [128], and poor survival of MSCs after transplantation [129]. The effect of MSCs largely depends on their capabilities to migrate, adhere, engraft to the injured site. Notably, the freshly isolated cells cultured in presence of specific cytokines or hypoxic conditions have higher engraftment efficiency [130]. Furthermore, aggregate culture conditions used for MSC production may improve secretory capacity [128]. The use of different MSC derivatives, such as extracellular vesicles and exosomes, may be more effective and preferable than the use of MSCs. There is still a long way to go before considering MSCs as an ideal clinical tool for aging frailty.
Challenges for Clinical Application of MSC Therapy
Efficacy
MSCs have the distinct advantages of rapid expansion, multi-lineage differentiation and potent ability of secreting tropic and immunomodulatory cytokines. For years, transplantation of MSCs has evolved as the promising therapeutic strategy for regenerative medicine and tissue engineering [131]. However, there are major limits to MSCs utilization. The allogeneic MSCs derived from different donors display different biological properties. Aged MSCs tend to exhibit the cellular senescence associated phenotypes, including the enhanced senescence-associated β-galactosidase activity, decreased stemness of stem cells, increased p16 expression, and apoptosis of cells as well as telomere attrition [132, 133]. With telomeres shortening, aged MSCs gradually cease to proliferate after a certain number of cell divisions. The proliferation and differentiation potential of MSCs progressively decline with age of donor and passage number of MSCs cultured in vitro [134, 135]. Cellular senescence impairs the self-renewal and differentiation potential of MSCs, which limit their therapeutic effects [136]. The replicative senescence of MSCs significantly limits their expansion to the large quantity necessary for clinical applications that need hundreds of millions of MSCs for per treatment [137]. Moreover, there are limits for autologous MSC applications. It is difficult to obtain sufficient amount of healthy MSCs from patients with some systemic diseases. Additionally, the process of autologous extraction is time-consuming, which is difficult to be utilized for the acute treatment of life-threatening diseases [131]. Other concerns regarding the efficacy of MSCs are their persistence after transplantation. These issues need to be addressed prior to widespread clinical application to enhance the efficacy of MSC therapy.
Safety Concerns
MSCs are emerging as the promising sources of cell-based therapy due to their pluripotency and ease of expansion. However, ethical issues regarding to security remain inadequately addressed. It has been noted that long-term MSC expansion in vitro can lead to chromosomal abnormalities [138, 139], which may induce tumors in vivo [140]. In the tumor microenvironment, MSCs possess immunosuppressive effects, which promote the progression of tumors [141, 142]. MSCs show the potential to differentiate into multiple tissues, such as bone and cartilage, so the unwanted differentiation of transplanted MSCs may promote tumor growth [143]. Furthermore, it is well accepted that angiogenesis exerts an important role in invasion and metastasis of tumors. MSCs can differentiate into vascular endothelial cells, secreting several growth factors including VEGF and PDGF (platelet-derived growth factor), which promote tumor angiogenesis and invasive behavior [144]. MSCs also involve in the tumor invasion and metastasis known as epithelial to mesenchymal transition (EMT), a process driving tumor cells to lose polarity and acquire invasive phenotype [145, 146]. In this regard, the tumorigenic potential of MSCs may become a major safety concern for the use of MSCs in clinical practice. MSC-based therapy may be a double-edged sword; the application of MSCs in clinical setting should be evaluated cautiously due to security concerns. Of note, as paracrine effect of MSCs plays a pivotal role, the bioactive secretions of MSCs have good efficacy and safety. For instance, extracellular vesicles, exosomes, and cytokines can avoid the risk of genetic instability and potential malignant transformation may be developed as a safe and effective agent in the regenerative medicine.
Conclusions and Future Perspectives
Frailty syndrome is a nonspecific state of increased vulnerability to stressors and is much more common in the old populations. Frailty is strongly associated with adverse outcomes, which may place a heavy burden on society in the coming years. As there is no specific approved treatment for frail patients, deeper understanding of the biological mechanisms of aging frailty to explore effective interventions is of great significance. Notably, multiple pathologic changes develop with age, aside from DNA damage and chronic inflammation that may contribute to aging frailty, endogenous stem cell exhaustion may be involved in the process of aging frailty. The frail patients may display the disruption of physiological homeostasis with decline in functions of several organs.
MSCs are emerging as the ideal sources of cells to solve the multi-organ problems. MSCs have potent self-renewal and differentiation capability. They are easy to be harvested from many tissues and can engraft to injured sites. In addition, the immune privileged state and anti-inflammatory property make MSC-based therapy as a promising tool in systemic applications. Current evidence has showed that MSCs could ameliorate status of frailty by promoting the functions of multiple important organs, including brain, muscles, heart, and endocrine system. To date, allo-hMSCs had undergone the phase I/II trials in which the safety and efficacy of MSC-based therapy for aging frailty were initially demonstrated. MSCs could attenuate symptoms of frail patients and no treatment-related serious adverse event was reported.
Transplantation of MSCs has generated great interests in regenerative medicine. However, the disputes arise regarding lack of efficacy as well as tumorigenic potential of MSCs on basis of current evidence. Although many findings shed a new light on MSC-based therapy for aging frailty, the scales and numbers of current clinical trials remain small, much further studies are warranted to elucidate if such therapeutic strategy could be safe and effective on regenerative medicine. The underlying mechanism of MSCs transplantation for the intervention of aging frailty should also be investigated.
Acknowledgements
This work was supported by grants from Important Weak Subject Construction Project of Pudong Health and Family Planning Commission of Shanghai (Grant No. PWZbr2017-06) and Major Program of Development Fund for Shanghai Zhangjiang National Innovation Demonstration Zone<Stem Cell Strategic Biobank and Stem Cell Clinical Technology Transformation Platform> (ZJ2018-ZD-004).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mathers CD, Stevens GA, Boerma T, White RA, Tobias MI. Causes of international increases in older age life expectancy. Lancet. 2015;385:540-8
2. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J. et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-56
3. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433-41
4. Li X, Ploner A, Karlsson IK, Liu X, Magnusson PKE, Pedersen NL. et al. The frailty index is a predictor of cause-specific mortality independent of familial effects from midlife onwards: a large cohort study. BMC Med. 2019;17:94
5. Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A. et al. Frailty and Subsequent Disability and Mortality among Patients with Critical Illness. Am J Respir Crit Care Med. 2017;196:64-72
6. Duarte GP, Santos JLF, Lebrão ML, Duarte YAO. Relationship of falls among the elderly and frailty components. Rev Bras Epidemiol. 2019;21(Suppl 02):e180017
7. Buckinx F, Rolland Y, Reginster JY, Ricour C, Petermans J, Bruyere O. Burden of frailty in the elderly population: perspectives for a public health challenge. Arch Public Health. 2015;73:19
8. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365-75
9. Walston J, Buta B, Xue QL. Frailty Screening and Interventions: Considerations for Clinical Practice. Clin Geriatr Med. 2018;34:25-38
10. Pahor M, Kritchevsky SB, Waters DL, Villareal DT, Morley J, Hare JM. et al. Designing Drug Trials for Frailty: ICFSR Task Force 2018. J Frailty Aging. 2018;7:150-4
11. Van der Elst M, Schoenmakers B, Duppen D, Lambotte D, Fret B, Vaes B. et al. Interventions for frail community-dwelling older adults have no significant effect on adverse outcomes: a systematic review and meta-analysis. BMC Geriatr. 2018;18:249
12. Li CM, Chen CY, Li CY, Wang WD, Wu SC. The effectiveness of a comprehensive geriatric assessment intervention program for frailty in community-dwelling older people: a randomized, controlled trial. Arch Gerontol Geriatr. 2010;50(Suppl 1):S39-42
13. Saler M, Caliogna L, Botta L, Benazzo F, Riva F, Gastaldi G. hASC and DFAT, Multipotent Stem Cells for Regenerative Medicine: A Comparison of Their Potential Differentiation In vitro. Int J Mol Sci. 2017 18
14. Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019 8
15. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722-7
16. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752-62
17. Saum KU, Dieffenbach AK, Müller H, Holleczek B, Hauer K, Brenner H. Frailty prevalence and 10-year survival in community-dwelling older adults: results from the ESTHER cohort study. Eur J Epidemiol. 2014;29:171-9
18. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487-92
19. Andrade JM, Duarte YAO, Alves LC, Andrade FCD, Souza Junior PRB, Lima-Costa MF. et al. Frailty profile in Brazilian older adults: ELSI-Brazil. Rev Saude Publica. 2018;52(Suppl 2):17s
20. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R. et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392-7
21. Viña J, Tarazona-Santabalbina FJ, Pérez-Ros P, Martínez-Arnau FM, Borras C, Olaso-Gonzalez G. et al. Biology of frailty: Modulation of ageing genes and its importance to prevent age-associated loss of function. Mol Aspects Med. 2016;50:88-108
22. Bektas A, Schurman SH, Sen R, Ferrucci L. Aging, inflammation and the environment. Exp Gerontol. 2018;105:10-8
23. Dato S, Montesanto A, Lagani V, Jeune B, Christensen K, Passarino G. Frailty phenotypes in the elderly based on cluster analysis: a longitudinal study of two Danish cohorts. Evidence for a genetic influence on frailty. Age (Dordr). 2012;34:571-82
24. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194-217
25. Seo AY, Leeuwenburgh C. The Role of Genome Instability in Frailty: Mitochondria versus Nucleus. Nestle Nutr Inst Workshop Ser. 2015;83:19-27
26. Pusceddu I, Farrell CJ, Di Pierro AM, Jani E, Herrmann W, Herrmann M. The role of telomeres and vitamin D in cellular aging and age-related diseases. Clin Chem Lab Med. 2015;53:1661-78
27. Booth LN, Brunet A. The Aging Epigenome. Mol Cell. 2016;62:728-44
28. Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res Rev. 2011;10:205-15
29. Álvarez-Satta M, Berna-Erro A, Carrasco-Garcia E, Alberro A, Saenz-Antoñanzas A, Vergara I. et al. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging (Albany N Y). 2020;12:9982-99
30. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505-22
31. Gomez-Cabrera MC, Sanchis-Gomar F, Garcia-Valles R, Pareja-Galeano H, Gambini J, Borras C. et al. Mitochondria as sources and targets of damage in cellular aging. Clin Chem Lab Med. 2012;50:1287-95
32. Sun XL, Hao QK, Tang RJ, Xiao C, Ge ML, Dong BR. Frailty and Rejuvenation with Stem Cells: Therapeutic Opportunities and Clinical Challenges. Rejuvenation Res. 2019;22:484-97
33. Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311:1257
34. LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular Senescence and the Biology of Aging, Disease, and Frailty. Nestle Nutr Inst Workshop Ser. 2015;83:11-8
35. Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966-72
36. Yu KR, Kang KS. Aging-related genes in mesenchymal stem cells: a mini-review. Gerontology. 2013;59:557-63
37. Wang MJ, Chen J, Chen F, Liu Q, Sun Y, Yan C. et al. Rejuvenating Strategies of Tissue-specific Stem Cells for Healthy Aging. Aging Dis. 2019;10:871-82
38. Warren LA, Rossi DJ. Stem cells and aging in the hematopoietic system. Mech Ageing Dev. 2009;130:46-53
39. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760-4
40. Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat Cell Biol. 2011;13:506-12
41. Aunan JR, Watson MM, Hagland HR, Søreide K. Molecular and biological hallmarks of ageing. Br J Surg. 2016;103:e29-46
42. Schultz MB, Sinclair DA. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development. 2016;143:3-14
43. Golpanian S, DiFede DL, Pujol MV, Lowery MH, Levis-Dusseau S, Goldstein BJ. et al. Rationale and design of the allogeneiC human mesenchymal stem cells (hMSC) in patients with aging fRAilTy via intravenoUS delivery (CRATUS) study: A phase I/II, randomized, blinded and placebo controlled trial to evaluate the safety and potential efficacy of allogeneic human mesenchymal stem cell infusion in patients with aging frailty. Oncotarget. 2016;7:11899-912
44. Raggi C, Berardi AC. Mesenchymal stem cells, aging and regenerative medicine. Muscles Ligaments Tendons J. 2012;2:239-42
45. Schulman IH, Balkan W, Hare JM. Mesenchymal Stem Cell Therapy for Aging Frailty. Front Nutr. 2018;5:108
46. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D. et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-7
47. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403
48. Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6:2173-85
49. Rodriguez AM, Elabd C, Amri EZ, Ailhaud G, Dani C. The human adipose tissue is a source of multipotent stem cells. Biochimie. 2005;87:125-8
50. Zhang X, Hirai M, Cantero S, Ciubotariu R, Dobrila L, Hirsh A. et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J Cell Biochem. 2011;112:1206-18
51. Sinclair K, Yerkovich ST, Chambers DC. Mesenchymal stem cells and the lung. Respirology. 2013;18:397-411
52. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD. et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-7
53. Cook D, Genever P. Regulation of mesenchymal stem cell differentiation. Adv Exp Med Biol. 2013;786:213-29
54. Majore I, Moretti P, Hass R, Kasper C. Identification of subpopulations in mesenchymal stem cell-like cultures from human umbilical cord. Cell Commun Signal. 2009;7:6
55. Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I. et al. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev Rep. 2009;5:378-86
56. Nitzsche F, Müller C, Lukomska B, Jolkkonen J, Deten A, Boltze J. Concise Review: MSC Adhesion Cascade-Insights into Homing and Transendothelial Migration. Stem Cells. 2017;35:1446-60
57. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005;2:8
58. Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829-48
59. Das M, Mayilsamy K, Mohapatra SS, Mohapatra S. Mesenchymal stem cell therapy for the treatment of traumatic brain injury: progress and prospects. Rev Neurosci. 2019;30:839-55
60. Mukhamedshina Y, Shulman I, Ogurcov S, Kostennikov A, Zakirova E, Akhmetzyanova E. et al. Mesenchymal Stem Cell Therapy for Spinal Cord Contusion: A Comparative Study on Small and Large Animal Models. Biomolecules. 2019 9
61. Goradel NH, Hour FG, Negahdari B, Malekshahi ZV, Hashemzehi M, Masoudifar A. et al. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J Cell Biochem. 2018;119:95-104
62. Doeppner TR, Hermann DM. Mesenchymal stem cells in the treatment of ischemic stroke: progress and possibilities. Stem Cells Cloning. 2010;3:157-63
63. Mahmood A, Seetharaman R, Kshatriya P, Patel D, Srivastava AS. Stem Cell Transplant for Advanced Stage Liver Disorders: Current Scenario and Future Prospects. Curr Med Chem. 2019
64. Malmstrom TK, Morley JE. The frail brain. J Am Med Dir Assoc. 2013;14:453-5
65. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment-a review of the evidence and causal mechanisms. Ageing research reviews. 2013;12:840-51
66. Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006;59:166-73
67. Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep. 2018;22:269-85
68. Lo Furno D, Mannino G, Giuffrida R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J Cell Physiol. 2018;233:3982-99
69. Duncan T, Valenzuela M. Alzheimer's disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8:111
70. Ren C, Guingab-Cagmat J, Kobeissy F, Zoltewicz S, Mondello S, Gao M. et al. A neuroproteomic and systems biology analysis of rat brain post intracerebral hemorrhagic stroke. Brain Res Bull. 2014;102:46-56
71. Matsushita T, Kibayashi T, Katayama T, Yamashita Y, Suzuki S, Kawamata J. et al. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci Lett. 2011;502:41-5
72. Liu L, Eckert MA, Riazifar H, Kang DK, Agalliu D, Zhao W. From blood to the brain: can systemically transplanted mesenchymal stem cells cross the blood-brain barrier? Stem Cells Int. 2013;2013:435093
73. Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102:18171-6
74. Tan B, Luan Z, Wei X, He Y, Wei G, Johnstone BH. et al. AMP-activated kinase mediates adipose stem cell-stimulated neuritogenesis of PC12 cells. Neuroscience. 2011;181:40-7
75. Bao X, Wei J, Feng M, Lu S, Li G, Dou W. et al. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011;1367:103-13
76. Ruan GP, Han YB, Wang TH, Xing ZG, Zhu XB, Yao X. et al. Comparative study among three different methods of bone marrow mesenchymal stem cell transplantation following cerebral infarction in rats. Neurol Res. 2013;35:212-20
77. Pizza V, Agresta A, D'Acunto CW, Festa M, Capasso A. Neuroinflamm-aging and neurodegenerative diseases: an overview. CNS Neurol Disord Drug Targets. 2011;10:621-34
78. Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W. et al. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588-602
79. Kudlik G, Hegyi B, Czibula Á, Monostori É, Buday L, Uher F. Mesenchymal stem cells promote macrophage polarization toward M2b-like cells. Exp Cell Res. 2016;348:36-45
80. Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K. et al. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552-70
81. Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017 18
82. Elobeid A, Libard S, Leino M, Popova SN, Alafuzoff I. Altered Proteins in the Aging Brain. J Neuropathol Exp Neurol. 2016;75:316-25
83. Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539:180-6
84. Polidoro A, Stefanelli F, Ciacciarelli M, Pacelli A, Di Sanzo D, Alessandri C. Frailty in patients affected by atrial fibrillation. Arch Gerontol Geriatr. 2013;57:325-7
85. Stewart R. Cardiovascular Disease and Frailty: What Are the Mechanistic Links? Clin Chem. 2019;65:80-6
86. Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R. et al. Associations of subclinical cardiovascular disease with frailty. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56:M158-M66
87. Strandberg TE, Pitkälä KH, Tilvis RS, O'Neill D, Erkinjuntti TJ. Geriatric syndromes-vascular disorders? Ann Med. 2013;45:265-73
88. Oka T, Komuro I. Molecular mechanisms underlying the transition of cardiac hypertrophy to heart failure. Circ J. 2008 72 Suppl A: A13-6
89. Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011;89:265-72
90. Zhang J, Wu Y, Chen A, Zhao Q. Mesenchymal stem cells promote cardiac muscle repair via enhanced neovascularization. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;35:1219-29
91. Ward MR, Abadeh A, Connelly KA. Concise Review: Rational Use of Mesenchymal Stem Cells in the Treatment of Ischemic Heart Disease. Stem Cells Transl Med. 2018;7:543-50
92. Gallina C, Turinetto V, Giachino C. A New Paradigm in Cardiac Regeneration: The Mesenchymal Stem Cell Secretome. Stem Cells Int. 2015;2015:765846
93. Makkar RR, Price MJ, Lill M, Frantzen M, Takizawa K, Kleisli T. et al. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. J Cardiovasc Pharmacol Ther. 2005;10:225-33
94. Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C. et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121:1192-204
95. Lalu MM, Mazzarello S, Zlepnig J, Dong YYR, Montroy J, McIntyre L. et al. Safety and Efficacy of Adult Stem Cell Therapy for Acute Myocardial Infarction and Ischemic Heart Failure (SafeCell Heart): A Systematic Review and Meta-Analysis. Stem Cells Transl Med. 2018;7:857-66
96. Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci. 2001;938:221-30
97. Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M. et al. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670-6
98. Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL. et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54-63
99. Panda NC, Zuckerman ST, Mesubi OO, Rosenbaum DS, Penn MS, Donahue JK. et al. Improved conduction and increased cell retention in healed MI using mesenchymal stem cells suspended in alginate hydrogel. J Interv Card Electrophysiol. 2014;41:117-27
100. Yun CW, Lee SH. Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease. Int J Mol Sci. 2019;20:982
101. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-23
102. Rosenberg IH. Sarcopenia: origins and clinical relevance. The Journal of nutrition. 1997;127:990S-1S
103. Landi F, Cruz-Jentoft AJ, Liperoti R, Russo A, Giovannini S, Tosato M. et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing. 2013;42:203-9
104. Vetrano DL, Landi F, Volpato S, Corsonello A, Meloni E, Bernabei R. et al. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: results from the CRIME study. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69:1154-61
105. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E. et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clinical nutrition (Edinburgh, Scotland). 2012;31:652-8
106. Marzetti E, Calvani R, Tosato M, Cesari M, Di Bari M, Cherubini A. et al. Sarcopenia: an overview. Aging Clin Exp Res. 2017;29:11-7
107. Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:192
108. Wang Q-Q, Jing X-M, Bi Y-Z, Cao X-F, Wang Y-Z, Li Y-X. et al. Human Umbilical Cord Wharton's Jelly Derived Mesenchymal Stromal Cells May Attenuate Sarcopenia in Aged Mice Induced by Hindlimb Suspension. Medical science monitor: international medical journal of experimental and clinical research. 2018;24:9272-81
109. Li T-S, Shi H, Wang L, Yan C. Effect of Bone Marrow Mesenchymal Stem Cells on Satellite Cell Proliferation and Apoptosis in Immobilization-Induced Muscle Atrophy in Rats. Medical science monitor: international medical journal of experimental and clinical research. 2016;22:4651-60
110. Clegg A, Hassan-Smith Z. Frailty and the endocrine system. The lancet Diabetes & endocrinology. 2018;6:743-52
111. Diamanti-Kandarakis E, Dattilo M, Macut D, Duntas L, Gonos ES, Goulis DG. et al. MECHANISMS IN ENDOCRINOLOGY: Aging and anti-aging: a Combo-Endocrinology overview. Eur J Endocrinol. 2017;176:R283-R308
112. Brown M. Skeletal muscle and bone: effect of sex steroids and aging. Adv Physiol Educ. 2008;32:120-6
113. Zhang ZY, Xing XY, Ju GQ, Zhong L, Sun J. Mesenchymal stem cells from human umbilical cord ameliorate testicular dysfunction in a male rat hypogonadism model. Asian J Androl. 2017;19:543-7
114. Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73:1081-8
115. Odeh HM, Kleinguetl C, Ge R, Zirkin BR, Chen H. Regulation of the proliferation and differentiation of Leydig stem cells in the adult testis. Biol Reprod. 2014;90:123
116. Chen B, Chen D, Jiang Z, Li J, Liu S, Dong Y. et al. Effects of estradiol and methoxychlor on Leydig cell regeneration in the adult rat testis. Int J Mol Sci. 2014;15:7812-26
117. Abbatecola AM, Paolisso G. Is there a relationship between insulin resistance and frailty syndrome? Curr Pharm Des. 2008;14:405-10
118. Pérez-Tasigchana RF, León-Muñoz LM, Lopez-Garcia E, Gutierrez-Fisac JL, Laclaustra M, Rodríguez-Artalejo F. et al. Metabolic syndrome and insulin resistance are associated with frailty in older adults: a prospective cohort study. Age Ageing. 2017;46:807-12
119. Volpato S, Ferrucci L, Blaum C, Ostir G, Cappola A, Fried LP. et al. Progression of lower-extremity disability in older women with diabetes: the Women's Health and Aging Study. Diabetes Care. 2003;26:70-5
120. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87-91
121. Sun X, Hao H, Han Q, Song X, Liu J, Dong L. et al. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther. 2017;8:241 -
122. Kintscher U, Hartge M, Hess K, Foryst-Ludwig A, Clemenz M, Wabitsch M. et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304-10
123. Liu X, Zheng P, Wang X, Dai G, Cheng H, Zhang Z. et al. A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther. 2014;5:57
124. Golpanian S, DiFede DL, Khan A, Schulman IH, Landin AM, Tompkins BA. et al. Allogeneic Human Mesenchymal Stem Cell Infusions for Aging Frailty. J Gerontol A Biol Sci Med Sci. 2017;72:1505-12
125. Tompkins BA, DiFede DL, Khan A, Landin AM, Schulman IH, Pujol MV. et al. Allogeneic Mesenchymal Stem Cells Ameliorate Aging Frailty: A Phase II Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Gerontol A Biol Sci Med Sci. 2017;72:1513-22
126. Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D. et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360-8
127. Tisato V, Naresh K, Girdlestone J, Navarrete C, Dazzi F. Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft-versus-host disease. Leukemia. 2007;21:1992-9
128. Doron G, Klontzas ME, Mantalaris A, Guldberg RE, Temenoff JS. Multiomics characterization of mesenchymal stromal cells cultured in monolayer and as aggregates. Biotechnol Bioeng. 2020;117:1761-78
129. Hu C, Zhao L, Wu D, Li L. Modulating autophagy in mesenchymal stem cells effectively protects against hypoxia- or ischemia-induced injury. Stem Cell Res Ther. 2019;10:120
130. Musiał-Wysocka A, Kot M, Majka M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019;28:801-12
131. Zhang J, Huang X, Wang H, Liu X, Zhang T, Wang Y. et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther. 2015;6:234
132. Zhang Y, Xu J, Liu S, Lim M, Zhao S, Cui K. et al. Embryonic stem cell-derived extracellular vesicles enhance the therapeutic effect of mesenchymal stem cells. Theranostics. 2019;9:6976-90
133. Ahmed AS, Sheng MH, Wasnik S, Baylink DJ, Lau KW. Effect of aging on stem cells. World J Exp Med. 2017;7:1-10
134. Payne KA, Didiano DM, Chu CR. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage. 2010;18:705-13
135. Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14
136. Ganguly P, El-Jawhari JJ, Giannoudis PV, Burska AN, Ponchel F, Jones EA. Age-related Changes in Bone Marrow Mesenchymal Stromal Cells: A Potential Impact on Osteoporosis and Osteoarthritis Development. Cell Transplant. 2017;26:1520-9
137. Han J, Liu JY, Swartz DD, Andreadis ST. Molecular and functional effects of organismal ageing on smooth muscle cells derived from bone marrow mesenchymal stem cells. Cardiovasc Res. 2010;87:147-55
138. Ueyama H, Horibe T, Hinotsu S, Tanaka T, Inoue T, Urushihara H. et al. Chromosomal variability of human mesenchymal stem cells cultured under hypoxic conditions. J Cell Mol Med. 2012;16:72-82
139. Froelich K, Mickler J, Steusloff G, Technau A, Ramos Tirado M, Scherzed A. et al. Chromosomal aberrations and deoxyribonucleic acid single-strand breaks in adipose-derived stem cells during long-term expansion in vitro. Cytotherapy. 2013;15:767-81
140. Bagley RG, Weber W, Rouleau C, Yao M, Honma N, Kataoka S. et al. Human mesenchymal stem cells from bone marrow express tumor endothelial and stromal markers. Int J Oncol. 2009;34:619-27
141. Li W, Zhou Y, Yang J, Zhang X, Zhang H, Zhang T. et al. Gastric cancer-derived mesenchymal stem cells prompt gastric cancer progression through secretion of interleukin-8. J Exp Clin Cancer Res. 2015;34:52
142. Hmadcha A, Martin-Montalvo A, Gauthier BR, Soria B, Capilla-Gonzalez V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front Bioeng Biotechnol. 2020;8:43
143. Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N. et al. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36-45
144. Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Tirino V. Concise Review: Cancer Cells, Cancer Stem Cells, and Mesenchymal Stem Cells: Influence in Cancer Development. Stem Cells Transl Med. 2017;6:2115-25
145. Kabashima-Niibe A, Higuchi H, Takaishi H, Masugi Y, Matsuzaki Y, Mabuchi Y. et al. Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci. 2013;104:157-64
146. Zhao X, Wu X, Qian M, Song Y, Wu D, Zhang W. Knockdown of TGF-β1 expression in human umbilical cord mesenchymal stem cells reverts their exosome-mediated EMT promoting effect on lung cancer cells. Cancer Lett. 2018;428:34-44
Author contact
![]() Corresponding authors: Hua Jiang, Ph.D. Department of Geriatrics, Shanghai East Hospital, Tongji University School of Medicine. Address: 150 Jimo Road, Shanghai 200123, China. E-mail: huajiang2013edu.cn; Hailiang Liu, Ph.D. Institute for Regenerative Medicine, Shanghai East Hospital, Tongji University School of Medicine. Address: 150 Jimo Road, Shanghai 200123, China. E-mail: hailiang_1111edu.cn.
Corresponding authors: Hua Jiang, Ph.D. Department of Geriatrics, Shanghai East Hospital, Tongji University School of Medicine. Address: 150 Jimo Road, Shanghai 200123, China. E-mail: huajiang2013edu.cn; Hailiang Liu, Ph.D. Institute for Regenerative Medicine, Shanghai East Hospital, Tongji University School of Medicine. Address: 150 Jimo Road, Shanghai 200123, China. E-mail: hailiang_1111edu.cn.
 Global reach, higher impact
Global reach, higher impact