13.3
Impact Factor
Theranostics 2021; 11(12):5634-5649. doi:10.7150/thno.57243 This issue Cite
Research Paper
An exosomal-carried short periostin isoform induces cardiomyocyte proliferation
1. Laboratory of Cellular and Molecular Cardiology, Istituto Cardiocentro Ticino, Lugano, Switzerland.
2. Center for Molecular Cardiology, University of Zurich, Zurich, Switzerland.
3. Laboratory of Cardiovascular Research, Lausanne University Hospital, Lausanne, Switzerland.
4. Victor Babes National Institute of Pathology, Bucharest, Romania.
5. Laboratory for Cardiovascular Theranostics, Istituto Cardiocentro Ticino, Lugano, Switzerland.
6. Department of Clinical Gene Therapy, Osaka University Graduate School of Medicine, Suita, Osaka, Japan.
7. Proteomics and Metabolomic Lab, ITB-CNR, Segrate, Italy.
8. Royal Brompton & Harefield Hospital, Imperial College, London, UK.
9. Institute of Life Science, Scuola Superiore Sant'Anna, Pisa, Italy.
10. Faculty of Biomedicine, Università della Svizzera Italiana (USI), Lugano, Switzerland.
Abstract
Although a small number of cardiomyocytes may reenter the cell cycle after injury, the adult mammalian heart is incapable of a robust cardiomyocyte proliferation. Periostin, a secreted extracellular matrix protein, has been implicated as a regulator of cardiomyocyte proliferation; however, this role remains controversial. Alternative splicing of the human periostin gene results in 6 isoforms lacking sequences between exons 17 and 21, in addition to full-length periostin. We previously showed that exosomes (Exo) secreted by human cardiac explant-derived progenitor cells (CPC) carried periostin. Here, we aimed to investigate their cell cycle activity.
Methods: CPC were derived as the cellular outgrowth of ex vivo cultured cardiac atrial explants. Exo were purified from CPC conditioned medium using size exclusion chromatography. Exosomal periostin was analyzed by Western blotting using a pair of antibodies (one raised against aa 537-836, and one raised against amino acids mapping at exon 17 of human periostin), by ELISA, and by cryo-EM with immune-gold labeling. Cell cycle activity was assessed in neonatal rat cardiomyocytes, in human induced pluripotent stem cell (iPS)-derived cardiomyocytes, and in adult rat cardiomyocytes after myocardial infarction. The role of periostin in cell cycle activity was investigated by transfecting donor CPC with a siRNA against this protein.
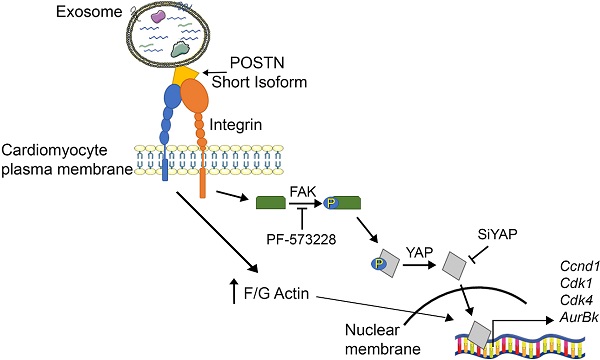
Results: Periostin expression in CPC-secreted exosomes was detected using the antibody raised against aa 537-836 of the human protein, but not with the exon 17-specific antibody, consistent with an isoform lacking exon 17. Periostin was visualized on vesicle surfaces by cryo-EM and immune-gold labeling. CPC-derived exosomes induced cell proliferation in neonatal rat cardiomyocytes both in vitro and in vivo, in human iPS-derived cardiomyocytes, and in adult rat cardiomyocytes after myocardial infarction. Exo promoted phosphorylation of focal adhesion kinase (FAK), actin polymerization, and nuclear translocation of Yes-associated protein (YAP) in cardiomyocytes. Knocking down of periostin or YAP, or blocking FAK phosphorylation with PF-573228 nullified Exo-induced proliferation. A truncated human periostin peptide (aa 22-669), but not recombinant human full-length periostin, mimicked the pro-proliferative activity of exosomes.
Conclusions: Our results show, for the first time, that CPC-secreted exosomes promote cardiomyocyte cell cycle-reentry via a short periostin isoform expressed on their surfaces, whereas recombinant full-length periostin does not. These findings highlight isoform-specific roles of periostin in cardiomyocyte proliferation.
Keywords: extracellular vesicles, exosomes, periostin, isoforms, cardiomyocyte, proliferation, Hippo pathway
 Global reach, higher impact
Global reach, higher impact