13.3
Impact Factor
Theranostics 2021; 11(11):5525-5538. doi:10.7150/thno.57510 This issue Cite
Research Paper
Homogeneous tumor targeting with a single dose of HER2-targeted albumin-binding domain-fused nanobody-drug conjugates results in long-lasting tumor remission in mice
1. Cell Biology, Neurobiology and Biophysics, Department of Biology, Faculty of Science, Utrecht University, Utrecht, The Netherlands.
2. LinXis B.V., Amsterdam, The Netherlands.
3. Pharmaceutics, Department of Pharmaceutical Sciences, Faculty of Science, Utrecht University, Utrecht, The Netherlands.
Abstract
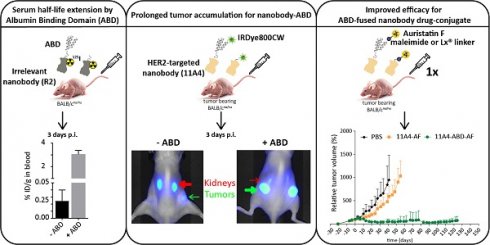
Background: The non-homogenous distribution of antibody-drug conjugates (ADCs) within solid tumors is a major limiting factor for their wide clinical application. Nanobodies have been shown to rapidly penetrate into xenografts, achieving more homogeneous tumor targeting. However, their rapid renal clearance can hamper their application as nanobody drug conjugates (NDCs). Here, we evaluate whether half-life extension via non-covalent interaction with albumin can benefit the efficacy of a HER2-targeted NDC.
Methods: HER2-targeted nanobody 11A4 and the irrelevant nanobody R2 were genetically fused to an albumin-binding domain (ABD) at their C-terminus. Binding to both albumin and tumor cells was determined by ELISA-based assays. The internalization potential as well as the in vitro efficacy of NDCs were tested on HER2 expressing cells. Serum half-life of iodinated R2 and R2-ABD was studied in tumor-free mice. The distribution of fluorescently labelled 11A4 and 11A4-ABD was assessed in vitro in 3D spheroids. Subsequently, the in vivo distribution was evaluated by optical molecular imaging and ex vivo by tissue biodistribution and tumor immunohistochemical analysis after intravenous injection of IRDye800-conjugated nanobodies in mice bearing HER2-positive subcutaneous xenografts. Finally, efficacy studies were performed in HER2-positive NCI-N87 xenograft-bearing mice intravenously injected with a single dose (250 nmol/kg) of nanobodies conjugated to auristatin F (AF) either via a maleimide or the organic Pt(II)‑based linker, coined Lx®.
Results: 11A4-ABD was able to bind albumin and HER2 and was internalized by HER2 expressing cells, irrespective of albumin presence. Interaction with albumin did not alter its distribution through 3D spheroids. Fusion to ABD resulted in a 14.8-fold increase in the serum half-life, as illustrated with the irrelevant nanobody. Furthermore, ABD fusion prolonged the accumulation of 11A4-ABD in HER2-expressing xenografts without affecting the expected homogenous intratumoral distribution. Next to that, reduced kidney retention of ABD-fused nanobodies was observed. Finally, a single dose administration of either 11A4-ABD-maleimide-AF or 11A4-ABD-Lx-AF led to long-lasting tumor remission in HER2-positive NCI-N87 xenograft-bearing mice.
Conclusion: Our results demonstrate that genetic fusion of a nanobody to ABD can significantly extend serum half-life, resulting in prolonged and homogenous tumor accumulation. Most importantly, as supported by the impressive anti-tumor efficacy observed after a single dose administration of 11A4-ABD-AF, our data reveal that monovalent internalizing ABD-fused nanobodies have potential for the development of highly effective NDCs.
Keywords: nanobody, single-domain antibodies, albumin-binding domain, half-life extension, nanobody-drug conjugates
 Global reach, higher impact
Global reach, higher impact