13.3
Impact Factor
Theranostics 2021; 11(11):5430-5446. doi:10.7150/thno.53961 This issue Cite
Review
Sialylated immunoglobulin G: a promising diagnostic and therapeutic strategy for autoimmune diseases
1. Department of Dermatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
2. Hubei Engineering Research Center for Skin Repair and Theranostics, Wuhan, China.
3. Britton Chance Center for Biomedical Photonics at Wuhan National Laboratory for Optoelectronics-Hubei Bioinformatics & Molecular Imaging Key Laboratory, Systems Biology Theme, Department of Biomedical Engineering, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, China.
4. Department of Dermatology, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology (HUST), Wuhan, China.
*These authors contributed equally to this work.
Received 2020-9-30; Accepted 2021-3-4; Published 2021-3-13
Abstract
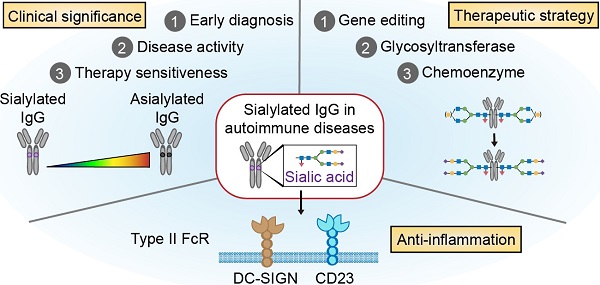
Human immunoglobulin G (IgG), especially autoantibodies, has major implications for the diagnosis and management of a wide range of autoimmune diseases. However, some healthy individuals also have autoantibodies, while a portion of patients with autoimmune diseases test negative for serologic autoantibodies. Recent advances in glycomics have shown that IgG Fc N-glycosylations are more reliable diagnostic and monitoring biomarkers than total IgG autoantibodies in a wide variety of autoimmune diseases. Furthermore, these N-glycosylations of IgG Fc, particularly sialylation, have been reported to exert significant anti-inflammatory effects by upregulating inhibitory FcγRIIb on effector macrophages and reducing the affinity of IgG for either complement protein or activating Fc gamma receptors. Therefore, sialylated IgG is a potential therapeutic strategy for attenuating pathogenic autoimmunity. IgG sialylation-based therapies for autoimmune diseases generated through genetic, metabolic or chemoenzymatic modifications have made some advances in both preclinical studies and clinical trials.
Keywords: sialylation, immunoglobulin G, glycosylation, autoimmune diseases, precision medicine
Introduction
Autoimmune diseases are a heterogeneous group of debilitating and painful conditions characterized by immune dysregulation resulting in inflammation and multiorgan involvement [1-3]. The presence of pathogenic autoantibodies is one of the characteristics of autoimmune diseases and is usually regarded as a diagnostic biomarker [4,5]. In addition to early diagnosis, these biomarkers could provide clinicians with useful information about risk stratification, therapeutic efficacy, and prognosis. However, it has been reported that up to 20-30% of the healthy population is positive for antinuclear antibodies [6]. Moreover, patients with certain conditions, such as laboratory sine syndromes and anti-citrullinated protein antibody (ACPA)-negative rheumatoid arthritis (RA), are negative for commonly specific autoantibodies [7]. For example, 15-25% of RA patients are negative for rheumatoid factor (RF) and ACPA [8,9].
In addition, although the advent and development of biologic agents, especially IgG-type monoclonal antibodies (mAbs), marks an unparalleled opportunity to advance therapeutic strategies for rheumatic diseases, some of these recombinant antibodies might aggravate the inflammatory response or induce new onset of pathological conditions. The exacerbation or new onset of psoriasis-like skin lesions is a common adverse event associated with TNF-α inhibitors [10,11]. Nearly 2-5% of patients with Crohn's disease and RA receiving anti-TNF-α mAbs (infliximab, adalimumab and etanercept) were associated with an increased risk of paradoxical psoriasis due to prolonged type I interferon production by immature plasmacytoid dendritic cells (pDCs) in the dermis [10,12]. Thus, there is an urgent need for the identification of more precise diagnostic biomarkers with higher sensitivity and specificity as well as more effective therapeutic strategies with fewer proinflammatory adverse events based on IgG-type autoantibodies and mAbs.
Glycosylation fingerprints are now considered critical regulators to fine-tune the steric conformation and function of IgG subclasses. All IgG molecules contain a biantennary oligosaccharide attached to the conserved N-linked glycosylation site (Asn-297) of each Fc-CH2 domain [13,14]. This glycan possesses a conserved heptamer consisting of N-acetylglucosamine (GlcNAc) and mannose residues, which can be further decorated by fucose, bisecting GlcNAc, galactose and sialic acid (Figure 1) [15-17]. Since the 1980s, mounting evidence has favored the presence of unique glycosylation patterns of IgG (mainly sialylation, fucosylation and galactosylation) in autoimmune diseases [18]. It is noteworthy that certain types of glycosylations of IgG, particularly sialylated autoantibodies, can help accurately diagnose antiphospholipid syndrome (APS) and RA [9,19]. In addition, decreased levels of IgG sialylation were observed in samples from RA patients compared to arthralgia samples and may predict therapy resistance in Kawasaki disease [20,21]. Furthermore, sialylated intravenous immunoglobulin (IVIg) and autoantibodies lead to significantly reduced joint inflammation or cytotoxicity of anti-platelet antibodies in RA or immune thrombocytopenic purpura (ITP) compared to those associated with their nonglycosylated counterparts [22]. Defucosylated anti-C-C chemokine receptor 4 (CCR4) IgG1 has been approved by the US Food and Drug Administration (FDA) for the treatment of relapsed or refractory mycosis fungoides or Sézary syndrome [23,24]. The presence of the above glycans further dictates specific Fc-Fc gamma receptor (FcγR) interactions, thus governing complement and immune cell activation in different ways [25,26]. Reduced core fucosylation was found in SLE, which resulted in an enhanced capacity to activate natural killer (NK) cells and macrophages [27,28]. In contrast, binding of the terminal sialic acid on IgG Fc to type II FcγRs can suppress proinflammatory effects, such as B cell receptor (BCR) signaling, by increasing inhibitory FcγRIIb expression [29]. Furthermore, the significant role played by defucosylation in promoting antitumor mAbs in antibody-dependent cellular cytotoxicity (ADCC) has been extensively demonstrated, while sialylated autoantibodies and/or IgG are a promising strategy during the process of diagnosing and treating autoimmune diseases because of their anti-inflammatory effect [30].
In this review, we summarize the influence of IgG Fc N-linked glycan in autoimmune diseases, focusing on recent advances in understanding the role of sialylated autoantibodies and IgG in clinical diagnosis and treatment. We also touch upon current glycoengineering approaches used in this area and future perspectives.
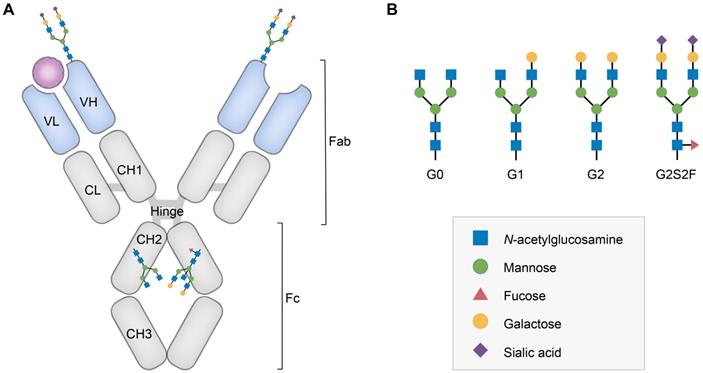
Structure and glycan composition of IgG. A. Schematic representation of immunoglobulin G with heavy chains and light chains in a general Y-shaped structure. Fab glycans are usually more galactosylated and sialylated but less fucosylated than Fc N-glycans. Fc glycans are rarely fully processed and can differ in composition between each CH2 domain of the same IgG molecule. B. The classification of Fc Asn-297 glycans by different glycoforms. N-glycans can also be galactosylated, sialylated or fucosylated, and these modifications alter the anti-inflammatory activity of IgG. The anti-inflammatory activity of immunoglobulin with different Fc N-glycan modifications increases from G0 to G2S2F. Ig, immunoglobulin; CH, constant heavy; CL, constant light; VH, variable heavy; VL, variable light; G, galactose; S, sialic acid; F, fucose.
IgG Fc and FcγRs
Structure and function of IgG Fc and FcγRs
The antigen-binding fragment (Fab) regions of IgG dictate target specificity, while the crystallizable fragment (Fc) regions contain interaction sites for sensors that elicit clearance, destruction or immunomodulatory signals [31]. Based on their binding stoichiometry and recognized Fc sites, FcγRs are mainly divided into types I and II, with distinct expression patterns across cell subsets and tissues [32]. Type I FcγRs fall within the immunoglobulin receptor superfamily and are classified as activating or inhibitory FcγRs on the basis of their intracellular domain signaling motifs [13,33]. Activating type I FcγRs bind Fc near its hinge-proximal region, driving proinflammatory signaling pathways [34,35]. The downstream effects of activating type I FcγRs include ADCC, antibody-dependent cellular phagocytosis (ADCP), leukocyte activation and proinflammatory cytokine release [15]. It is worth mentioning that FcγRIIb is the only inhibitory receptor among type I FcγRs, being able to antagonize and balance signals of activating FcγRI or BCR [31]. XmAb5871 is an engineered humanized anti-CD19 mAb that can also bind to FcγRIIb with 400-fold higher affinity than native IgG1 via its modified Fc. Thus, XmAb587 can suppress BCR signaling in B cells by promoting the coengagement of FcγRIIb with CD19. To date, it has realized promising therapeutic efficacy in a phase 2 clinical trial recruiting patients with IgG4-related diseases (NCT02725476) [36,37]. Of note, each FcγR has a unique pattern of cellular expression. For example, B cells express inhibitory FcγRIIb as their sole type I FcγRs, whereas NK cells exclusively express activating FcγRs (FcγRIIIa) [38]. FcγRIIIa on NK cells contributes to ADCC, while the upregulation of FcγRIIb on effector macrophages is achieved by sialylated IVIg [34]. Most other immune cells express a combination of diverse FcγRs, pairing activating and inhibitory receptors to trigger specific effector functions.
Type II FcγRs belong to the C-type lectin receptor family and have an extracellular oligomeric structure to bind Fc at the CH2-CH3 interface [34,35]. Dendritic cell-specific ICAM-3-grabbing nonintegrin (DC-SIGN, SIGN-R1 in mice) and CD23 are representative type II FcγRs. SIGN-R1 is expressed mainly on macrophages in mice, whereas DC-SIGN is expressed primarily by monocytes, dendritic cells and macrophages in humans [39]. CD23 exists in two splice variants, CD23a and CD23b, with their first seven amino acid residues differing. The former is constitutively expressed by mature B cells, whereas the latter is only expressed in association with IL-4 on a variety of leukocytes, including monocytes, macrophages, eosinophils and basophils [40]. Regarding functions, DC-SIGN plays a role in the anti-inflammatory activity of sialylated Fc by raising the threshold for myeloid cell activation, while CD23 mediates the selection of higher affinity antibodies for antigens by elevating FcγRIIb expression on B cells [29,41]. In addition to the above proteins, Fc domain-interacting proteins also include (1) C1q, the classical promoter of the complement cascade; (2) neonatal crystallizable fragment receptor (FcRn), a recycling or transcytosis receptor for IgG; and (3) tripartite motif-containing 21 (TRIM21), a cytosolic receptor required for antibody-dependent pathogen constraint [42,43]. Thus, the interaction between Fc and FcγRs can trigger diverse immune responses whose types are affected by the affinity of Fc.
Glycosylation of IgG can affect the binding affinity between Fc and FcγRs
It has been reported that different subclasses of IgG demonstrate distinct binding affinity for FcγRs. For example, IgG1 and IgG3 exhibit the highest affinity for type I FcγRs, while IgG2 and IgG4 have lower affinities [31,32,44]. In addition, Fc-associated glycoforms have been shown to profoundly impact these interactions. Although 10% of IgG3 molecules possess O-linked glycosylation at their hinge region, N-linked glycosylation can be found at Asn-297 of the CH2 domains of all other human IgG subclasses. The majority of these covalently attached N-glycans are complex-type biantennary structures containing a core heptasaccharide comprised of GlcNAc and mannose residues [45-47]. Variable addition or removal of sugar moieties (e.g., fucose, galactose, bisecting GlcNAc, or sialic acid) gives rise to Fc diversity. As a result, over 36 distinct glycans have been found in healthy individuals' sera [48].
The presence of these glycans is critical for the flexibility of the Fc regions, which is a determinant for FcγR binding affinity, and for maintaining the CH2 domains at a conformation suitable for Fc-FcγR interactions [32]. The affinity of core-fucosylated IgG1 for FcγRIIIa is 100-fold lower than that of the defucosylated version due to the steric hindrance between the Asn162-glycan of FcγRIIIa and the Asn297-linked carbohydrate of Fc chain A [49]. On the one hand, the fucose residue prohibits the protrusion of FcγRIIIa Asn162-glycan into the central cavity formed by two CH2 domains. Therefore, the dynamics of the receptor binding site could negatively regulate the Fc-FcγR interaction [50,51]. On the other hand, the reduction in the formation of hydrogen bonds between Fc-Gln295 and mannose 5 on receptor carbohydrates could also affect this interaction [49]. In addition, sialylation of Fc reduces its affinity for type I FcγRs, whereas its affinity for DC-SIGN or CD23 increases. Upon sialylation, the CH2 domain of Fc adopts a flexible conformation suitable for binding to type II FcγRs, while the binding site for canonical FcγRs near the hinge-proximal surface is occluded [52,53]. Overall, it is generally thought that IgG N-glycosylation profoundly affects immune responses and is involved in the occurrence and development of autoimmune disease.
Sialylated IgG in autoimmune diseases
N-Acetylneuraminic acid (Neu5Ac), also called sialic acid, is attached to the galactose residue and constitutes the nonreducing end of IgG Fc N-glycans [54]. The Neu5Ac-galactose linkage is an α2,6-linkage in humans and an α2,3-linkage in Chinese hamster ovary (CHO) cells [55]. Although serum IgG contains less than 15% sialylated IgG, sialylation has been shown to be a vital player in mediating anti-inflammatory activities in autoimmunity [16].
Sialylated IgG as a biomarker for diagnosis and monitoring
The IgG sialylation level changes during the development and progression of a wide variety of autoimmune diseases (Table 1) [54]. Low levels of sialylated glycans in serum total IgG or autoantibodies have been reported in a number of autoimmune diseases, including SLE, granulomatosis with polyangiitis (GPA), Kawasaki disease, RA, and Crohn's disease [20, 21, 56-58]. In 2015, Lauc et al. demonstrated that the levels of major sialylated glycans (FA2G1S1, FA2BG2S1, and FA2G2S2) in total IgG decreased in three SLE patient cohorts of different ethnicities (Latin American Mestizo, Trinidad and China) [56]. IgG1 and IgG2 sialylation also decreased in GPA, as confirmed by liquid chromatography coupled with mass spectrometry [57]. Su et al. observed a negative correlation between the serum levels of total sialylated IgG and RF autoantibodies in RA patients, suggesting that sialylation levels may be promising serum biomarkers for RA disease activity and clinical diagnosis [59].
Clinical significance of sialylated IgG in human autoimmune diseases
| Disease | Serum levels of sialylated IgG (s-IgG) | Clinical significance | Ref. |
|---|---|---|---|
| Rheumatoid arthritis (RA) | Total s-IgG↓ RF s-IgG↓ ACPA s-IgG↓ | Enhanced diagnostic sensitivity: Sulfated FA2G2S1 was identified as a biomarker to distinguish RA patients from the ACPA and RF-negative ones with high sensitivity Therapeutic response: Serum s-IgG was significantly elevated after methotrexate therapy in RA patients; Negative correlations of s-IgG were observed between DAS 28 and Sambucus nigra before and after therapy. | [9,59,65,77,78] |
| Juvenile idiopathic arthritis (JIA) | Total s-IgG↓ | Total IgG sialylation was reduced in JIA patients' sera compared with that of healthy controls | [79] |
| Systemic lupus erythematosus (SLE) | Total s-IgG↓ Anti-histone s-IgG↓ | Three major sialylated glycans in total IgG were decreased in SLE patients of African Caribbean populations, Latin Americans of Mestizo ethnicity and Han Chinese populations Disease intensity: Monosialylated glycans in total s-IgG were negatively associated with symptom profiles of SLE patients in African Caribbean and Latin American cohorts. | [56,80] |
| Anti-phospholipid syndrome (APS) | Anti-β2GP1 s-IgG↓ | Disease activity: A significant negative correlation was observed between BVAS score and the sialylation ratio of PR3-ANCA. Closing diagnostic gaps-asymptomatic individuals: Sera from healthy children showed the highest Sambucus nigra/anti-β2GP1 s-IgG ratio. The ratio decreased from aaPL to SLE + aPL to SAPS to PAPS. | [19,64] |
| Granulomatosis with polyangiitis (GPA) | Total s-IgG↓ PR3-ANCA s-IgG1↓ | Total s-IgG1 and s-IgG2 were reduced in GPA patients compared to healthy controls Enhanced diagnostic specificity: PR3-ANCA s-IgG was used to determine GPA activity with higher specificity and sensitivity than nonsialylated IgG. Relapse: Patients with low total s-IgG1 were highly prone to relapse after an ANCA increases. The degree of total s-IgG1 Fc was found to differentiate relapsing patients from nonrelapsing ones with high sensitivity and specificity. | [57,64,69] |
| Kawasaki disease (KD) | Total s-IgG↓ | Therapeutic response: IVIg-resistant KD patients had lower levels of total s-IgG than IVIg-responsive KD patients at both pretreatment and one-year time points | [20] |
| Crohn's disease (CD) | Total s-IgG↓ | The proportion of total s-IgG was significantly decreased in CD patients | [58,81] |
| Fetal and neonatal alloimmune thrombocytopenia (FNAIT) | Anti-HPA-1a s-IgG1↑ | Anti-HPA-1a s-IgG1 increased up to 30% compared to total IgG sialylation of less than 10% in FNAIT patients. | [73] |
| Hemolytic disease of the fetus and newborn (HDFN) | Anti-D s-IgG↑ Anti-c s-IgG1↑ | Disease activity: High Fc sialylation of anti-c was correlated with HDFN disease severity. | [75,82] |
| Chronic inflammatory demyelinating polyneuropathy (CIDP) | Total s-IgG Fc↓ | s-IgG Fc was reduced in CIDP patients Therapeutic response: Reduction in clinical disease severity scores upon IVIg therapy was significantly associated with an induction of s-IgG Fc. | [70,83] |
| Guillain-Barré syndrome (GBS) | s-IgG2↓ | Therapeutic response: IVIg therapy resulted in increased s-IgG1 and s-IgG2 in GBS patients A higher level of s-IgG1 and IgG2 Fc was associated with reduced disease severity and improved outcomes of GBS patients after IVIg therapy. | [70,84] |
| Alzheimer's disease (AD) | s-IgG1↓ | S-IgG1 (FA2G2S1) was reduced in AD patients compared to patients with SMCI | [85] |
| Parkinson's disease (PD) | Total s-IgG↓ | s-IgG sialylation decreased in PD patients Disease activity: FG2S1 was negatively associated with case status, with high sensitivity and specificity. | [86] |
ACPA, anti-citrullinated protein antibody; anti-βGP1, anti-beta-2-glycoprotein 1; BVAS, Birmingham vasculitis activity score; PR3-ANCA, anti-neutrophilic cytoplasmic autoantibodies targeting proteinase 3; aPL, antiphospholipid antibody; aaPL, asymptomatic carriers of aPL; SLE + aaPL, patients with SLE without symptoms of APS harboring circulating aPL; SAPS, patients with APS and SLE as an underlying disease; PAPS, patients with primary APS; IVIg, intravenous immunoglobulin; HPA, human platelet antigen; anti-D, anti-rhesus D (RhD); SMCI, stable mild cognitive impairment.
Anti-beta-2-glycoprotein 1 (anti-β2GP1) antibodies are commonly found in patients with APS and are associated with increased thrombotic risk [60]. However, anti-β2GP1 antibodies can be detected in 3% of asymptomatic healthy controls [61]. The frequency of anti-β2GP1 antibodies in healthy children was 6.6%, according to an investigation by Avcin and coworkers enrolling 61 subjects [62]. Therefore, biomarkers with higher specificity are needed to distinguish seropositive healthy individuals from asymptomatic patients. Fickentscher et al. found significantly decreased serum sialylation levels in APS patients and asymptomatic carriers compared with those in a healthy population, which indicated that hyposialylated anti-β2GP1 IgG could be a new measure to distinguish healthy and asymptomatic populations [19]. Serologic markers, such as RF and ACPA autoantibodies, are often used in the diagnosis of RA [8]. However, approximately 15-25% of RA patients are negative for either autoantibody. Utilizing an innovative platform based on TiO2-PGC chips, Wang and coworkers identified sulfated FA2G2S1 (termed SGm2), a sulfated sialylated glycan on serum IgG, as a biomarker for RF and ACPA-negative patients, with a high prediction accuracy of 88% [9]. Thus, altered sialylation could provide a better way to close the diagnostic gap in asymptomatic individuals and to enhance diagnostic sensitivity in autoimmune diseases where autoantibodies are less easy to detect.
Anti-proteinase 3 (anti-PR3) antibody, which is correlated with the oxidative burst of neutrophils, is a diagnostic and pathogenic hallmark of GPA [63]. Kemna et al. observed that the mean sialylation ratio of anti-PR3 antibodies not only decreased in GPA patients but was also inversely related to the Birmingham vasculitis activity score, indicating that the IgG sialylation level may be utilized to assess disease severity. Importantly, this reduced sialylation level was confirmed by an in vitro study showing that desialylated anti-PR3 antibodies could significantly increase the production of H2O2 by neutrophils, which contributes to GPA progression [64].
In addition to serving as a promising diagnostic biomarker complementing or even surpassing the present methods in some aspects, changes in sialylated IgG levels in serum could also have the potential to monitor therapeutic efficacy in autoimmune diseases. Gińdzieńska et al. found that the total serum IgG sialylation level was elevated after 12 months of methotrexate therapy in RA patients [65]. Engdahl et al. observed increased sialylation levels of both total IgG and ACPA autoantibodies in postmenopausal women with RA after estrogen (E2) replacement therapy [66]. In chronic inflammatory demyelinating polyneuropathy patients, sialylated IgG Fc was reported to be superior to other biomarkers for evaluating treatment response, such as polymorphism of the transient axonal glycoprotein-1 gene or expression of inhibitory FcγRIIb on B cells, due to its less time-consuming, easily accessible and reliable detection process [67].
In addition, sialylated IgG shows great promise for confirming remission, as well as predicting or confirming flares [68]. The prognostic capacity of the total IgG sialylation profile preceding relapse has been pronounced by Kemna et al. [69]. Total IgG1 sialylation in GPA patients started to decrease from the time of relapse, while it remained relatively unchanged in nonrelapsing patients, indicating that low sialyation of total IgG1 could predict disease relapse [69]. In Guillain-Barré syndrome (GBS), it has been observed that patients with higher galactosylated and sialylated IgG1 and IgG2 levels require ventilator support less often and that they require less time to recover walking ability six months after receiving a high-dose IVIg treatment regimen [70]. In contrast, patients with persistent low galactosylated and sialylated IgG1 and IgG2 levels had the most severe forms of GBS and showed poor response to IVIg treatment, suggesting that serum IgG Fc glycosylation in GBS is related to clinical recovery [70]. Similarly, a persistent low level of serum sialylated IgG predicted IVIg resistance in patients with Kawasaki disease [20].
However, negative or inconsistent observations have been reported. For example, Wuhrer et al. found no significant changes in IgG Fc glycosylation in serum samples from multiple sclerosis patients compared to samples from healthy controls [71]. Myrthe et al. observed that high serum Fc galactosylation and sialylation of anti-c autoantibodies positively correlated with disease severity (P=0.008 and P=0.039 respectively) in hemolytic disease of the fetus and newborn (HDFN). The increased galactosylation of anti-c is in line with studies showing a positive correlation between galactosylation and the binding of IgG1 to FcγRIIa and FcγRIIIa, ADCC activity and clinical outcome in fetal or neonatal alloimmune thrombocytopenia [72-74]. However, as no major effect of sialylation on FcγR binding affinity has been reported in the literature, this positive correlation may not be direct and could be explained by significantly increased levels of galactosylation, as well as the close relationship between galactose and sialic acid as terminal sugars [75]. Moreover, similar to other posttranslational modifications, IgG sialylation also has tissue specificity. Scherer et al. found low levels of sialylation in both total and ACPA IgG1 in RA patient sera, while decreased sialylation was found only in ACPA IgG1 in paired synovial fluid [76].
Sialylated IgG as a therapeutic strategy
Sialylated IgG is a dynamic marker that has value for precision medicine, considering its close relationship with disease presence, progression, recurrence and response to therapeutics. Changes in the level of sialylation are also factors that shape the development of certain autoimmune diseases. Sialylated IgG-based therapeutics developed through glycoengineering present an exciting approach, as they can reset the threshold for immune cell activation [87,88]. This strategy could correct rather than suppress the immune imbalance with minimal side effects and long-term efficacy [88]. In the past ten years, preclinical and clinical progress has been made (Table 2), as we describe in detail below.
Therapeutic efficacy of sialylated IVIg
IVIg consists of pooled IgG from healthy donor plasma and is approved by the FDA for the treatment of acute or chronic inflammatory diseases. Although over 100 tons of IVIg is consumed worldwide every year, its high costs and finite supply limit its clinical applications [89-91]. The immunomodulatory power of IVIg lies in its interference with multiple innate and adaptive cells. For example, IVIg induced autophagy in peripheral blood mononuclear cells, which restricted to monocytes, dendritic cells and M1 macrophages but not in M2 macrophages [92]. Phagocytic capabilities of human monocyte-derived or splenic macrophages were also inhibited by IVIg [93,94]. And IVIg activated Wnt-β-catenin pathway in human dendritic cells (DCs), while the transcription factor blocks inflammatory mediators and favored tolerogenic DCs [95]. As for adaptive cellular immunity, IVIg expanded Tregs via induction of cyclooxygenase-2-dependent prostaglandin E2 in human DCs [96]. Besides, mechanisms of IVIg activity have been subdivided into Fab- and Fc-dependent, considering IgG's function domains. Among them, sialic acid-enriched Fc has been demonstrated as an active component of high-dose IVIg with potent anti-inflammatory properties [39]. In 2006, Kaneko and coworkers fractionated sialic acid-modified structures from IVIg through a Sambucus nigra agglutinin (SNA)-lectin affinity column. They found that 0.1 g/kg SNA IVIg could function as efficiently as 1 g/kg unfractionated IVIg [97]. Recent evidence has also confirmed that sialylated IgG is indispensable for protecting mice from allergic bronchopulmonary aspergillosis, as desialylated IVIg fails to suppress Th2, Th17 and IgE responses [98]. In vitro, sialylated IVIg inhibited complement activation in sera from patients with Guillain-Barré syndrome, Miller Fisher syndrome and multifocal motor neuropathy, while galactosylated, degalactosylated, or deglycosylated IVIg could not [99]. Taking advantage of glycosyltransferase engineering, both α2,6 sialyltransferase (ST) recombinant Fc (rFc) and α2,3ST rFc were generated. Surprisingly, a one-thirtieth dosage of α2,6ST rFc reduced joint swelling to an extent similar to 1 g/kg IVIg, with increased IL-33 production and FcγRIIb upregulation in a mouse model of arthritis [22,28]. Despite these benefits, however, minor sialylated IgG still lacks quantitative controls in the manufacturing process. In 2015, Nathaniel et al. prepared tetrasialylated IVIg (s4-IVIg) with minimal byproducts using a flow-through method. S4-IVIg showed at least 10-fold enhanced anti-inflammatory activities in mouse models of CIA, K/BxN arthritis, ITP, and epidermolysis bullosa acquisita, subsequently inhibiting the progression of the diseases [100]. Of note, this process generating sialylated IgG products with consistent anti-inflammatory efficacy is suitable for clinical development. Furthermore, M254, the commercial derivative of s4-IVIg, is undergoing a randomized clinical trial (NCT03866577) [101]. This trial is evaluating the safety, tolerability, pharmacokinetics, and pharmacodynamics of M254 in a total of 70 healthy volunteers and ITP patients. Additionally, the outcome of a head-to-head comparison between M254 and IVIg remains to be seen [102]. In addition to the abovementioned glycoengineering strategies, Fiebiger and coworkers generated an IgG Fc variant with a point mutation at residue F241 (F241A), which mimics the conformation of sialylated IgG. Mechanistically, in contrast to the increased expression of FcγRIIb through α2,6ST rFc, F241A protected against the development of experimental autoimmune encephalomyelitis (EAE) in a mouse model, along with the expansion of Treg cells and the suppression of CD4+ effector T cells in the draining lymph nodes. Treg cell-depleted mice were devoid of F241 protection. Therefore, this point mutation F241A suppresses the pathogenic CD4+ T cell response by activating and expanding Treg cells [103].
However, it has to mention that the necessity of sialylated Fc has also met challenges in IVIg-mediated anti-inflammatory effects. Recent studies included Fc-sialylation-independent mechanisms of IVIg actions in the therapeutic collagen Ab-induced arthritis, EAE and ITP mouse model, as no difference in the therapeutic ability of IVIg was observed between sialic acid-enriched and -depleted groups [104-106]. Additional studies from a human in vitro whole blood assay, found anti-inflammatory effects were most robust with Fab-sialylated fractions, rather than Fc-sialylated fragments [107]. It is likely that more than one of the IVIg potential modes, together with pathogenetic heterogeneity from disease to disease, accounts for these differences.
Although most therapeutic attempts to enhance sialylation of both total IgG and autoantibodies are still in the preclinical stage, they have shown great potential to weaken and transform pathogenic antibodies and to reset the immune threshold. It is certain that additional drug candidates will emerge in the near future through advanced glycoengineering. It would be intriguing to unravel the broader indications and accurate usages of sialylated IVIg and autoantibodies, as well as the feasibility of their use in humans.
Therapeutic efficacy of sialylated endogenous IgG and pathogenic autoantibodies
In addition to exogenous IgG supplementation, studies have also been conducted to increase the sialylation of endogenous antibodies. For instance, collagen-induced arthritis (CIA) mice were orally fed the sialic acid precursor N-acetylmannosamine (ManNAc) by Ulrike et al., which significantly increased serum IgG1 Fc sialylation. ManNAc-fed mice had a decreased CIA incidence, delayed arthritis onset and mitigated bone loss compared with mannose-fed or water-fed mice [108]. This finding demonstrated a protective role of sialylated endogenous IgG against immune-mediated bone loss.
Autoantibodies are thought to be more crucial drivers in the pathogenesis of autoimmune diseases than total IgG [109]. Compared with untreated antigen-induced arthritis (AIA)-IgG, the administration of sialylated (AIA) IgG before injecting methylated bovine serum albumin into BALB/c mouse knee joints could reduce arthritogenic activity and CIA severity [108]. Consistently, sialylated anti-2,4,6-trinitrophenyl IgG1 could extend the survival of mice with nephrotoxic nephritis (NTN) beyond 18 days [110]. By fusing human IgG1 Fc to ST6GAL1 and/or B4GALT1, a soluble form of the membrane proteins was engineered by Anthony et al. B4ST6FC but not B4Fc or ST6Fc led to an increase in sialylation of pathogenic IgG in K/BxN-treated mouse paws and NTN-induced mouse kidneys compared to that in mice treated with PBS or IVIg. Mechanistically, the anti-inflammatory activities of B4ST6Fc require the activation of DC-SIGN and STAT6 as well as the inhibitory FcγRIIb signaling pathway [87,111]. Additionally, Ohmi and coworkers demonstrated that the observed anti-inflammatory effects were specific to citrullinated collagen type II (Col II) [78].
Therapeutic effect of IgG sialylation in experimental models of autoimmune diseases
| Sialylated IgG | Method | Autoimmune disease | Outcome in vivo | Mechanism | Ref. |
|---|---|---|---|---|---|
| SNA-enriched IVIg | Fractionate IVIg on an SNA-lectin affinity column | RA | The reduction in clinical scores of arthritis was enhanced | Anti-inflammatory activity was enhanced through increased expression of inhibitory FcγRIIb on effector macrophages | [99] |
| α2,6 ST IVIg or rFc | Treat Fc with α2,3/6 sialidase, β1,4 GT and α2,3 or α2,6 ST in turn | RA | The reduction in clinical scores of arthritis was enhanced | Sialylation of Fc altered the binding affinity to FcγR, leading to a reduced ratio of binding to activating over inhibitory FcγRs | [22] |
| Sialylated 6A6-IgG2b | ITP | Platelet counts were significantly increased in vivo | Cytotoxicity of 6A6-IgG2b-mediated platelet consumption was reduced | ||
| Sialylated AIA-IgG | Incubate IgG with CMP-sialic acid and α2,6ST | RA | Sialylated AIA-IgG showed excellent efficacy in preventing arthritis progression | Osteoclast differentiation and bone loss were prevented | [108] |
| Sialylated anti-Col II IgG1 | Incubate IgG1 with β1,4 GT and α2,6 ST in the presence of UDP-galactose and CMP-sialic acid | RA | Sialylated Col II-reactive IgG autoAbs reduced the mean clinical score of arthritis symptoms | Activation of inflammatory DC and subsequent Th17 cell differentiation were inhibited and production of proinflammatory cytokines, such as IL-6 and IL-17, was reduced | [110] |
| Sialylated anti-TNP IgG1 | NTN | Sialylated anti-TNP IgG1 in ICs reduced nephritis-induced mortality | |||
| Sialylated anti-Col II antibody | Transfect mB4galt1 and mSt6gal1 cDNAs into anti-Col II lgG1 hybridomas | RA | Sialylated anti-Col II IgG antibodies prevented the development and progression of CAIA | Sialylated antibody treatment significantly increased the sialylation levels of anti-Col II IgG during an antigen-specific event, thus inducing regulatory activity | [78] |
| Sialylated autoantibodies | Generate recombinant glycosyltransferase enzymes by fusing human lgG1 Fc to ST6GAL1 and B4GALT1 | RA | Elevated levels of sialylated autoantibodies induced by B4ST6Fc reduced joint inflammation | B4ST6Fc converted pathogenic IgG into anti-inflammatory IgG | [111] |
| NTN | BUN level and kidney damage were reduced, and survival rate was increased | ||||
| s4 IVIg | Incubate recombinant human IgG1 Fc with β1,4 GT and α2,6 ST in the presence of UDP-galactose and CMP-sialic acid | RA | Enhanced potency when treated prophylactically compared to that of conventional IVIg | Neutrophil infiltration in ankle joints was inhibited | [100] |
| ITP | Platelet levels were restored with enhanced efficacy | ||||
| EBA | Therapeutic effect was enhanced when dosed prophylactically | Recruitment of inflammatory effector cells was inhibited and skin inflammation was reduced | |||
| M254 (commercialized s4-IVIg) | Same as above | ITP | An ongoing randomized clinical trial (NCT03866577) is enrolling patients with ITP, expected to be completed in 2021 | [102] |
SNA, Sambucus nigra agglutinin; IVIg, intravenous immunoglobulin; RA, rheumatoid arthritis; rFc, recombinant Fc; ST, sialyltransferase; GT, galactosyltransferase; ITP, idiopathic thrombocytopenic purpura; CMP, cytidine monophosphate; UDP, uridine 5'-diphosphate; AIA, antigen-induced arthritis; Col II, collagen type II; TNP, 2,4,6-trinitrophenyl; IC, immune complex; IL, interleukin; Th, T helper; BUN, blood urea nitrogen; NTN, nephron toxic nephritis; EBA, epidermolysis bullosa acquisita.
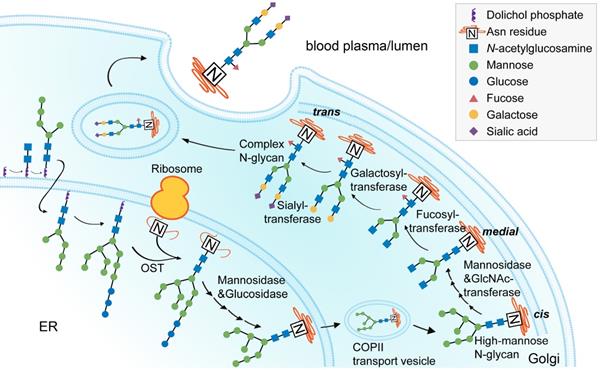
Process of IgG N-glycan biosynthesis in the secretory pathway. The process of N-glycosylation begins in the endoplasmic reticulum (ER) and ends in the Golgi. A lipid-linked precursor oligosaccharide is synthesized and transferred to the Asn residue in the ER, followed by initial trimming, transfer to the Golgi, modification with terminal sugar residues, and finally secreted into the lumen or blood plasma. OST, oligosaccharyltransferase.
Mechanisms of the anti-inflammatory effects of sialylated IgG in autoimmune diseases
ST6GAL1 is the key enzyme involved in the process and biosynthesis of sialylated IgG
The intracellular process of glyco1sylation begins in the endoplasmic reticulum and ends in the Golgi (Figure 2), during which sialylation is catalyzed by sialyltransferases [45,112]. ST6GAL1 is the rate-limiting enzyme that transfers α2,6-linked sialic acids from a donor to the oligosaccharide chains of a glycoprotein [113,114]. There are two forms of ST6GAL1: the secreted, soluble form and the membrane-bound form. The soluble form of ST6GAL1 can be produced by cells lining the central veins of the liver, with enzymatic activity to catalyze IgG sialylation in circulation, while the membrane-bound form of ST6GAL1 is widely expressed in B cells and typically found in the Golgi apparatus [115]. The two forms of ST6GAL1 can both be altered by inflammatory challenges under autoimmune conditions. Hormone levels are associated with notable changes in this enzyme. In ovariectomized mice, E2 increased ST6GAL1 expression in plasmablasts as well as Fc sialylation of ovalbumin-specific IgG [66]. In addition to hormones, aberrant blood factors have also been reported. In the CIA mouse model, activated T helper 17 (Th17) cells regulated ST6GAL1 expression on newly differentiated antibody-producing cells in an interleukin-21 (IL-21)- and IL-22-dependent manner, which subsequently changed the glycosylation profile and the activity of IgG produced by plasma cells.
This sequence of events clearly shows the correlation between the glycosylation machinery of B cells and inflammation initiation [116].
Mechanisms of the anti-inflammatory effects of sialylated IgG
To understand how sialylated IgG exerts anti-inflammatory effects in autoimmune diseases, two main cell subsets should be considered: myeloid cells and B cells.
Several studies have demonstrated that a high level of IgG Fc sialylation was associated with decreased ADCC or complement-dependent cytotoxicity (CDC) activity due to the reduced affinity of human polyclonal IgG1 for FcγRIIIa or C1q (Figure 3A) [83,117]. Compared with the asialylated G2F glycovariant, S2G2F IgG showed reduced affinity for the FcγRIIIa-V158 and F158 alleles. Thus, compared with administration of the core-fucosylated mAb in FcγR-humanized mice, S2G2F anti-mCD4 administration could prevent CD4+ T cell depletion in the blood [26]. Researchers have proposed the requirement of sialylated IgG Fc with SIGN-R1 in models of inflammatory arthritis and skin blistering diseases under therapeutic conditions by using either mice deficient in SIGNR1 or SIGNR1-blocking Abs [118]. The capability of its human ortholog DC-SIGN to replace SIGN-R1 in sialylated IVIg-mediated therapeutics effects has been verified in K/BxN arthritis models [41]. However, the indispensability of these receptors has been challenged, as different groups have reported mouse SIGNR1 or its human ortholog DC-SIGN-independent protective effects of IVIg. For example, IVIg activity remained in SIGNR1-deficient mice or splenectomized C57BL/6 mice lacking SIGNR1-positive splenic marginal zone macrophages in ITP under therapeutic treatment conditions [118]. Glycan array studies indicated that Fab fragments of IVIg were responsible for the binding between hypersialylated IgG and DC-SIGN [119]. Moreover, Massoud et al. observed the interaction of sialylated IVIg with dendritic cell immunoreceptors (DCIRs) constructed on DCs, mediating Treg cell expansion and airway hyperresponsiveness attenuation [120]. These differences may be attributed to the involvement of different immunoregulatory pathways between humans and mice, as well as the existence of disease-specific mechanisms for given clinical states.
After the engagement of human DC-SIGN with sialylated IgG, a Th2-dependent pathway was triggered, involving the production of IL-33 and the expansion of IL-4-produing basophils. Upon stimulation with IL-4, effector macrophages upregulated the expression of inhibitory FcγRIIb and increased the threshold of immune complex (IC)-mediated activation [41]. Again, differences between model systems have been noted. Plasma IL-33 of rheumatic patients increased after infused with high-dose IVIg but was insufficient to mediate basophil expansion [121]. Similarly, expansion of peripheral Treg cells, but not the up-regulation of plasma IL-33, was associated with clinical recovery following IVIg therapy in GBS patients [122]. Recent evidence also showed that IVIg activated IL-3-primed human basophils and subsequent IL-4 secretion by directly interacting with surface-bound IgE Fab fragments independent of IL-33, FcγRIIb and C-type lection receptors [123]. Overall, the action of Fc-sialylated IVIg does not involve a single mechanism. These board mechanisms need to be tested in distinct and specific models of autoimmune disorders.
In addition, Th17 cells also seem to be associated with downstream pathways. Bartsch et al. found that sialylated anti-Col II IgG1 antibodies significantly attenuated Col II-induced arthritis in FcγRIIb-deficient mice, with decreased accumulations of Th17 cells in the popliteal and brachial lymph nodes, in line with the in vitro finding that coculturing bone marrow-derived DCs from FcγRIIb-deficient mice with sialylated ICs inhibited secretion of IL-6, the critical cytokine for Th17 generation [110]. However, additional studies are still required to identify the exact mechanism through which sialylated IgG impacts Th17 cells.
The function and underlying mechanisms of sialylated IgG (s-IgG) in myeloid and B cells. A. Upon IL-33 secretion by myeloid cells after s-IgG binding to DC-SIGN/SIGN-R1 (1), basophils produce IL-4, thus upregulating inhibitory FcγRIIb on CSF1-independent effector macrophages and resulting in increased anti-inflammatory effects. Additionally, CDC and ADCC activity decreases due to the reduced affinity of s-IgG for C1q (2) and activating FcγRs (3), respectively, which lead to a reduced proinflammatory effect. B. s-IgG can bind to CD23 (4) and increase the expression of inhibitory FcγRIIb on B cells. S-IgGs can also bind to CD22 (5) in an Fc-dependent manner and induce inhibitory SHP-1 recruitment, which inhibits BCR and TLR signaling pathways in B cells, further inhibiting the NF-κB signaling pathway, downregulating the expression of MHC II, CD40, CD80, and CD86 molecules and decreasing the expression of IL-6. ADCC, antibody-dependent cellular cytotoxicity; NK cell, natural killer cell; MBL, mannose-binding lectin; CDC, complement-dependent cytotoxicity; DC-SIGN, dendritic cell-specific ICAM-3-grabbing nonintegrin; SIGN-R1, specific ICAM-3-grabbing nonintegrin-receptor 1; IL, interleukin; CSF1, colony-stimulating factor 1; CD, cluster of differentiation; BCR, B cell receptor; SHP-1, SH2 domain-containing phosphatase 1; PAMP, pathogen-associated molecular pattern; MHC, major histocompatibility complex; TLR, toll-like receptor; NF-κB, nuclear factor-κB.
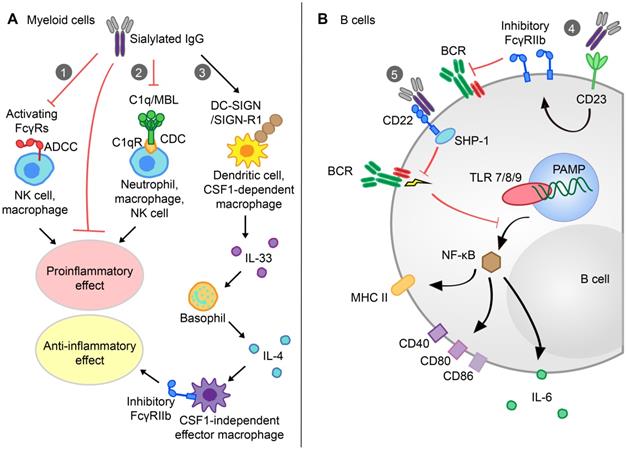
B cells can also be specifically modulated by sialylated IgG (Figure 3B). CD23 is a type II FcγR expressed on activated B cells. Wang et al. elaborated that after sialylated Fc glycans (sFc) bound to CD23, the inhibitory FcγRIIb on activated B cells was elevated, which resulted in B cells selecting higher affinity BCRs [29]. CD22 is another inhibitory receptor expressed by B cells. Seite and colleagues observed that specific binding of sialylated IVIg to CD22 inhibited the BCR signaling pathway by downregulating tyrosine phosphorylation on numerous kinases [124]. After treatment with sialylated IVIg, B cells became nonresponsive, with decreased antigen presentation ability, reduced expression of costimulatory molecules (MHC II, CD40, CD80, and CD86) and decreased secretion of inflammatory cytokines (IL-6/IL-10).
Other glycans
In addition to sialylation, IgG fucosylation or galactosylation has been widely documented across various autoimmune diseases [15]. Glycosyltransferases conjugate sugar moieties to nascent proteins and lipids, thus generating a wide variety of glycans [125]. Meta-analysis of genome-wide association studies identified four glycosyltransferase (ST6GAL1, B4GALT1, FUT8 and MGAT3)-coding loci that are strongly associated with autoimmune conditions [126].
Galactose
Galactose, which links to GlcNAc, is a widely studied glycan moiety in autoimmunity [54]. Since the 1980s, the majority of galactosylation-related studies have focused on the impact and correlation of decreased IgG N-linked galactosylation in RA. A high percentage of IgG G0 (no galactose) glycoforms was positively correlated with the RA state and severity, which reverted to normal levels upon MTX treatment or pregnancy-induced remission [65,127-129]. This G0 change occurred preferentially in ACPA, as SF ACPA-IgG1 but not with total SF IgG1 was highly agalactosylated [76,129]. Importantly, ACPA-IgG1 Fc exhibited decreased galactosylation three months prior to RA diagnosis, but this was not observed in undifferentiated arthritis populations [21]. Overall, the level of galactosylation could be informative with respect to pathogenetically relevant inflammatory processes pre-RA and may be of value in early intervention. In addition, hypogalactosylation of both total IgG and specific autoantibodies has also been reported in spondyloarthropathy, SLE, ANCA-associated systemic vasculitis, inflammatory bowel disease, myasthenia gravis and primary Sjögren's syndrome [3,56,81,130-132]. Through detailed studies of the Fc portion, the observation of galactosylation defects was expanded to GPA and autoimmune hemolytic anemia [69,133].
Despite the alterations in galactosylation patterns in a variety of autoimmune diseases, inconsistencies have also been observed among the immune responses triggered by galactosylated IgG. Early research discovered that IgG with G0F glycans exhibited a high affinity for FcγRIII, contributing to antibody-mediated inflammation [74,134]. However, the binding rate of hypofucosylated and hypergalactosylated IgG1 to FcγRIIIa increased 40-fold, while hypofucosylated IgG1 showed a 17-fold affinity increase, resulting in enhanced NK cell-mediated ADCC against red blood cells [135]. Hypergalactosylated IgG1 ICs facilitated the association of FcγIIb with dectin-1, leading to the blockage of C5a-dependent inflammatory responses in vitro and in vivo [136]. Moreover, hypergalactosylated IgG1 showed increased binding to C1q, with the de novo ability to lyse Burkitt lymphoma-derived Raji cells [135,137]. Further studies are needed to resolve these inconsistencies and to assess the roles of galactosylation in therapy.
Fucose
In the serum IgG pool, nearly 90% of Fc-tail glycoforms are core fucosylated. Serum Fc fucosylation is relatively stable throughout life and in the majority of autoimmune conditions [54]. Some studies have reported skewed fucosylation found in fetal and neonatal alloimmune thrombocytopenia and HDFN apart from SLE [27,56,73,75]. Lower core fucosylation was positively correlated with clinical severity, a feature found for both anti-human platelet antigen (HPA)-1a and anti-D IgG1 antibodies [73,75,138,139]. Anti-c and anti-E antibodies were undetectable or showed far lower levels, while low fucosylation was observed for anti-D antibodies [75]. Importantly, decreased fucosylation of IgG increased its affinity for human FcγRIIIa/IIIb, in line with a 50-fold higher ADCC activity of less-fucosylated humanized mAbs through more flexible glycan-glycan interactions between the Fc domain and FcγRIII [28,140]. Such observations led to the development of defucosylated or hypofucosylated antibodies, especially for antitumor therapy, where cytotoxicity is needed [141]. For example, mogamulizumab, a defucosylated mAb targeting CC‑chemokine receptor 4, broadens the spectrum of treatment options for adult T-cell leukemia/lymphoma and cutaneous T-cell lymphoma [142].
Comparison of glycoengineering methods
| Genetic glycoengineering | Glycosyltransferase glycoengineering | Chemoenzymatic glycoengineering | |
|---|---|---|---|
| Strategies | Modify intracellular glycosylation pathways and enzymes via genetic engineering. | Extend monosaccharide residues by glycosyltransferases in vitro. | Modify sugar chains by endoglycosidases and their mutants, together with chemically synthesized active glycan oxazolines. |
| Methods | Remold sialyltransferases; increase CMP-Neu5Ac-associated enzymes or transporters; inhibit or eliminate sialidases; introduce new N-glycosylation sites. | Construct one-pot system with monosaccharide precursors and glycosyltransferases. | Deglycosylate IgG by an ENGase, prepare oxazoline derivatives as sugars donors via chemical methods, and transglycosylate oxazoline donor to glycoprotein. |
| Pros | Versatility | Simplicity and relatively purified products. | Simplicity; relatively purified and unlimited products. |
| Cons | Low efficiency and hybrid glyco-products | Limited glyco-products; difficulty and high cost of active glycan substrates. | Unavoidable hydrolytic activity of ENGase mutant; difficult to achieve oligosaccharide substrates in a large scale. |
CMP, cytidine monophosphate; Neu5Ac, N-acetylneuraminic acid; ENGase, endoglycosidase.
IgG sialylation glycoengineering approaches
In 2006, Jeffrey Ravetch's team reported that the anti-inflammatory activity of high-dose IVIg depended on terminal α2,6-sialylated IgG [18,97]. Then, recombinant glycosylated IgG with tailored effector function was demonstrated to be a promising therapeutic strategy [111]. Recent progress in glycoengineering has provided strategies to produce glycan-defined homogeneous antibodies. They include an in vivo genetic method focusing on N-glycan biosynthetic system manipulation and in vitro metabolic remodeling via glycosyltransferase or endoglycosidase activity (Table 3). The following sections will discuss recent advances in these areas [143].
Genetic glycoengineering
Host cell engineering through genetically modifying mediators in the intracellular glycan biosynthetic pathway has been the most widely used approach [55]. Various permanent edits in glycosylation can be achieved via genome mutation and knockout, as well as upregulation or downregulation of glycosylation enzymes [144]. α2,6 ST and α2,3 ST, which can catalyze the addition of monosaccharide residues, are the most frequently studied glycosylation enzymes. Raymond and coworkers coexpressed ST6 and β1,4-galactosyltransferase 1 in CHO cells to produce F241A mutant IgG1 with 85% α2,6-linked sialic acid [145]. Other attempts include transfection of cytidine monophosphate-sialic acid transporter (CMP-SAT) to increase precursor sugar levels or inhibit sialidase to eliminate terminal carbohydrate hydrolysis. Likewise, advances in genome editing technologies, such as CRISPR/Cas9, have made it more feasible to produce pure or enriched α2,6-sialylated mAbs [146,147]. Blundell et al. introduced new N-glycosylation sites to hexa-Fcs, which enhanced its sialylated glycan composition up to 75% [148].
However, the glycoengineering output quality and efficiency may deviate from the desired level due to the restrictions of mammalian host cells. CHO cells are the preferred in vitro expression platforms, allowing for high levels of human-like posttranslational modifications of proteins. However, N-glycolylneuraminic acid (Neu5Gc), a nonhuman sialic acid, can be synthesized by CHO cells to occupy the same epitopes as Neu5Ac. Neu5Gc-glycoprotein injection gives rise to an abnormal anti-Neu5Gc antibody response, and the combination of Neu5Gc-containing epitopes and anti-Neu5Gc antibodies may exacerbate colorectal cancer and atherosclerosis [149-151]. Variability in cell culture parameters could also affect glycosylated protein expression levels. Lower cell culture temperature was reported to be associated with an increase in IgG3 sialylation [152]. Furthermore, glycoproteins are produced as glycoformic mixtures that have the same protein backbone but differ in the attached sugar chains. It is still difficult to isolate pure glycoproteins using current chromatographic techniques [153].
Glycosyltransferase glycoengineering
Parallel to in vivo genetic approaches, in vitro glycan chain extension by glycosyltransferase has paved the way for producing homogenous glycoproteins with structurally defined oligosaccharides [143,154]. A one-pot system composed of two enzymes (ST6GAL1 and B4GAL1) as well as the corresponding substrate molecules (uridine-5'-diphospho-galactose and CMP-sialic acid) was used to generate tetra-Fc-sialylated IVIg. Washburn and coworkers maximized enzymatic incorporation while minimizing byproducts by optimizing the ratio of CMP-sialic acid to ST6GAL1 [100]. Likewise, 74% sialylated human IgG has been generated by Dekkers utilizing recombinant ST6GAL1 and CMP-sialic acid substrates [155]. Glycosyltransferase-based strategies have made it much simpler to acquire defined glycoproteins quickly. Despite these advances, the obtained glycoforms lack diversity due to the limited number of commonly used glycosyltransferases. Parameters, including enzymatic substrate specificity and favorable catalytic temperature, should be considered in next-generation glycosyltransferase glycoengineering [156]. Another difficulty in acquiring expensive sugar nucleotides or sugar phosphate as active donor substrates limits its application in large-scale manufacturing.
Chemoenzymatic glycoengineering
While the aforementioned approaches are impeded by the limited quality and diversity of glycoforms, chemoenzymatic glycoengineering, remodeling based on endoglycosidase (ENGase) for glycans and the chemical synthesis of highly active glycan oxazolines, has attracted considerable attention since 2008 [143,153]. Briefly, chemoenzymatic glycoengineering consists of two enzymatic steps: deglycosylation to trim heterogeneous IgG by ENGase, leaving the innermost GlcNAc residues, and subsequent addition of a predefined oligosaccharide substrate to the GlcNAc peptide by glycosynthase mutants [143,157]. Both purified mAbs and antibody mixtures, such as IVIg, have been successfully modified using chemoenzymatic glycoengineering.
Contrary to the narrow substrate specificity of other endoglycosidases, Endo-S secreted by Streptococcus pyogenes shows high specificity for IgG and can act only on biantennary complex glycans [158,159]. The pair of Endo-S and its mutant (Endo-S-D233Q) showed great efficiency in generating bisialylated mAbs, including rituximab and trastuzumab [25,160,161]. A glycosylase mutant (Endo-S2-D184M) from Endo-S2 was developed by Wang et al. [162,163], with improved efficiency of transglycosylation. Additionally, Endo-S2 can cleave all three types of Fc N-glycans, including hybrid, high mannose and complex types [164-166]. Furthermore, Endo-S2-processed rituximab with a homogeneous terminal α2,6-sialylation showed clearly reduced affinity for FcγRIIIaV158 compared to that of asialylated antibodies [26]. Utilizing Endo-S and Endo-S-D233Q, Wang and coworkers successfully purified minor α2,6-sialic acid from IVIg, increasing its purity from less than 10% to more than 90%. Moreover, the N-glycans on the Fab domains remained intact, which was confirmed by fluorescent HPLC profiles [167].
Chemoenzymatic glycoengineering is a versatile approach to optimize sizable homogenous IgG glycoforms. Its preparation time is relatively short, with 5 to 8 days to prepare glycan substrates and 3 to 4 days to engineer IgG glycosylation [168]. However, the synthesis and purification of large quantities of oligosaccharide substrates are still in their infancy [169,170]. Recent developments, such as the production of Man3GlcNAc oxazoline from egg yolks and Man9GlcNAc2 from soybean flour, have shown progress [171,172].
To summarize, glycoengineering will deepen our understanding of antibody glycosylation and facilitate the production of therapeutics modulating IgG sialylation.
Conclusions and perspectives
The ultimate goal of ongoing research on autoimmune diseases is to improve diagnostic accuracy and specifically attenuate pathogenic self-reactive components while maintaining normal immune defense and surveillance [7,88]. Great attention has been paid to IgG Fc sialylation, the alterations of which have been observed in a wide range of immune disorders [45]. These changes in sialic acid can be used as biomarkers to facilitate diagnosis and prognosis, to monitor disease progression and to evaluate therapeutic efficacy. Furthermore, developments in glycoengineering have ushered in a series of sialylated IgG-based therapeutics.
The glycome is affected by genetic and environmental factors, and changes have been associated with the development of inflammation. Prior research, therefore, focused mainly on the cross-sectional and longitudinal associations between the biomarkers of autoimmune diseases, disease activity, therapeutic efficacy and sialylated IgG. However, the interactions between sialylated IgG and immune cells as well as the underlying mechanisms of the regulation of sialylated IgG on immune responses in autoimmune disease have not been fully elucidated and need further investigation. Moreover, as rheumatic diseases are often systemic, abnormal immune tolerance can lead to multimorbidity [173]. Interestingly, links between cardiovascular or metabolic disease and IgG sialylation are emerging: in high-fat diet-fed mice, ManNAc restored IgG sialylation and prevented obesity-related insulin resistance, as well as obesity-induced hypertension [174,175]. In this respect, it would be helpful to elucidate additional roles of sialylated IgG in rheumatic patients with multimorbidity. In addition, the latest literature has indicated that these sialylated IgG therapeutic interventions may also attenuate IgE-mediated allergic reactions by upregulating inhibitory FcγRIIb on immune cells [176]. Their application range may be expanded to allergies, infections, and even tumors, while their detailed mechanisms are still under study [177].
Although advancements in glycoscience have led to progress in glycan-based therapeutics and continued to provide exciting prospects, further studies are expected to achieve commercially available glycoengineered antibodies and site-specific antibody-drug conjugation [178]. With the advancement of glycobiology and glycomedicine, we anticipate that in the near future, glycan analysis will become integral to the diagnosis, prognosis and management of autoimmune diseases.
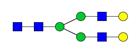
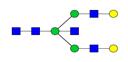
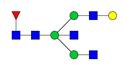
Overview of the glycan structures described in this review. For all glycoforms, blue square: N-acetylglucosamine, green circle: mannose, yellow circle: galactose, red triangle: fucose, purple diamond: N-acetylneuraminic acid. Glycan compositions are depicted with hexose (H), N-acetylhexosamine (N), deoxyhexose (F), and N-acetylneuraminic acid (S). Short names are given in terms of the diantennary (A2), galactosylated (G), sialylated (S), bisecting β (1,4) GlcNAc (B) and α (1,6) fucose (F) glycan attached to core GlcNAc.
| Glycan structure | Compound name | Glycan short name |
|---|---|---|
 | H5N4-IgG | A2G2 |
 | H5N5-IgG | A2BG2 |
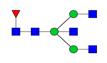 | H3N5F1-IgG | FA2B |
 | H4N5F1-IgG | FA2BG1 |
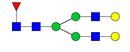 | H5N4F1-IgG | FA2G2 |
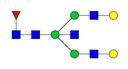 | H5N5F1-IgG | FA2BG2 |
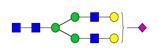 | H5N4S1-IgG | A2G2S |
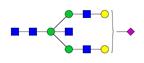 | H5N5S1-IgG | A2BG2S |
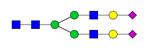 | H4N5S2-IgG | A2G2S2 |
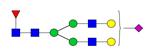 | H5N4F1S1-IgG | FA2G2S |
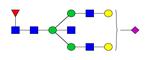 | H5N5F1S1-IgG | FA2BG2S |
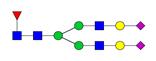 | H5N4F1S2-IgG | FA2G2S2 |
Abbreviations
IgG: immunoglobulin G; Fc: crystallizable fragment; FcγR: Fc gamma receptor; RA: rheumatoid arthritis; RF: rheumatoid factor; ACPA: anti-citrullinated protein antibody; mAbs: monoclonal antibodies; TNF: tumor necrosis factor; DC: dendritic cell; PML: progressive multifocal leukoencephalopathy; SLE: systemic lupus erythematosus; APS: antiphospholipid syndrome; IVIg: intravenous immunoglobulin; ITP: immune thrombocytopenic purpura; CCR: C-C chemokine receptor 4; FDA: Food and Drug Administration; NK: natural killer; BCR: B cell receptor; ADCC: antibody-dependent cellular cytotoxicity; Fab: antigen-binding fragment; ADCP: antibody-dependent cellular phagocytosis; DC-SIGN: Dendritic cell-specific ICAM-3-grabbing nonintegrin; FcRn: neonatal crystallizable fragment receptor; TRIM21: tripartite motif-containing 21; CH: constant heavy; CHO: Chinese hamster ovary; GPA: granulomatosis with polyangiitis; GBS: Guillain-Barré syndrome; HDFN: hemolytic disease of the fetus and newborn; FNAIT: Fetal and neonatal alloimmune thrombocytopenia; JIA: Juvenile idiopathic arthritis; CIDP: Chronic inflammatory demyelinating polyneuropathy; AD: Alzheimer's disease; PD: Parkinson's disease; KD: Kawasaki disease; CD: Crohn's disease; CIA: collagen-induced arthritis; AIA: antigen-induced arthritis; NTN: nephrotoxic nephritis; SNA: Sambucus nigra agglutinin; IL: interleukin; Th: T helper; CDC: complement-dependent cytotoxicity; IC: immune complex; ANCA: anti-neutrophilic cytoplasmic autoantibodies; ST: sialyltransferase; GT: galactosyltransferase.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No's. 81573047).
Authors' contributions
Danqi Li and Yuchen Lou contributed equally to researching data for the article and writing the manuscript; Yamin Zhang, Si Liu, Jun Li and Juan Tao reviewed and revised the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Murphy G, Lisnevskaia L, Isenberg D. Systemic lupus erythematosus and other autoimmune rheumatic diseases: challenges to treatment. Lancet. 2013;382:809-818
2. Papotto PH, Marengo EB, Sardinha LR, Goldberg AC, Rizzo LV. Immunotherapeutic strategies in autoimmune uveitis. Autoimmun Rev. 2014;13:909-916
3. Scherlinger M, Mertz P, Sagez F. et al. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun Rev. 2020;19:102531
4. Goldblatt F, O'Neill SG. Clinical aspects of autoimmune rheumatic diseases. Lancet. 2013;382:797-808
5. Derksen VFAM, Huizinga TWJ, van der Woude D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin Immunopathol. 2017;39:437-446
6. Pisetsky DS. Antinuclear antibody testing - misunderstood or misbegotten? Nat Rev Rheumatol. 2017;13:495-502
7. Conrad K, Shoenfeld Y, Fritzler MJ. Precision health: A pragmatic approach to understanding and addressing key factors in autoimmune diseases. Autoimmun Rev. 2020;19:102508
8. Verheul MK, Böhringer S, van Delft MAM. et al. Triple Positivity for Anti-Citrullinated Protein Autoantibodies, Rheumatoid Factor, and Anti-Carbamylated Protein Antibodies Conferring High Specificity for Rheumatoid Arthritis: Implications for very early identification of at-risk individuals. Arthritis Rheumatol. 2018;70:1721-1731
9. Wang JR, Gao WN, Grimm R. et al. A method to identify trace sulfated IgG N-glycans as biomarkers for rheumatoid arthritis. Nat Commun. 2017;8:631
10. Brown G, Wang E, Leon A. et al. Tumor necrosis factor-α inhibitor-induced psoriasis: Systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341
11. Moran B, Gallagher C, Tobin AM, Fletcher JM. Enrichment of Polyfunctional IL-17-Producing T Cells in Paradoxical Psoriasis Skin Lesions. J Invest Dermatol. 2020;140:1094-1097
12. Conrad C, Di Domizio J, Mylonas A. et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25
13. Pincetic A, Bournazos S, DiLillo DJ. et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15:707-716
14. Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176-189
15. Alter G, Ottenhoff THM, Joosten SA. Antibody glycosylation in inflammation, disease and vaccination. Semin Immunol. 2018;39:102-110
16. Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21-50
17. Maverakis E, Kim K, Shimoda M. et al. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review. J Autoimmun. 2015;57:1-13
18. Cobb BA. The history of IgG glycosylation and where we are now. Glycobiology. 2020;30:202-213
19. Fickentscher C, Magorivska I, Janko C. et al. The Pathogenicity of Anti-β2GP1-IgG Autoantibodies Depends on Fc Glycosylation. J Immunol Res. 2015;2015:638129
20. Ogata S, Shimizu C, Franco A. et al. Treatment response in Kawasaki disease is associated with sialylation levels of endogenous but not therapeutic intravenous immunoglobulin G. PLoS One. 2013;8:e81448
21. Rombouts Y, Ewing E, van de Stadt LA. et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis. 2015;74:234-241
22. Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373-376
23. Kasamon YL, Chen H, de Claro RA. et al. FDA Approval Summary: Mogamulizumab-kpkc for Mycosis Fungoides and Sézary Syndrome. Clin Cancer Res. 2019;25:7275-7280
24. Carter PJ, Lazar GA. Next generation antibody drugs: pursuit of the 'high-hanging fruit'. Nat Rev Drug Discov. 2018;17:197-223
25. Lin CW, Tsai MH, Li ST. et al. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc Natl Acad Sci U S A. 2015;112:10611-10616
26. Li T, DiLillo DJ, Bournazos S, Giddens JP, Ravetch JV, Wang LX. Modulating IgG effector function by Fc glycan engineering. Proc Natl Acad Sci U S A. 2017;114:3485-3490
27. Sjöwall C, Zapf J, von Löhneysen S. et al. Altered glycosylation of complexed native IgG molecules is associated with disease activity of systemic lupus erythematosus. Lupus. 2015;24:569-581
28. Shinkawa T, Nakamura K, Yamane N. et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466-3473
29. Wang TT, Maamary J, Tan GS. et al. Anti-HA Glycoforms Drive B Cell Affinity Selection and Determine Influenza Vaccine Efficacy. Cell. 2015;162:160-169
30. Pereira NA, Chan KF, Lin PC, Song Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs. 2018;10:693-711
31. Bournazos S, Wang TT, Dahan R, Maamary J, Ravetch JV. Signaling by Antibodies: Recent Progress. Annu Rev Immunol. 2017;35:285-311
32. Bournazos S, Ravetch JV. Fcγ Receptor Function and the Design of Vaccination Strategies. Immunity. 2017;47:224-233
33. Schroeder HW Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125:S41-S52
34. Bournazos S, Ravetch JV. Fcγ receptor pathways during active and passive immunization. Immunol Rev. 2015;268:88-103
35. Bournazos S, Ravetch JV. Diversification of IgG effector functions. Int Immunol. 2017;29:303-310
36. Chu SY, Yeter K, Kotha R. et al. Suppression of rheumatoid arthritis B cells by XmAb5871, an anti-CD19 antibody that coengages B cell antigen receptor complex and Fcγ receptor IIb inhibitory receptor. Arthritis Rheumatol. 2014;66:1153-1164
37. Zhao Q. Bispecific Antibodies for Autoimmune and Inflammatory Diseases: Clinical Progress to Date. BioDrugs. 2020;34:111-119
38. Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol. 2020;20:633-643
39. Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105:19571-19578
40. Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205-217
41. Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110-113
42. Pyzik M, Sand KMK, Hubbard JJ, Andersen JT, Sandlie I, Blumberg RS. The Neonatal Fc Receptor (FcRn): A Misnomer? Front Immunol. 2019;10:1540
43. Rhodes DA, Isenberg DA. TRIM21 and the function of antibodies inside cells. Trends Immunol. 2017;38:916-926
44. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520
45. Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol. 2019;15:346-366
46. Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18:46-61
47. Plomp R, Dekkers G, Rombouts Y. et al. Hinge-region O-Glycosylation of Human Immunoglobulin G3 (IgG3). Mol Cell Proteomics. 2015;14:1373-1384
48. Russell A, Adua E, Ugrina I, Laws S, Wang W. Unravelling Immunoglobulin G Fc N-Glycosylation: A Dynamic Marker Potentiating Predictive, Preventive and Personalised Medicine. Int J Mol Sci. 2018;19:390
49. Ferrara C, Grau S, Jäger C. et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669-12674
50. Ferrara C, Stuart F, Sondermann P, Brünker P, Umaña P. The carbohydrate at FcgammaRIIIa Asn-162. An element required for high affinity binding to non-fucosylated IgG glycoforms. J Biol Chem. 2006;281:5032-5036
51. Mizushima T, Yagi H, Takemoto E. et al. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells. 2011;16:1071-1080
52. Ahmed AA, Giddens J, Pincetic A. et al. Structural characterization of anti-inflammatory immunoglobulin G Fc proteins. J Mol Biol. 2014;426:3166-3179
53. Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci U S A. 2013;110:9868-9872
54. Seeling M, Brückner C, Nimmerjahn F. Differential antibody glycosylation in autoimmunity: sweet biomarker or modulator of disease activity? Nat Rev Rheumatol. 2017;13:621-630
55. Buettner MJ, Shah SR, Saeui CT, Ariss R, Yarema KJ. Improving Immunotherapy through Glycodesign. Front Immunol. 2018;9:2485
56. Vučković F, Krištić J, Gudelj I. et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol. 2015;67:2978-2989
57. Wuhrer M, Stavenhagen K, Koeleman CA. et al. Skewed Fc glycosylation profiles of anti-proteinase 3 immunoglobulin G1 autoantibodies from granulomatosis with polyangiitis patients show low levels of bisection, galactosylation, and sialylation. J Proteome Res. 2015;14:1657-1665
58. Šimurina M, de Haan N, Vučković F. et al. Glycosylation of Immunoglobulin G associates with clinical features of Inflammatory Bowel Diseases. Gastroenterology. 2018;154:1320-1333
59. Su Z, Xie Q, Wang Y, Li Y. Abberant Immunoglobulin G Glycosylation in Rheumatoid Arthritis by LTQ-ESI-MS. Int J Mol Sci. 2020;21:2045
60. de Moerloose P, Fickentscher C, Boehlen F, Tiercy JM, Kruithof EKO, Brandt KJ. Patient-derived anti-β2GP1 antibodies recognize a peptide motif pattern and not a specific sequence of residues. Haematologica. 2017;102:1324-1332
61. Biggioggero M, Meroni PL. The geoepidemiology of the antiphospholipid antibody syndrome. Autoimmun Rev. 2010;9:A299-A304
62. Avcin T, Ambrozic A, Kuhar M, Kveder T, Rozman B. Anticardiolipin and anti-beta(2) glycoprotein I antibodies in sera of 61 apparently healthy children at regular preventive visits. Rheumatology (Oxford). 2001;40:565-573
63. Weppner G, Ohlei O, Hammers CM. et al. In situ detection of PR3-ANCA+ B cells and alterations in the variable region of immunoglobulin genes support a role of inflamed tissue in the emergence of auto-reactivity in granulomatosis with polyangiitis. J Autoimmun. 2018;93:89-103
64. Espy C, Morelle W, Kavian N. et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener's). Arthritis Rheum. 2011;63:2105-2115
65. Gińdzieńska-Sieśkiewicz E, Radziejewska I, Domysławska I. et al. Changes of glycosylation of IgG in rheumatoid arthritis patients treated with methotrexate. Adv Med Sci. 2016;61:193-197
66. Engdahl C, Bondt A, Harre U. et al. Estrogen induces St6gal1 expression and increases IgG sialylation in mice and patients with rheumatoid arthritis: a potential explanation for the increased risk of rheumatoid arthritis in postmenopausal women. Arthritis Res Ther. 2018;20:84
67. Wong AH, Fukami Y, Sudo M, Kokubun N, Hamada S, Yuki N. Sialylated IgG-Fc: a novel biomarker of chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2016;87:275-279
68. Štambuk T, Klasić M, Zoldoš V, Lauc G. N-glycans as functional effectors of genetic and epigenetic disease risk. Mol Aspects Med. 2020 [Epub ahead of print]
69. Kemna MJ, Plomp R, van Paassen P. et al. Galactosylation and Sialylation levels of IgG Predict relapse in patients with PR3-ANCA Associated Vasculitis. EBioMedicine. 2017;17:108-118
70. Fokkink WJ, Selman MH, Dortland JR. et al. IgG Fc N-glycosylation in Guillain-Barré syndrome treated with immunoglobulins. J Proteome Res. 2014;13:1722-1730
71. Wuhrer M, Selman MH, McDonnell LA. et al. Pro-inflammatory pattern of IgG1 Fc glycosylation in multiple sclerosis cerebrospinal fluid. J Neuroinflammation. 2015;12:235
72. Thomann M, Schlothauer T, Dashivets T. et al. In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS One. 2015;10:e0134949
73. Houde D, Peng Y, Berkowitz SA, Engen JR. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol Cell Proteomics. 2010;9:1716-1728
74. Sonneveld ME, Natunen S, Sainio S. et al. Glycosylation pattern of anti-platelet IgG is stable during pregnancy and predicts clinical outcome in alloimmune thrombocytopenia. Br J Haematol. 2016;174:310-320
75. Sonneveld ME, Koelewijn J, de Haas M. et al. Antigen specificity determines anti-red blood cell IgG-Fc alloantibody glycosylation and thereby severity of haemolytic disease of the fetus and newborn. Br J Haematol. 2017;176:651-660
76. Scherer HU, van der Woude D, Ioan-Facsinay A. et al. Glycan profiling of anti-citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum. 2010;62:1620-1629
77. Matsumoto A, Shikata K, Takeuchi F, Kojima N, Mizuochi T. Autoantibody activity of IgG rheumatoid factor increases with decreasing levels of galactosylation and sialylation. J Biochem. 2000;128:621-628
78. Ohmi Y, Ise W, Harazono A. et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun. 2016;7:11205
79. Cheng HD, Stöckmann H, Adamczyk B. et al. High-throughput characterization of the functional impact of IgG Fc glycan aberrancy in juvenile idiopathic arthritis. Glycobiology. 2017;27:1099-1108
80. Magorivska I, Muñoz LE, Janko C. et al. Sialylation of anti-histone immunoglobulin G autoantibodies determines their capabilities to participate in the clearance of late apoptotic cells. Clin Exp Immunol. 2016;184:110-117
81. Trbojević Akmačić I, Ventham NT, Theodoratou E. et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm Bowel Dis. 2015;21:1237-1247
82. Winkler A, Berger M, Ehlers M. Anti-rhesus D prophylaxis in pregnant women is based on sialylated IgG antibodies. F1000Res. 2013;2:169
83. Quast I, Keller CW, Maurer MA. et al. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J Clin Invest. 2015;125:4160-4170
84. Fokkink WJ, Selman MH, Wuhrer M, Jacobs BC. Immunoglobulin G Fc N-glycosylation in Guillain-Barré syndrome treated with intravenous immunoglobulin. Clin Exp Immunol. 2014;178(Suppl 1):105-107
85. Lundström SL, Yang H, Lyutvinskiy Y. et al. Blood plasma IgG Fc glycans are significantly altered in Alzheimer's disease and progressive mild cognitive impairment. J Alzheimers Dis. 2014;38:567-579
86. Russell AC, Šimurina M, Garcia MT. et al. The N-glycosylation of immunoglobulin G as a novel biomarker of Parkinson's disease. Glycobiology. 2017;27:501-510
87. McHugh J. Autoimmunity: Glycoengineering has therapeutic potential. Nat Rev Rheumatol. 2018;14:121
88. Kerrigan SA, McInnes IB. Reflections on 'older' drugs: learning new lessons in rheumatology. Nat Rev Rheumatol. 2020;16:179-183
89. Perez EE, Orange JS, Bonilla F. et al. Update on the use of immunoglobulin in human disease: A review of evidence. J Allergy Clin Immunol. 2017;139(s):1-46
90. João C, Negi VS, Kazatchkine MD, Bayry J, Kaveri SV. Passive Serum Therapy to Immunomodulation by IVIG: A Fascinating Journey of Antibodies. J Immunol. 2018;200:1957-1963
91. Forbat E, Ali FR, Al-Niaimi F. Intravenous immunoglobulins in dermatology. Part 1: biological mechanisms and methods of administration. Clin Exp Dermatol. 2018;43:513-517
92. Das M, Karnam A, Stephen-Victor E. et al. Intravenous immunoglobulin mediates anti-inflammatory effects in peripheral blood mononuclear cells by inducing autophagy. Cell Death Dis. 2020;11:50
93. Nagelkerke SQ, Dekkers G, Kustiawan I. et al. Inhibition of FcγR-mediated phagocytosis by IVIg is independent of IgG-Fc sialylation and FcγRIIb in human macrophages. Blood. 2014;124:3709-3718
94. Audia S, Santegoets K, Laarhoven AG. et al. Fcγ receptor expression on splenic macrophages in adult immune thrombocytopenia. Clin Exp Immunol. 2017;188:275-282
95. Karnam A, Rambabu N, Das M. et al. Therapeutic normal IgG intravenous immunoglobulin activates Wnt-β-catenin pathway in dendritic cells. Commun Biol. 2020;3:96
96. Trinath J, Hegde P, Sharma M. et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2-dependent prostaglandin E2 in human dendritic cells. Blood. 2013;122:1419-1427
97. Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670-673
98. Bozza S, Käsermann F, Kaveri SV, Romani L, Bayry J. Intravenous immunoglobulin protects from experimental allergic bronchopulmonary aspergillosis via a sialylation-dependent mechanism. Eur J Immunol. 2019;49:195-198
99. Sudo M, Yamaguchi Y, Späth PJ. et al. Different IVIG glycoforms affect in vitro inhibition of anti-ganglioside antibody-mediated complement deposition. PLoS One. 2014;9:e107772
100. Washburn N, Schwab I, Ortiz D. et al. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc Natl Acad Sci U S A. 2015;112:E1297-E1306
101. Zuercher AW, Spirig R, Baz Morelli A, Rowe T, Käsermann F. Next-generation Fc receptor-targeting biologics for autoimmune diseases. Autoimmun Rev. 2019;18:102366
102. US National Library of Medicine. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/record/NCT03866577
103. Fiebiger BM, Maamary J, Pincetic A, Ravetch JV. Protection in antibody- and T cell-mediated autoimmune diseases by antiinflammatory IgG Fcs requires type II FcRs. Proc Natl Acad Sci U S A. 2015;112:E2385-E2394
104. Leontyev D, Katsman Y, Ma XZ, Miescher S, Käsermann F, Branch DR. Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin. Transfusion. 2012;52:1799-1805
105. Othy S, Topçu S, Saha C. et al. Sialylation may be dispensable for reciprocal modulation of helper T cells by intravenous immunoglobulin. Eur J Immunol. 2014;44:2059-2063
106. Campbell IK, Miescher S, Branch DR. et al. Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. J Immunol. 2014;192:5031-5038
107. Käsermann F, Boerema DJ, Rüegsegger M. et al. Analysis and functional consequences of increased Fab-sialylation of intravenous immunoglobulin (IVIG) after lectin fractionation. PLoS One. 2012;7:e37243
108. Harre U, Lang SC, Pfeifle R. et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun. 2015;6:6651
109. Bondt A, Hafkenscheid L, Falck D. et al. ACPA IgG galactosylation associates with disease activity in pregnant patients with rheumatoid arthritis. Ann Rheum Dis. 2018;77:1130-1136
110. Bartsch YC, Rahmöller J, Mertes MMM. et al. Sialylated Autoantigen-Reactive IgG Antibodies Attenuate Disease Development in Autoimmune Mouse Models of Lupus Nephritis and Rheumatoid Arthritis. Front Immunol. 2018;9:1183
111. Pagan JD, Kitaoka M, Anthony RM. Engineered Sialylation of Pathogenic Antibodies in vivo attenuates Autoimmune Disease. Cell. 2018;172:564-577
112. Cherepanova N, Shrimal S, Gilmore R. N-linked glycosylation and homeostasis of the endoplasmic reticulum. Curr Opin Cell Biol. 2016;41:57-65
113. Li F, Ding J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell. 2019;10:550-565
114. Jones MB. IgG and leukocytes: Targets of immunomodulatory α2,6 sialic acids. Cell Immunol. 2018;333:58-64
115. Jones MB, Oswald DM, Joshi S, Whiteheart SW, Orlando R, Cobb BA. B-cell-independent sialylation of IgG. Proc Natl Acad Sci U S A. 2016;113:7207-7212
116. Pfeifle R, Rothe T, Ipseiz N. et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat Immunol. 2017;18:104-113
117. Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44:1524-1534
118. Schwab I, Mihai S, Seeling M, Kasperkiewicz M, Ludwig RJ, Nimmerjahn F. Broad requirement for terminal sialic acid residues and FcγRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo. Eur J Immunol. 2014;44:1444-1453
119. Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J Mol Biol. 2013;425:1253-1258
120. Massoud AH, Yona M, Xue D. et al. Dendritic cell immunoreceptor: a novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J Allergy Clin Immunol. 2014;133:853-63
121. Sharma M, Schoindre Y, Hegde P. et al. Intravenous immunoglobulin-induced IL-33 is insufficient to mediate basophil expansion in autoimmune patients. Sci Rep. 2014;4:5672
122. Maddur MS, Stephen-Victor E, Das M. et al. Regulatory T cell frequency, but not plasma IL-33 levels, represents potential immunological biomarker to predict clinical response to intravenous immunoglobulin therapy. J Neuroinflammation. 2017;14:58
123. Galeotti C, Stephen-Victor E, Karnam A. et al. Intravenous immunoglobulin induces IL-4 in human basophils by signaling through surface-bound IgE. J Allergy Clin Immunol. 2019;144:524-535
124. Séïté JF, Cornec D, Renaudineau Y, Youinou P, Mageed RA, Hillion S. IVIg modulates BCR signaling through CD22 and promotes apoptosis in mature human B lymphocytes. Blood. 2010;116:1698-1704
125. Lee-Sundlov MM, Ashline DJ, Hanneman AJ. et al. Circulating blood and platelets supply glycosyltransferases that enable extrinsic extracellular glycosylation. Glycobiology. 2017;27:188-198
126. Lauc G, Huffman JE, Pučić M. et al. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 2013;9:e1003225
127. Förger F, Villiger PM. Immunological adaptations in pregnancy that modulate rheumatoid arthritis disease activity. Nat Rev Rheumatol. 2020;16:113-122
128. Parekh RB, Roitt IM, Isenberg DA, Dwek RA, Ansell BM, Rademacher TW. Galactosylation of IgG associated oligosaccharides: reduction in patients with adult and juvenile onset rheumatoid arthritis and relation to disease activity. Lancet. 1988;1:966-969
129. Ercan A, Cui J, Chatterton DE. et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2010;62:2239-2248
130. Holland M, Takada K, Okumoto T. et al. Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin Exp Immunol. 2002;129:183-190
131. Pilkington C, Yeung E, Isenberg D, Lefvert AK, Rook GA. Agalactosyl IgG and antibody specificity in rheumatoid arthritis, tuberculosis, systemic lupus erythematosus and myasthenia gravis. Autoimmunity. 1995;22:107-111
132. Leirisalo-Repo M, Hernandez-Munoz HE, Rook GA. Agalactosyl IgG is elevated in patients with active spondyloarthropathy. Rheumatol Int. 1999;18:171-176
133. Sonneveld ME, de Haas M, Koeleman C. et al. Patients with IgG1-anti-red blood cell autoantibodies show aberrant Fc-glycosylation. Sci Rep. 2017;7:8187
134. Nimmerjahn F, Anthony RM, Ravetch JV. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci U S A. 2007;104:8433-8437
135. Dekkers G, Treffers L, Plomp R. et al. Decoding the Human Immunoglobulin G-Glycan repertoire reveals a Spectrum of Fc-Receptor- and Complement-mediated-effector activities. Front Immunol. 2017;8:877
136. Karsten CM, Pandey MK, Figge J. et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med. 2012;18:1401-1406
137. Peschke B, Keller CW, Weber P, Quast I, Lünemann JD. Fc-Galactosylation of Human Immunoglobulin Gamma Isotypes improves C1q binding and enhances Complement-dependent Cytotoxicity. Front Immunol. 2017;8:646
138. Kapur R, Kustiawan I, Vestrheim A. et al. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood. 2014;123:471-480
139. Kapur R, Della Valle L, Sonneveld M. et al. Low anti-RhD IgG-Fc-fucosylation in pregnancy: a new variable predicting severity in haemolytic disease of the fetus and newborn. Br J Haematol. 2014;166:936-945
140. Majewska NI, Tejada ML, Betenbaugh MJ, Agarwal N. N-Glycosylation of IgG and IgG-Like Recombinant Therapeutic Proteins: Why is it important and how can we control it? Annu Rev Chem Biomol Eng. 2020;11:311-338
141. Gupta K, Parasnis M, Jain R, Dandekar P. Vector-related stratagems for enhanced monoclonal antibody production in mammalian cells. Biotechnol Adv. 2019;37:107415
142. Ogura M, Ishida T, Hatake K. et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32:1157-1163
143. Wang LX, Tong X, Li C, Giddens JP, Li T. Glycoengineering of antibodies for modulating functions. Annu Rev Biochem. 2019;88:433-459
144. Sha S, Agarabi C, Brorson K, Lee DY, Yoon S. N-Glycosylation Design and Control of Therapeutic Monoclonal Antibodies. Trends Biotechnol. 2016;34:835-846
145. Raymond C, Robotham A, Spearman M, Butler M, Kelly J, Durocher Y. Production of α2,6-sialylated IgG1 in CHO cells. MAbs. 2015;7:571-583
146. Chung CY, Wang Q, Yang S, Yin B, Zhang H, Betenbaugh M. Integrated Genome and Protein Editing Swaps α-2,6 Sialylation for α-2,3 Sialic Acid on Recombinant Antibodies from CHO. Biotechnol J. 2017;12:10
147. Schulz MA, Tian W, Mao Y. et al. Glycoengineering design options for IgG1 in CHO cells using precise gene editing. Glycobiology. 2018;28:542-549
148. Blundell PA, Le NPL, Allen J, Watanabe Y, Pleass RJ. Engineering the fragment crystallizable (Fc) region of human IgG1 multimers and monomers to fine-tune interactions with sialic acid-dependent receptors. J Biol Chem. 2017;292:12994-13007
149. Samraj AN, Bertrand KA, Luben R. et al. Polyclonal human antibodies against glycans bearing red meat-derived non-human sialic acid N-glycolylneuraminic acid are stable, reproducible, complex and vary between individuals: Total antibody levels are associated with colorectal cancer risk. PLoS One. 2018;13:e0197464
150. Pham T, Gregg CJ, Karp F. et al. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. 2009;114:5225-5235
151. Yehuda S, Padler-Karavani V. Glycosylated Biotherapeutics: Immunological Effects of N-Glycolylneuraminic Acid. Front Immunol. 2020;11:21
152. Agarabi CD, Schiel JE, Lute SC. et al. Bioreactor process parameter screening utilizing a Plackett-Burman design for a model monoclonal antibody. J Pharm Sci. 2015;104:1919-1928
153. Li C, Wang LX. Chemoenzymatic Methods for the Synthesis of Glycoproteins. Chem Rev. 2018;118:8359-8413
154. Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog. 2005;21:1644-1652
155. Dekkers G, Plomp R, Koeleman CA. et al. Multi-level glyco-engineering techniques to generate IgG with defined Fc-glycans. Sci Rep. 2016;6:36964
156. Wen L, Edmunds G, Gibbons C. et al. Toward automated enzymatic synthesis of oligosaccharides. Chem Rev. 2018;118:8151-8187
157. Mimura Y, Katoh T, Saldova R. et al. Glycosylation engineering of therapeutic IgG antibodies: challenges for the safety, functionality and efficacy. Protein Cell. 2018;9:47-62
158. Collin M, Olsén A. EndoS, a novel secreted protein from Streptococcus pyogenes with endoglycosidase activity on human IgG. EMBO J. 2001;20:3046-3055
159. Fairbanks AJ. The ENGases: versatile biocatalysts for the production of homogeneous N-linked glycopeptides and glycoproteins. Chem Soc Rev. 2017;46:5128-5146
160. Parsons TB, Struwe WB, Gault J. et al. Optimal Synthetic Glycosylation of a Therapeutic Antibody. Angew Chem Int Ed Engl. 2016;55:2361-2367
161. Iwamoto M, Sekiguchi Y, Nakamura K, Kawaguchi Y, Honda T, Hasegawa J. Generation of efficient mutants of endoglycosidase from Streptococcus pyogenes and their application in a novel one-pot transglycosylation reaction for antibody modification. PLoS One. 2018;13:e0193534
162. Li T, Li C, Quan DN, Bentley WE, Wang LX. Site-specific immobilization of endoglycosidases for streamlined chemoenzymatic glycan remodeling of antibodies. Carbohydr Res. 2018;458-459:77-84
163. Li T, Tong X, Yang Q, Giddens JP, Wang LX. Glycosynthase mutants of endoglycosidase S2 show potent Transglycosylation Activity and remarkably relaxed substrate specificity for Antibody Glycosylation Remodeling. J Biol Chem. 2016;291:16508-16518
164. Sjögren J, Lood R, Nägeli A. On enzymatic remodeling of IgG glycosylation; unique tools with broad applications. Glycobiology. 2020;30:254-267
165. Sjögren J, Cosgrave EF, Allhorn M. et al. EndoS and EndoS2 hydrolyze Fc-glycans on therapeutic antibodies with different glycoform selectivity and can be used for rapid quantification of high-mannose glycans. Glycobiology. 2015;25:1053-1063
166. Shivatare SS, Huang LY, Zeng YF. et al. Development of glycosynthases with broad glycan specificity for the efficient glyco-remodeling of antibodies. Chem Commun (Camb). 2018;54:6161-6164
167. Huang W, Giddens J, Fan SQ, Toonstra C, Wang LX. Chemoenzymatic glycoengineering of intact IgG antibodies for gain of functions. J Am Chem Soc. 2012;134:12308-12318
168. Tang F, Wang LX, Huang W. Chemoenzymatic synthesis of glycoengineered IgG antibodies and glycosite-specific antibody-drug conjugates. Nat Protoc. 2017;12:1702-1721
169. Fairbanks AJ. Chemoenzymatic synthesis of glycoproteins. Curr Opin Chem Biol. 2019;53:9-15
170. Van Landuyt L, Lonigro C, Meuris L, Callewaert N. Customized protein glycosylation to improve biopharmaceutical function and targeting. Curr Opin Biotechnol. 2019;60:17-28
171. Sun B, Bao W, Tian X. et al. A simplified procedure for gram-scale production of sialylglycopeptide (SGP) from egg yolks and subsequent semi-synthesis of Man3GlcNAc oxazoline. Carbohydr Res. 2014;396:62-69
172. Wang LX, Ni J, Singh S, Li H. Binding of high-mannose-type oligosaccharides and synthetic oligomannose clusters to human antibody 2G12: implications for HIV-1 vaccine design. Chem Biol. 2004;11:127-134
173. Radner H, Yoshida K, Smolen JS, Solomon DH. Multimorbidity and rheumatic conditions-enhancing the concept of comorbidity. Nat Rev Rheumatol. 2014;10:252-256
174. Tanigaki K, Sacharidou A, Peng J. et al. Hyposialylated IgG activates endothelial IgG receptor FcγRIIB to promote obesity-induced insulin resistance. J Clin Invest. 2018;128:309-322
175. Peng J, Vongpatanasin W, Sacharidou A. et al. Supplementation with the Sialic Acid precursor N-Acetyl-D-Mannosamine breaks the link between Obesity and Hypertension. Circulation. 2019;140:2005-2018
176. Petry J, Rahmöller J, Dühring L. et al. Enriched blood IgG sialylation attenuates IgG-mediated and IgG-controlled-IgE-mediated allergic reactions. J Allergy Clin Immunol. 2020 [Epub ahead of print]
177. Shade KC, Conroy ME, Washburn N. et al. Sialylation of immunoglobulin E is a determinant of allergic pathogenicity. Nature. 2020;582:265-270
178. Mastrangeli R, Palinsky W, Bierau H. Glycoengineered antibodies: towards the next-generation of immunotherapeutics. Glycobiology. 2019;29:199-210
Author contact
![]() Corresponding authors: E-mail address: goldcoindanielcom (J. Li), tjhappycom (J. Tao).
Corresponding authors: E-mail address: goldcoindanielcom (J. Li), tjhappycom (J. Tao).
 Global reach, higher impact
Global reach, higher impact