13.3
Impact Factor
Theranostics 2021; 11(11):5330-5345. doi:10.7150/thno.58337 This issue Cite
Research Paper
CD45RO-CD8+ T cell-derived exosomes restrict estrogen-driven endometrial cancer development via the ERβ/miR-765/PLP2/Notch axis
1. Laboratory for Reproductive Immunology, NHC Key Lab of Reproduction Regulation (Shanghai Institute of Planned Parenthood Research), Hospital of Obstetrics and Gynecology, Shanghai Medical School, Fudan University, Shanghai 200080, People's Republic of China.
2. Reproductive Medical Center, Department of Obstetrics and Gynecology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, People's Republic of China.
3. Department of Obstetrics and Gynecology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, No.100, Haining Road, Shanghai 200080, People's Republic of China.
4. Clinical Epidemiology, Hospital of Obstetrics and Gynecology, Shanghai Medical School, Fudan University, Shanghai 200011, People's Republic of China.
5. Division of Obstetrics and Gynecology, KK Women's and Children's Hospital, 229899, Singapore.
6. Department of Urology, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, No.100, Haining Road, Shanghai 200080, People's Republic of China.
7. Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, Hospital of Obstetrics and Gynecology, Shanghai Medical School, Fudan University, Shanghai, 200080, People's Republic of China.
#These authors contributed equally to this work.
Abstract
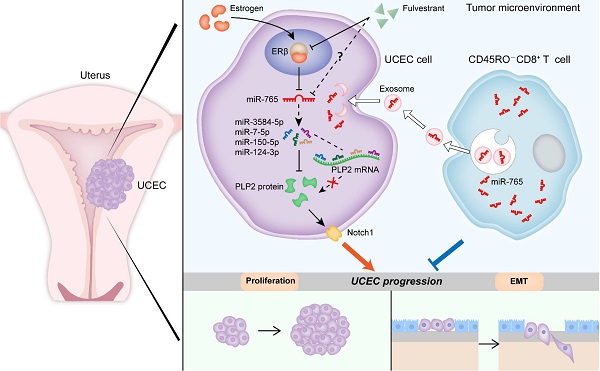
Rationale: Estrogen-dependent cancers (e.g., breast, endometrial, and ovarian cancers) are among the leading causes of morbidity and mortality in women worldwide. Recently, exosomes released by tumor-infiltrating CD8+ T cells have been under the spotlight in the field of cancer immunotherapy. Our study aims at elucidating the underlying mechanisms of the crosstalk between estrogen signaling and CD8+ T cells, and possible intervention values in uterine corpus endometrial cancer (UCEC).
Methods: Micro RNA-seq was conducted to screen differentially expressed micro RNA in UCEC. Bioinformatic analysis was processed to predict the target of miR-765. RNA silencing or overexpressing and pharmacologic inhibitors were used to assess the functions of ERβ/miR-765/PLP2/Notch axis in UCEC cell proliferation and invasion in vivo and in vitro. In vivo imaging was performed to evaluate the metastasis of tumor in mice. Combined fluorescent in situ hybridization for miR-765 and immunofluorescent labeling for CD8 was carried out to prove the co-localization between miR-765 and CD8+ T cells. Exosomes derived from CD45RO-CD8+ T cells were isolated to detect the regulatory effects on UCEC.
Results: miR-765 is characterized as the most downregulated miRNA in UCEC, and there is a negative correlation between miR-765 and Proteolipid protein 2 (PLP2) in UCEC lesion. Estrogen significantly down-regulates miR-765 level, and facilitates the development of UCEC by estrogen receptor (ER) β. Mechanistically, this process is mediated through the miRNAs (e.g., miR-3584-5p, miR-7-5p, miR-150-5p, and miR-124-3p) cluster-controlled regulation of the PLP2, which further regulates Ki-67 and multiple epithelial-mesenchymal transition (EMT)-related molecules (e.g, E-cadherin and Vimentin) in a Notch signaling pathway-dependent manner. Interestingly, the selective ER degrader Fulvestrant alleviates estrogen-mediated miR-765/PLP2 expression regulation and UCEC development in ERβ-dependent and -independent manners. Additionally, CD45RO-CD8+ T cell-derived exosomes release more miR-765 than that from CD45RO+CD8+ T cells. In therapeutic studies, these exosomes limit estrogen-driven disease development via regulation of the miR-765/PLP2 axis.
Conclusions: This observation reveals novel molecular mechanisms underlying estrogen signaling and CD8+ T cell-released exosomes in UCEC development, and provides a potential therapeutic strategy for UCEC patients with aberrant ERβ/miR-765/PLP2/Notch signaling axis.
Keywords: miR-765, PLP2, estrogen, T cell-derived exosomes, endometrial cancer
 Global reach, higher impact
Global reach, higher impact