13.3
Impact Factor
Theranostics 2021; 11(11):5296-5312. doi:10.7150/thno.55986 This issue Cite
Review
Role of serotonin receptor signaling in cancer cells and anti-tumor immunity
National Centre for Cell Science (NCCS), Ganeshkhind, Pune, MH-411007, India.
Received 2020-11-16; Accepted 2021-2-12; Published 2021-3-11
Abstract
Serotonin or 5-hydroxytryptamine (5-HT) is a neurotransmitter known to affect emotion, behavior, and cognition, and its effects are mostly studied in neurological diseases. The crosstalk between the immune cells and the nervous system through serotonin and its receptors (5-HTRs) in the tumor microenvironment and the secondary lymphoid organs are known to affect cancer pathogenesis. However, the molecular mechanism of - alteration in the phenotype and function of - innate and adaptive immune cells by serotonin is not well explored. In this review, we discuss how serotonin and serotonin receptors modulate the phenotype and function of various immune cells, and how the 5-HT-5-HTR axis modulates antitumor immunity. Understanding how 5-HT and immune signaling are involved in tumor immunity could help improve therapeutic strategies to control cancer progression and metastasis.
Keywords: 5-hydroxytryptamine, neuroimmune communication, neurotransmitter, serotonin receptor, serotonergic system
Introduction
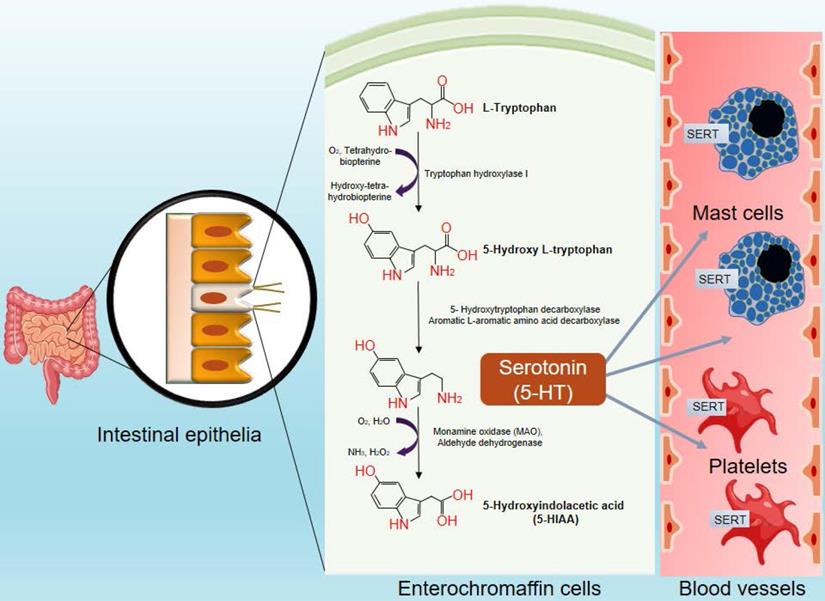
Serotonin (5-Hydroxytryptamine; 5-HT) is a neurotransmitter that regulates signal transduction in the central nervous system (CNS). Apart from its neurotransmitter function, it also provides a link between neuronal signals and an immune response, and establishes a well-defined neuroimmune communication network in the body. Serotonin is synthesized form the essential amino acid L-tryptophan by a two-step process catalyzed by the enzymes, tryptophan hydroxylase (TPH) and monoamine decarboxylase (also known as 5-hydroxytryptophan decarboxylase or DOPA decarboxylase), as depicted in Figure 1. TPH is the rate-limiting enzyme of this serotonin biosynthetic pathway. TPH enzyme exists in two isoforms, TPH1 and TPH2. TPH1 is expressed widely in the periphery, with highest expression in the enterochromaffin cells of the intestine [1]. The expression of TPH2 is restricted to neurons of the CNS. The existence of two isoforms of TPH indicates two different pools of serotonin in the body; one is restricted to the brain, and separated from the periphery by the blood-brain barrier, while the other pool is present in the peripheral systems. The enterochromaffin cells are the largest source of serotonin and accounts for almost 90-95% of the total serotonin production in the body. Mast cells and the brain are the second- and the third-largest sources of serotonin, respectively [2]. The crucial role of serotonin pathway metabolites as a molecular mediator between gut and brain and its contribution in neuroimmune communications are reviewed recently [3, 4].
Serotonin synthesized in the brain performs various neuro-psychotic functions such as interventions via serotonergic neurons, regulation of appetite, sleep, mood, pain and sexual behavior [5]. However, the serotonin produced in the periphery plays a wide array of functions such as vasoconstriction [6], regulating angiogenesis [7], controlling the bone density and osteoporosis [8], regulating diverse metabolic functions, including maintaining the blood glucose levels and obesity [9], and modulating gastrointestinal motility and gut microbiome content [10]. Serotonin also functions as a growth factor, and modulates the cell cycle [11], inflammation and immunity [12]. The serotonin synthesized by the enterochromaffin cells is secreted into the blood, most of which is taken up by the resident platelets and mast cells through the serotonin transporter (SERT) molecules present on their surface. The intracellular serotonin is then stored in dense granules at a high concentration of about 65 mmol/ml [12]. Normal serotonin levels in the blood fall in the ranges of from 0.7 to 2.5 µM. Serotonin levels can rise to concentrations in the millimolar range in different pathological conditions, and at the synapses of serotonergic neurons following stimulation [13]. It is released from the platelets and mast cells either upon their rupture or following crosslinking with IgE. After its physiological function is served, serotonin is degraded by the enzyme monoamine oxidase (MAO), resulting in the production of melatonin and 5-hydroxyindolacetic acid as metabolic byproducts [12].
Synthesis of peripheral serotonin. Serotonin is synthesized from L-tryptophan by the TPH1 enzyme in the enterochromaffin cells of the intestinal epithelium, which is taken up by platelets and mast cells present in the local circulation. They release the serotonin elsewhere in the body either upon stimulation or due to their rupture.
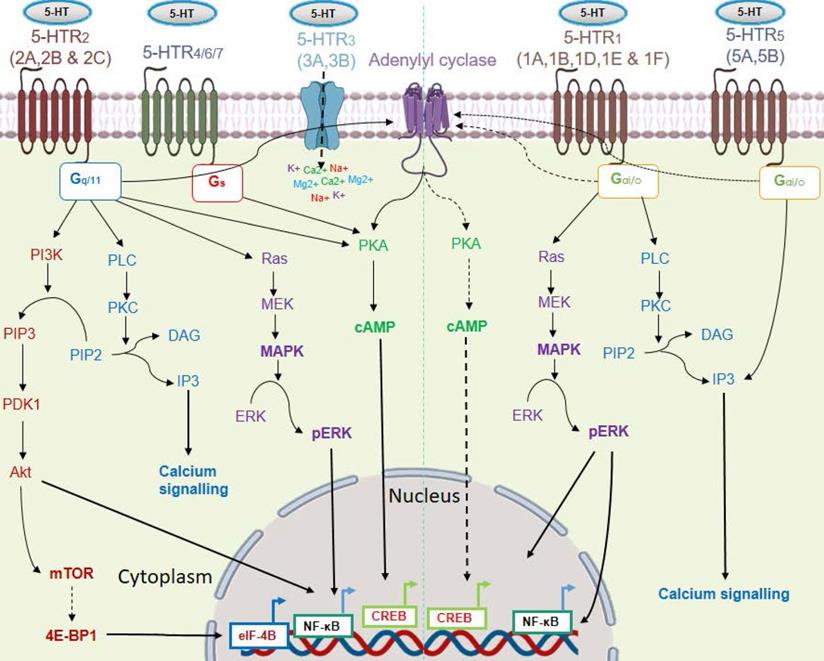
Response to serotonin is mediated via a wide repertoire of serotonin receptors and a serotonin transporter (SERT). At least 15 distinct subtypes of serotonin receptors (5-HTR1-7) are identified. Except for 5-HTR3, most of the 5-HTRs belong to the G protein-coupled receptor superfamily (GPCRs). The 5-HTR3 family of receptors (5-HTR3A & 5-HTR3B) forms a ligand-gated non-specific cation channel, which permits the passage of cations like Na+, K+, Ca2+, and Mg2+ upon serotonin binding. Among all the 5-HTRs, the 5-HTR1 (5-HTR1A-1F) and 5-HTR5 (5-HTR5A and 5-HTR5B) families of receptors are coupled with the intracellular G-protein Gαi/o. They inhibit adenylyl cyclase, resulting in subsequent downregulation of the PKA/cAMP activity [14]. Signaling through 5-HTR1A was also found to increase ERK phosphorylation in a PI3K/Akt-dependent pathway [15]. The 5-HTR2 subtypes coupled with an intracellular stimulatory G protein (Gq/11), which stimulates intracellular calcium signaling through the activation of phospholipase C. Serotonin also exerts its mitogenic activity through 5-HTR2, via mitogen-activated pathway kinase (MAPK) signaling [16]. 5-HTR4/6/7 triggers the PKA/cAMP axis via a stimulatory G protein (Gs) [17]. Signaling through its transporter involves entry of serotonin into the cells through SERT, followed by regulation of gene expression [18]. Within the cell, transglutaminase 2 (TGM2) incorporates serotonin into the glutamine residue of histone H3, which subsequently helps in transcription of the target gene via recruitment of the general transcription factor II D (TFIID) [18]. The various 5-HT/5-HTRs signaling pathways are shown in Figure 2. Due to its mitogenic property, serotonin also has a compelling role in cancer progression [19]. In this review, we have discussed the role played by serotonin and the influence of serotonin-associated signaling on immune cells and cancer cells, and their implications in anti-tumor immunity.
Effect of serotonin on various immune cells
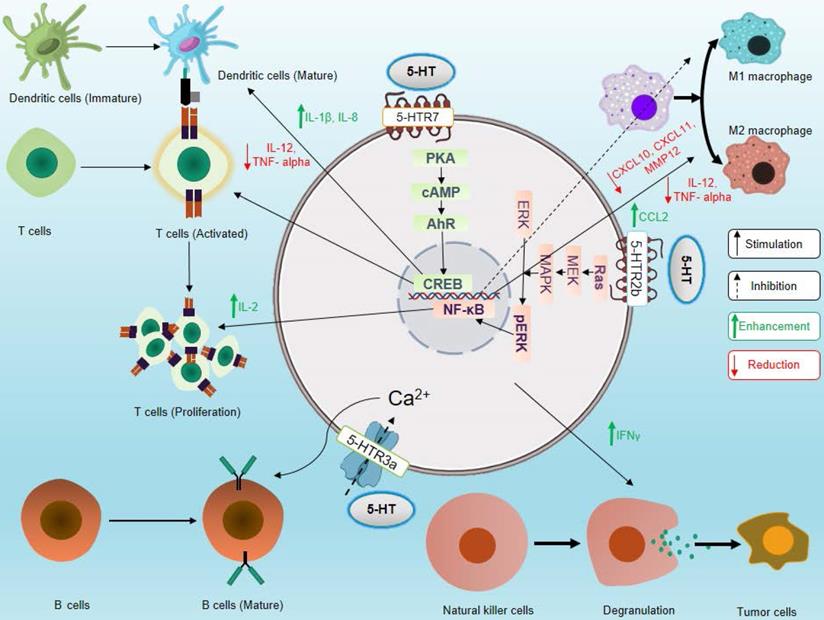
The role of serotonin in neuroimmune circuits in inter-organ communication and in inflammation and immunity is a new emerging area [20-22]. Several types of immune cells express the serotonergic components and modulate the effector and regulatory functions of immune cells. Recently, it has been shown that IL-33 is sensed by the enterochromaffin cells, which induces the release of 5-HT, in turn activates the enteric neurons, thus serving as a link for neuroimmune communication [23]. The effects of 5-HTRs signaling on various immune cells are depicted in Figure 3. The influence of serotonergic signaling on different immune cells and its importance in immunity are discussed below.
Signaling pathways of the serotonin receptor (5-HTR) subtypes. Serotonin signals through 15 receptor subtypes. Most of the receptors belong to GPCRs except the 5-HTR3 subfamily. These receptors activate four major interconnected signaling networks: PI3K/Akt, PKC/Ca2+, MAPK, and PKA-cAMP axis. Here, solid black arrows indicate stimulation of a pathway and black dashed arrows indicate inhibition of a pathway.
Effects of serotonin signaling on different components of the immune system. Serotonin signaling stimulates activation and proliferation of T cells, promotes maturation of DCs, supports B cell development, enhances cytotoxicity of NK cells, and stimulates polarization of macrophages toward the M2 phenotype. On the other hand, serotonin signaling inhibits M1 macrophage polarization. Here, solid black arrows indicate stimulation and black dashed arrows indicate inhibition, and green upward arrows indicate an increase in cytokine secretion, and red downward arrows indicate a decrease in cytokine secretion.
Macrophages
Macrophages are professional antigen-presenting cells (APCs) that form a bridge between the innate and adaptive immune systems. These cells process antigens and present antigenic peptides through MHC molecules to activate T cells. During development, depending on the nature of the microenvironment, macrophages can develop into either the pro-inflammatory (M1) or the anti-inflammatory (M2) phenotype [24]. While inducible nitric oxdide synthase (iNOS)+ M1 macrophages show pro-inflammatory properties [25, 26], M2 macrophages play a substantial role in the suppression of inflammation. M2 macrophages express arginase 1 (Arg1), which hydrolyses the amino acid L-arginine into urea and ornithine. The latter molecule is a precursor for L-proline and polyamines that play an important role in tissue repair, fibrosis and wound healing [27]. Tumor-associated macrophages (TAMs) also secrete anti-inflammatory cytokines like TGF-β and IL-10, which help in promoting the development of regulatory CD4+FoxP3+ T cells, and prevention of an exaggerated immune response. TAMs also overexpress immune checkpoint proteins like PD-L1 and B7-H4, which suppress Cytotoxic T lymphocyte (CTL) activity [28].
Macrophages express 5-HTR2B, 5-HTR7, and SERT [13, 29, 30]. Serotonin receptor signaling through 5-HTR2B in macrophages regulates the transcription of several genes (AP1, c/EBP, and SRF), which contributes to macrophage activation and polarization through phosphorylation of ERK1/2 molecules. 5-HTR2B stimulation also promotes M2 macrophage polarization, but inhibits M1 macrophage polarization [30, 31]. Casas-Engel et al. have demonstrated that 5-HTR2B stimulation increased the expression of M2-specific markers (SERINB2, COL23AI, THBS1, and STAB1) and reduced the expression M1-specific markers (ALDH1, CD1B, and MMP12) [30]. Serotonin also has an inhibitory effect on cytokine (TNF-α and IL-12) secretion by monocyte-derived M2 macrophages following lipopolysaccharide (LPS)-stimulation via the NF-κB pathway [30, 31]. Serotonin and its metabolites (N-acetyl serotonin and melatonin) are known to reduce nitric oxide (NO) production in the human macrophage cell line RAW264.7 by virtue of their reactive oxygen species (ROS) scavenging activity [32]. Serotonin impairs the expression of type-I IFN-dependent genes (CXCL10, CXCL11, IDO1, RSAP2, IL-27, and IFIT2) and stimulates TGF-β mRNA expression in the macrophages through the 5-HTR7-PKA axis [13]. Serotonin also helps macrophages to acquire the profibrogenic and anti-inflammatory phenotypes through 5-HTR7, and lack of 5-HTR7 expression results in diminished macrophage infiltration and enhanced skin fibrosis pathology in mice [13].
Dendritic cells (DC)
Though DCs are a relatively rare population of immune cells in the lymphoid organs, they are the most potent professional APCs that process and present antigens on their cell surface through MHC I and MHC II molecules, and activating CD8 and CD4 T cells, respectively [33]. DCs are characterized into 3 different subtypes - (i) Conventional DCs (cDCs), (i) Plasmacytoid DCs (pDCs), and (iii) Monocyte-derived or inflammatory DCs (MoDCs or iDCs). Two main subtypes of the DCs, type 1 cDCs (CD103a+ cDCs) and type 2 cDC (CD11b+ cDC2) develop from a common DC precursor [34]. pDCs are known to secrete type I IFNs and other tolerogenic factors such as TGF-β, IDO1 and IL-10, which promotes the induction of T cell anergy [34]. Inflammatory DCs are developed from monocytes under inflammatory conditions and migrate to the site of inflammation. The role of these monocyte-derived DCs in cancer is not yet well characterized [33]. Among the various subsets of DCs, type 1 cDCs are found to be systematically dysregulated in the early phase of pancreatic cancer, which leads to a poor prognosis [35]. Loss of abundance of type 1 cDCs is also associated with poor response to antitumor immunity in pancreatic ductal adenocarcinoma (PDAC) [36]. Type 1 cDCs are more potent activators of CD8 T cells and play a promising role in activating CTLs [33]. Recently, CD103+ cDC1 were generated in vitro, and their use as an immunotherapeutic agent showed promising results in cancer [37].
DCs are known to express different subtypes of 5-HTRs in a maturity-dependent manner. Immature DCs express 5-HTR1A, 5-HTR1E, 5-HTR2A and 5-HTR3; whereas mature DCs express 5-HTR2A, 5-HTR3, 5-HTR4, and 5-HTR7. DCs are also known to express SERT and the serotonin-degrading enzyme, MAO-A [38, 39]. Serotonin modulates cytokine secretion by DCs. It enhances the release of IL-1β and IL-8, and reduces the secretion of IL-12 and TNF-α from mature DCs by modulating a second messenger, cAMP, via 5-HTR4 and 5-HTR7 signaling. Serotonin also induces intracellular Ca2+ signaling in immature DCs via 5-HTR1 and 5-HTR2 signaling. Idzko et al. reported that IL-8 secretion is enhanced by serotonin through transcriptional regulation, whereas it modulates IL-1β secretion through a post-transcriptional regulation [38]. Serotonin through 5-HTR2B signaling, promotes the maturation of immature CD1a+ human monocyte-derived DCs upon TLR3 activation. Szabo et al. found that 5-HTR2B signaling in moDCs upregulates the expression of DC maturation markers like CD80, CD83, and CD86 [39]. Serotonin also promotes the maturation and migratory property of bone marrow-derived DCs (BMDCs) via 5-HTR7-mediated activation of a small GTPase, Cdc45. CCL19-induced chemotaxis of BMDCs is also enhanced by serotonin, probably, via the upregulation of CCR7 expression on the surface of DCs [40].
The serotonin-mediated downregulation of IL-12 in DCs has a widespread effect on T cell polarization. It has been shown that serotonin reduces DC-mediated IFNγ+Th1 polarization and IL-17+Th17 polarization of CD4 T cells, whereas it enhances the IL-4+Th2 polarization [38]. The expression of SERT on the surface of DCs enables them to take up peripheral serotonin and release it at immunological synapses formed between DCs and T cells, causing activation of T cells via 5-HTR1 signaling [41]. Though serotonin stimulates the maturation of CD1a+ moDC and BMDC, it suppresses the secretion of pro-inflammatory cytokines by DCs. The effect of serotonin on cDCs and pDCs is not well defined and needs further investigation.
Natural killer (NK) cells
NK cells are components of one of the most potent arms of the innate immune system against cancer. They impart its cytotoxicity against cancer cells by degranulation of cytolytic granules. Some cancer cells downregulate the expression of MHC I molecules on their surface, which helps them evade immune surveillance by CD8 cytotoxic T lymphocytes. However, NK cells can recognize the absence of MHC class I molecules on the surface of cancer cells and eliminate them [42, 43]. Though NK cells mainly kill tumor cells through contact-dependent cytotoxicity, they can also contribute to anti-tumor immunity by secreting cytokines like IFN-γ and modulating the functions of the innate and adaptive immune cells and contribute to anti-tumor immunity [44]. The effector functions of NK cells are characterized by the presence of activating receptors such as NKG2D, CD160, NKp46, NKp80, OX40, CD16 and 4-1BB, and inhibiting receptors such as NKG2A, KLRG1, CD161, TIGIT, CD96 and PD-1 on their surface [45]. Inhibitory receptors such as NKG2A recognizes the presence of MHC class I molecules on the surface of target cells and prevents NK cell degranulation. Conversely, activating receptors like NKG2D interacts with ligands such as H60 (a-c), RAE (α-ε), and those from the MULT1 family in mice, and with ligand like MIC (MICA and MICB) and those from the ULBP1-6 family in humans, and promote NK cell activation and degranulation [42].
While there is no clear evidence of the expression pattern of the serotonergic system on NK cells [46], it was observed that the numbers of NK cells were substantially higher in patients with major depressive disorder, which increased further upon treatment with serotonin reuptake inhibitor (SSRI) antidepressants [47]. Studies indicate that serotonin plays a role in enhancing the cytotoxicity, proliferation, and IFN-γ secretion potential of NK cells in the presence of autologous monocytes, through 5-HTR2 signaling [48, 49]. This influence, which can be inhibited by IFN-α, is probably effected through its depolarizing activity on the plasma membrane of NK cells [50]. Evans et al. showed that treatment with citalopram, an SSRI, enhanced NK cell-mediated cytotoxicity ex vivo, probably by causing an increase in the total available extracellular serotonin [51]. In the presence of serotonin, NK cells can also lyse Daudi cells that are preferentially resistant to NK cell-mediated killing [52]. In the tumor microenvironment, mononuclear phagocytes-like monocytes limit the cytotoxicity of NK cells by generating oxidative stress and releasing extracellular H2O2 and myeloperoxidase (MPO) [53]. The free radical-scavenging ability of serotonin efficiently protects NK cells from the inhibitory effect of H2O2, and peroxidases like MPO [54], as well as MPO-independent serotonin-sensitive oxygen radicals [55]. Though it has been shown that serotonin plays an important role in enhancing the cytotoxic potential of NK cells, the mechanism of serotonin signaling in these cells is not well studied, and needs to the explored.
T cells
T cells form the major pillar of the adaptive immune system. Upon activation, CD4 T cells differentiate into Th1, Th2, Th9, Th17, or Treg cells [56, 57]. CD8 T cells, on other hand, are the cytotoxic T lymphocytes (CTLs) that directly kill the target cells or antigen-expressing cells. Modern immunotherapeutic approaches have been designed to utilize the activities of effector T cells and suppress the regulatory T cells to eliminate cancer [58]. In patients with major depressive disorder, the blood levels of pro-inflammatory cytokines (IL-2, IL-12, and TNF-α) and monocyte chemoattractant protein-1 (MCP-1) are relatively higher, while, the blood levels of anti-inflammatory cytokines like IL-4 and TGF-β are relatively lower [59]. Reversal of this condition following treatment with antidepressants like SSRIs suggests that a role of serotonin plays a role in the modulation of cytokine secretion [59]. Serotonin is known to impede TNF-α and IL-1β production in human peripheral blood mononuclear cells (PBMCs) through 5-HTR2A signaling [60]. These studies suggested a strong correlation between blood serotonin levels and the inflammatory state of the body, which was also reflected in the Th1/Th2 balance [61]. Further, it was found that T cells have the ability to synthesize, store, degrade, and respond to serotonin and have a functional serotonergic system [41, 62]. The expression of components of the serotonergic system, such as TPH1, MAO-A, and VMAT1, is relatively higher in CD8 CTLs as compared to CD4 helper T cells [63, 64]. The expression of these enzymes increases further upon activation, and T cells actively respond to the autocrine-produced serotonin [63].
Serotonin causes activation of T cells via 5-HTR3-mediated upregulation of intracellular Na+ concentrations [65]. 5-HTR3 signaling also facilitates the proliferation of T cells [65]. 5-HTR1-mediated activation of cAMP signaling also activates T cells [41]. Leon-Ponte et al. showed that blocking in vivo synthesis of peripheral serotonin by para-chlorophenylalanine (PCPA) reduces the activation and proliferation of CD4+CD25+ T cells in the periphery [66]. Serotonin acts through 5-HTR7 signaling synergistically with TCR signaling and activates T cells. 5-HTR7 signaling activates NF-κB through ERK1/2 phosphorylation, which eventually drives T cells to secrete IL-2 and proliferate [66]. Serotonin also suppresses the ability of T cells to elicit delayed-type hypersensitivity reaction through 5-HTR2 signaling [67]. Many of the effects of signaling through serotonin receptors on T cell function were described using various chemical agonists and antagonists of 5-HTRs, which are discussed in Table 1 and Table 2. Though the role of serotonin signaling has been explored in T cells, the detailed mechanism of how this signaling influences the differentiation and functions of various subsets of T cells is not clearly understood.
Effects of serotonin receptor agonists in different immune cells
| Agonists | Receptors | Functions | References | ||||
|---|---|---|---|---|---|---|---|
| DC | Macrophage | T cells | NK cells | B Cells | |||
| S14506 | 5-HTR1A | -- | -- | Suppresses the rise of intracellular cAMP level in response to ATP | -- | -- | [133, 134] |
| 8-OH-DPAT | 5-HTR1A/7 | Enhances dose dependant secretion of IL-8 and IL-1β and suppressed IL-12 and TNF-α release from mature DCs. | -- | -- | Increases NK Cell cytotoxicity. | Increases proliferation of B cells, characterised by [3H] thymidine uptake and S phase transition. | [38, 48, 75] |
| (+)ALK | 5-HTR1A | -- | -- | -- | a) Stimulates NK cell mediated killing of NK insensitive Daudi cells in the presence of IL-1, TNF-α and monocyte b) Increases IFN-γ secretion in the presence of IL-2. | -- | [49, 52] |
| AnHCL | 5-HTR1B | Stimulates intracellular Ca2+ spike in immature DCs. | -- | -- | -- | -- | [38] |
| BRL5443 | 5-HTR1E/1F | Stimulates intracellular Ca2+ spike in immature DCs. | -- | -- | -- | -- | [38] |
| DOI | 5-HTR2 | Stimulates intracellular Ca2+ spike in immature DCs. | -- | Inhibits TNF-α production from PBMC. | -- | -- | [38, 60] |
| BW-723C86 | 5-HTR2B | -- | a) Stimulates M2 macrophage polarization by signalling through the ERK pathway b) Inhibits LPS stimulated TNF-α production through the NFκB pathway and stimulates LPS stimulated CCL2 production from monocyte-derived macrophage thus imparts anti-inflammatory effect through AhR activation. | -- | -- | -- | [ 31, 30] |
| 2-Methyl- Serotonin | 5-HTR3 | Enhances dose dependant secretion of IL-8 and IL-1β and suppresses IL-12 and TNF-α release from mature DCs. | -- | Activates T cells via increasing intracellular Na+ and thus helping the progression of T cell from S phase to G2/M phase. | -- | -- | [38, 65] |
| 2MHT | 5-HTR4 | Enhances dose dependant secretion of IL-8 and IL-1β and suppresses IL-12 and TNF-α release from mature DCs. | -- | -- | -- | -- | [38] |
| 5-CI | 5-HT1/4/7 | Enhances dose dependant secretion of IL-8 and IL-1β and suppresses IL-12 and TNF-α release from mature DCs. | -- | -- | -- | -- | [38] |
| AS19 | 5-HTR7 | -- | a) Enhances the expression of PDE2A and THBS1 through PKA-cAMP axis and thus promotes anti-inflammatory gene profile. b) Mimics the effect of 5-HT mediated downregulation of TNF-α and IL-12p40 secretion. | Stimulates T cell proliferation alike 5-HT. | -- | -- | [13, 66] |
B cells
The presence of B cells along with CD8 T cells in the tumor microenvironment (TME) has been reported, proved to be a better prognostic marker in many types of cancers, including melanoma, breast cancer and ovarian cancer [68, 69]. The ability of B cells to serve as antigen-presenting cells (APCs) may promote better outcomes through B cell-T cell cooperation [70]. One of the major functions of B cells is to produce antibodies upon activation. Antibodies against neoantigens that are sometimes detected in the serum, are considered as a good prognostic marker in some cancers [71]. Such antibodies can promote opsonization and boost antibody-mediated phagocytosis by macrophages, or ADCC-mediated killing of cancer cells by NK cells [72].
Both normal and neoplastic B cells in germinal center malignancies seem to express 5-HTR3A [73]. B cells express SERT and have the capacity to reuptake serotonin from the periphery [74]. Higher numbers of B cells have been reported in major depressive disorder (MDD) patients, following treatment with anti-depressant SSRIs [47]. Rinaldi et al. have shown that serotonin plays a role in B cell development through 5-HTR3A signaling in the germinal centers [73]. Serotonin also enhances the mitogen-stimulated proliferation of B cells [73, 75]. Serafin et al. have described the role of serotonin in the apoptosis of the Burkitt lymphoma cell line [76]. This may be mediated through SERT, since SSRI treatment completely abolished this effect [76]. B cells are the major source of humoral immunity in the body, and the impacts of serotonin on B cells needs to be explored further. Understanding the detailed molecular mechanisms will give greater insights into the interactions between serotonin signaling and B cells, and their importance in immunity.
Role of serotonin in cancer cells
Cancers originating in different tissues express different serotonin receptors, which are listed in (Table 3). The role of serotonin in various cancers is discussed below.
Hepatocellular carcinoma
Hepatocellular cancer (HCC) cells express different serotonin receptors, as seen from HCC cell lines and tumor tissue sections from HCC patients (Table 3). It has been observed that serum and platelet-driven serotonin levels are higher in HCC patients than in normal individuals [77]. Serum serotonin levels have also been shown to be upregulated in the chemical-induced HCC mouse model [78], suggesting its involvement in the prognosis and progression of HCC [77]. Through the 5-HTR1B and 5-HTR2B signaling pathways, serotonin promotes the proliferation of Huh7 and HepG2 human HCC cell lines under serum-deprived conditions by phosphorylation of ERK1/2 via the MAPK pathway [16, 79]. TPH1 knockout mice show defective peripheral serotonin levels and develop resistance to CCL4-mediated liver tumor formation, suggesting that serotonin may play an essential role in liver cancer development [80]. Serotonin is also found to affect autophagy in cancer cells. Autophagy or “self-eating” is a process that enables the cells to degrade or recycle damaged organelles and aged protein for maintenance of cellular homeostasis [81]. Autophagy plays a dual role in cancer cells by regulating both cell survival and death. It helps to promote cancer by enhancing their ability to sustain their high metabolic demand by recycling damaged organelles and proteins. On the other hand, autophagy also performs a tumor suppressive role via maintaining cellular homeostasis by reducing cellular damage resulting from cellular stress [81]. In pancreatic ductal adenocarcinoma (PDAC), autophagy leads to the degradation of MHC class I molecules, thus reducing antigen presentation by the cancer cells. These molecular changes result in resistance to immune checkpoint therapy, which can be reversed by combined treatment with an autophagy inhibitor or anti-CTLA4 antibodies [82]. Autophagy is regulated by the LC3β and ATG8 proteins. LC3β promotes membrane fusion, elongation, and sealing of the autophagosome during autophagosome biogenesis [83]. Another protein, p62 or sequestome-1, acts as a receptor that targets specific cargoes to autophagosomes by interacting with LC3β. When the process of autophagy is disrupted, p62 accumulates in the cytoplasm as ubiquitin-containing aggregates, which indicates inhibition of the autophagy in the cell [84]. In serum-deprived conditions, cancer cells survive by enhancing autophagy, marked by an increase in the LC3β levels and a decrease in p62 accumulation in the cytoplasm. Serotonin shows a somewhat paradoxical role in the regulation of autophagy in hepatocellular carcinoma. Soll et al. showed that serotonin inhibits autophagy in HCC cells [80]. On the contrary, Niture et al. showed that serotonin stimulates autophagy and induces apoptosis and steatosis (abnormal retention of lipids within the cell) in HepG2 and SK-Hep1 human HCC cell lines [80, 85]. 5-HTR1B and 5-HTR2B signaling promotes autophagy by upregulating LC3β and autophagy-related effector proteins like Beclin1, ATG3, 4EBP1, and s65 [85].
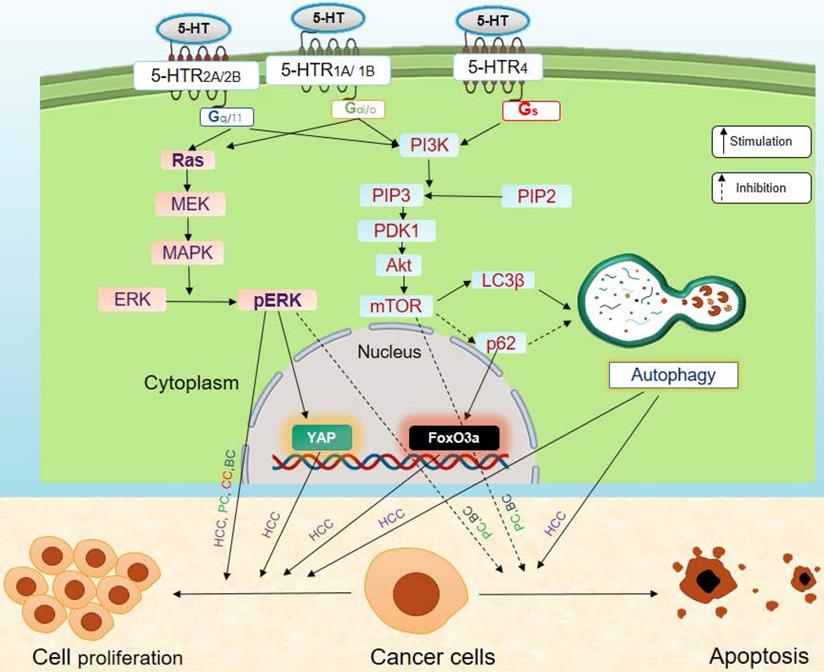
Serotonin modulates the development and progression of cancer via regulation of the oncogene Yes-associated protein (YAP), and the tumor suppressor, Vestigial-like family member 4 (VGLL4) protein [77, 86]. Interestingly, YAP is an effector of the Hippo pathway that increases cell proliferation and decreases apoptosis in cancer cells [87]. YAP binds to the transcription factor, TEF-1 and members of the abaA domain family member (TEAD). It promotes transcription of TEAD regulatory genes that are involved in increasing cell proliferation, suppressing apoptosis, and enhancing invasiveness and metastasis of cancer cells via epithelial-mesenchyme transition (EMT) (Figure 4). On the other hand, VGLL4 is a transcriptional cofactor that acts as a tumor suppressor. It binds to TEAD and inhibits the interactions between TEAD and YAP, thus suppressing TEAD-transcribed gene expression [88]. An increase in the YAP/VGLL4 ratio is associated with cancer progression. Peripheral serotonin increases the YAP/VGLL4 ratio via phosphorylation of ERK through 5-HTR2B signaling (Figure 4). This 5-HTR2B-pERK-YAP axis promotes the progression of tumors [77, 86]. Serotonin also increases expression and phosphorylation of the oncogene FoxO3a in nutrition-starved Huh7 cells, through 5-HTR2B. FoxO3a promotes the growth and proliferation of serum-deprived human HCC cells. Serotonin also promotes cellular survival by inhibiting apoptosis of HCC cells by stimulating Akt phosphorylation [37, 79]. Serotonin is known to induce oncogenic Notch signaling in HepG2 and Sk-Hep1 human HCC cell lines, likely through 5-HTR1B and 5-HTR2B. In turn, this increases the survival capacity of HCC cells [85].
Effects of serotonin receptor antagonists on immune cells
| Antagonists | Receptors | Functions | ||||
|---|---|---|---|---|---|---|
| DC | Macrophage | T cells | B cells | References | ||
| (+) WAY 100135 | 5-HTR1A | -- | -- | -- | Inhibits the proliferative effect of serotonin on B cells. | [75] |
| Methylseride, ketanserin | 5-HTR2 | Abolishes the 5-HT-mediated intracellular Ca2+ spike in immature DCs. | -- | a) Inhibits the capabilities of T cells to generate delayed type of hypersensitivity. b) Abolishes the 5-HT-mediated reduction in IL-1β secretion in PBMC. | -- | [38, 60, 67] |
| SB-204741 | 5-HTR2B | -- | a) Inhibits M2 macrophage specific gene (STAB1 & SERPINB2) expression. b) Reduces the expression of MMP12 and enhances the CD1B mRNA expression. | -- | -- | [30] |
| RS-39604 | 5-HTR4 | Inhibits the LPS mediated IL-8 and IL-1β release through 5-HTR4 stimulation from immature DCs. | -- | -- | -- | [38] |
| SB-269970, SB-258719 | 5-HTR7 | a) Inhibits the LPS mediated IL-8 and IL-1β release through 5-HTR7 stimulation from immature DCs. b) Abolishes the increase in the length of processes of mature DCs by 5-HTR7 agonism. | a) Inhibits 5-HT mediated upregulation of TGFβ1 mRNA in monocyte-derived macrophages. b) Dampens the effect of 5-HT on limitation of expression of type I IFN responsive gene via PKA-cAMP axis. c) Reverses the 5-HT mediated inhibitory effect on LPS-stimulated TNF-α and IL-12p40 release from monocyte-derived macrophage. d) Dampens the 5-HT-mediated upregulation of M2 macrophage specific gene expression (SERPINB2, THBSI, and COL23AI). | Inhibits 5-HT mediated phosphorylation of the ERK1/2 and Iκβα in T cells. | -- | [13, 30, 38, 40, 66, 30] |
Serotonergic system in different cancer cells
| Cancer type | Models used | Serotonin receptor (5-HTR) subtypes expressed | References |
|---|---|---|---|
| Hepatocellular Carcinoma | Human hepatocellular carcinoma cell lines (Huh7 and HepG2), human HCC tissues, and mouse xenograft models. | 1A, 1B, 2B, 7 | [16, 77, 79, 85] |
| Prostate Cancer | Prostate cancer cell lines (PC3, DU-145, and LCaNP), and human prostate cancer tissue. | 1A, 1B, 1D, 2A, 2B, 2C, 4 | [89, 91, 93] |
| Breast Cancer | Hormone responsive cell lines (MCF7 and T47D), Triple-negative breast cancer cell lines (MDA-MB-231, HCC-1395, and Hs578T), and human breast cancer tissues. | 1B, 2A, 2B, 3A, 4, 7 | [111, 131] |
| Colon Cancer | Mice colon cancer cell lines (CT26 and MC38), human colon cancer cell line (HT29), and human colon cancer tissue. | 1A, 1B, 3, 4 | [98-101] |
| Pancreatic cancer | Human pancreatic cancer cell lines (AsPC-1, BxPC-3, Capan-2, CFPAC-1, HPAC, PANC-1 and SW1990PANC-1 and MIAPaCa-2) and human pancreatic cancer tissue microarrays. | 1B, 1D, 2B | [115, 116, 132] |
| Ovary cancer | Human ovarian cancer cell lines (SKOV3, HEYA8, 2774, ES2, TOV112D, OV90, SW626, UWB1.298 and CaOV3) and human ovarian cancer tissues | 1A, 1B, 2A, 2B, 4 | [118, 119] |
| Melanoma | Uveal melanoma cell line, human skin melanoma cell line (IPC-298) | 2A, 2B, 2C | [120, 121] |
| Glioblastoma | Glioblastoma cell lines ((U-373 MG, U-138MG, U-87 MG, DBTRG-05MG, T98G, H4, CCF-STTG1 and Hs 683) and Human glioblastoma tissues. | 5A, 7 | [124, 125] |
| Lung Cancer | In vivo mouse model of Lewis lung cancer and patient lung adenocarcinoma samples with depression | 1B | [101, 105] |
| Bile duct cancer (Cholangiocarcinoma) | Cholangiocarcinoma cell lines (Mz-Cha-1, Huh-28, Hucc-T1, CCLP-1, SG231, TFK1) | 1A, 2A, 2B, 4, 6 | [96] |
| Urinary bladder cancer | Human bladder cancer cell line (HT1376) | 1A, 1B, 1D, 2A, 2B, 2C | [93, 127] |
| Placenta cancer (Choriocarcinoma) | Human trophoblast cell lines (JEG3 and BeWo) | 2A | [128] |
Effects of serotonin signaling on cancer. Serotonin signaling promotes tumor progression by stimulating proliferation and inhibiting apoptosis of cancer cells via MAPK and PI3K/Akt signaling axis. Here, solid black arrows indicate stimulation and dashed black arrows indicate inhibition. Abbreviations: BC, Breast cancer; CC, Colon cancer; HCC, Hepatocellular carcinoma; PC, Prostate cancer.
Prostate cancer
Various serotonin receptor subtypes are expressed on androgen-sensitive (PC3, DU145) and androgen-insensitive (LCaNP, hPCP) prostate cancer cell lines. Serotonin receptor subtypes are expressed at both the primary site and the metastatic sites in humans [89-91]. Serotonin stimulates the growth and proliferation of human prostate cancer cell lines (PC3, DU-145, LCaNP, hPCP, etc.) [89-92]. The proliferative effect of serotonin is more prominent in androgen-insensitive prostate cancer cells than in androgen-sensitive prostate cancer cells. Serotonin mediates its pro-proliferative effect on prostate cancer cells through 5-HTR1A, 5-HTR1B, 5-HTR2B, and 5-HTR4 signaling [89-92]. Serotonin modulates the mitogenic MAPK/ERK and PI3K/Akt signaling pathways in prostate cancer, and stimulates phosphorylation of ERK1/2 through the MAPK pathway in the PC3 prostate cancer cell line (Figure 4). This effect of serotonin is likely mediated through 5-HTR1A signaling, though the possibility of the involvement of other receptor subtypes cannot be ruled out. Serotonin also increases the phosphorylation of Akt, with concomitant activation of the PI3K/Akt pathway in the DU-145 prostate cancer cells (Figure 4). This modulation of the Akt pathway by serotonin also suppresses apoptosis in prostate cancer cell lines, possibly by signaling through 5-HTR1B [37, 90, 93]. Together, these studies suggest that serotonin influences prostate cancer progression. However, there is no conclusive evidence of the modulation of the PI3K/Akt pathway in PC3 and LNCaP prostate cancer cell lines, since they have a constitutive activation of PI3K/Akt pathway due to the deletion of the tumor suppression gene, PTEN [15, 94].
Cholangiocarcinoma (Bile duct carcinoma)
Cholangiocarcinoma is one of the most devastating intrahepatic and extrahepatic bile duct cancer [95]. In cholangiocarcinoma, serotonin metabolism is found to be dysregulated. Higher levels of serotonin have been detected in the serum of cholangiocarcinoma patients, as compared to healthy individuals. This phenomenon can be explained by the fact that cholangiocarcinoma cell lines (Mz-Cha-1, HuH-28, HuCC-T1, CCLP-1, SG231, and TFK1) show higher expression of the serotonin biosynthetic enzyme (TPH1), and lower expression of the serotonin degrading enzyme (MAO-A) [96]. Serotonin promotes the growth and proliferation of cholangiocarcinoma cells via 5-HTR1A, 5-HTR2A, 5-HTR2B, 5-HTR4, and 5-HTR6 signaling [96]. As cholangiocarcinoma cells can synthesize serotonin, they can also respond to serotonin in an autocrine manner. Alipini et al. demonstrated that blocking serotonin synthesis with the TPH1 blocker, PCPA, reduces the proliferation of cholangiocarcinoma cells [96]. Blocking of TPH1 with PCPA also led to reduced tumor growth, increased area of tumor necrosis, and increased fibrosis in the tumor [96]. McMillin et al. showed that an increase in the free serotonin levels in the circulation caused by treatment with antidepressant SSRIs (sertraline and fluoxetine) enhances tumor progression [96]. These effects may be mediated through the interaction of SSRIs with the components of the TME, rather than with the cancer cells. Alpini et al. showed that serotonin treatment increase BrdU incorporation and cell cycle progression in Mz-ChA-1 cells, which can be blocked by the TPH1 inhibitor, PCPA [96].
Colon cancer
In colon cancer, serotonin modulates cancer cell proliferation and angiogenesis, which ultimately culminates in tumor progression. Serum serotonin levels in colon cancer patients are elevated, suggesting that serotonin may be a potential prognostic marker for colon cancer progression [97]. The human colon cancer cell line HT29 also shows the expression of 5-HTR1, 5-HTR3, and 5-HTR4 [98, 99]. Serotonin exerts mitogenic and anti-apoptotic effects on the HT29 cells mainly through 5-HTR1A, 5-HTR1B, 5-HTR3, and 5-HTR4 [98-100]. This effect is also mimicked in mice colon cancer cell lines (CT26 and MC38) [101]. Nocito et al. showed that peripheral serotonin deficiency slows tumor growth in mouse models with colon cancer allografts, and that external serotonin administration can reverse this condition [101]. However, this effect of serotonin is not a consequence of its direct influence on cancer cells. Instead, serotonin increases angiogenesis by decreasing matrix metalloproteinase 12 (MMP12) expression in tumor-infiltrating macrophages. MMP12 keeps angiogenesis in check by increasing the circulatory levels of angiostatin, a potent inhibitor of angiogenesis [101]. In colon cancer, serotonin modulates cancer cell proliferation and angiogenesis that ultimately culminates in tumor progression.
Kannen et al. showed that treatment with the antidepressant fluoxetine (an SSRI) treatment inhibits the proliferation of the mouse colon cancer cell line HT29 [102], and inhibits carcinogen, dimethyl hydrazine-induced colon cancer in mice. This effect of the SSRI is mainly mediated through its inhibitory effect on micro-vessel formation and reduction of angiogenesis in the TME [103], and by affecting the energy-generating machinery of the cancer cells [104]. Further studies are required to explain the detailed mechanism of serotonin's effect on colon cancer.
Lung cancer
Clinicians have observed significantly higher serum serotonin levels in lung adenocarcinoma patients with depression, as compared to individuals without depression. Additionally, the expression of serotonin receptor subtypes like 5-HTR1A and 5-HTR1B in tumor tissues are also higher in patients with depression [105]. Serotonin also plays a significant role in tumor progression in mice. In the Lewis lung cancer (LLC) allograft model, mice deprived of peripheral serotonin show significantly reduced tumor growth. This suggests that serotonin has a role in lung cancer progression [101]. Serotonin controls tumor growth by stimulating angiogenesis via modulation of the MMP12/angiostatin axis [101]. Lung adenocarcinoma is also known to express 5-HTR3A, and knockdown of this receptor reduces the proliferation of lung adenocarcinoma cells [106]. Liu et al. showed a negative correlation between 5-HTR1A and 5-HTR1B expression in lung adenocarcinoma tumors in patients with depression, and the number of tumor-specific T cells as well as the ratio of CD8/CD4 T cells present in the TME. Patients with higher expression of intratumoral 5-HTR1A and 5-HTR1B, exhibit more CD4+CD25+FoxP3+ Treg cells, and higher levels of expression of PD-L1 and pSTAT3 molecules in the TME [105]. These studies indicate that sustained depression leads to the development of an immunosuppressive tumor microenvironment. This is associated with higher tumor-favoring cytokine release and transition of effector T cells to the regulatory phenotype [105]. These reports thus indicate the involvement of serotonin in both tumor growth and immune modulation of the TME.
Breast cancer
High levels of expression of TPH1 in the human breast cancer cell lines MCF7 and T47D indicate that they can synthesize large amounts of serotonin [107]. MCF7 and human breast cancer tissues express the serotonin receptor subtype 5-HTR2A and 5-HTR3A [108, 109]. Human cancer tissues from ductal carcinoma and invasive lobular cancer also express many of serotonin receptor subtypes [110], which are listed in Table 3. Serotonin enhances the rate of proliferation of MCF7 cells via 5-HTR2A and 5-HTR3A signaling [108, 111]. Inhibiting these receptors with chemical antagonists was found to be reduce the proliferation and induced apoptosis of the MCF-7 cell lines [108]. The triple-negative breast cancer (TNBC) cell lines (MDA-MB-231, HCC-1395, and Hs578T) shows higher expression of TPH1 and exhibit a stronger response to serotonin via 5-HTR7 in an autocrine manner, as compared to hormone-sensitive cell lines (MCF7, T47D). 5-HTR7 signaling activates the adenylyl cyclase, MAP kinase, and PI3K/Akt pathways [107]. A dysregulated serotonin system enable proliferation, EMT, and survival in cancer cells via suppression of apoptosis [107]. Treatment with SSRI and TPH1 inhibitors has been reported to suppress the growth of breast tumor-initiating cells (BTIC), and synergizes with chemotherapy, inhibiting breast cancer xenograft growth in mice [112]. Recently, Gwynne et al. showed that the 5-HTR4A antagonist, SB699551, targets human breast tumor-initiating cells through the canonical Gαi/o-coupled pathway and the PI3K/AKT/mTOR axis [113].
Sola-Penna et al. showed that serotonin promotes proliferation of the MCF-7 and MDA-MB-23 cell lines, and inhibits apoptosis in these cells by promoting glycolysis and oxidative phosphorylation. This action is performed through 5-HTR2A/2C signaling-mediated ERK1/2 and Akt phosphorylation, and HIF1α expression. Serotonin also stimulates mitochondrial biogenesis in MCF-7 and MDA-MB-23 cells through activation of the cofactor PGC1α via the PKA-cAMP axis, through 5-HTR2A/2C signaling [114]. These phenomena may ultimately help mitigate a more invasive and aggressive forms of metastatic breast carcinoma.
Pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) tissues show a dysregulated serotonergic system. Microarrays of PDAC tissues from mice as well as in humans show higher expression of the serotonin-synthesizing enzyme TPH1, and lower expression of the serotonin-degrading enzyme MAO-A, as compared to control pancreas tissues [115]. This indicates increased serotonin synthesis and reduced serotonin degradation in PDAC tumors. Among 13 different subtypes of 5-HTRs, 5-HTR1B, 5-HTR1D, and 5-HTR2B were found to be overexpressed in PDAC tissues as well as in pancreatic cancer cell lines, as compared to normal pancreas, whereas the expression of other subtypes of 5-HTRs was almost undetectable [115, 116]. Overexpression of 5-HTR2B in PDAC is associated with a poorer prognosis [115, 117].
Jiang et al observed that serotonin provides protection to the pancreatic cancer cell lines (BxPC-3, HPAC, PANC-1, and SW1990) by increasing cell viability and decreasing cellular apoptosis [115]. Serotonin promotes the Warburg effect in pancreatic cancer cell lines by promoting glycolysis. Serotonin also increases the expression of HIF1α and c-MYC in pancreatic cancer cells. The protective effect of serotonin through 5-HTR2B signaling on pancreatic cancer cell lines is mediated via the PI3K/Akt/mTOR axis. This cancer-promoting effect has also been observed in the pancreatic cancer xenograft model, where the antagonism of this receptor decreased cancer growth and metastasis [115]. In addition to 5-HTR2B signaling, 5-HTR1B and 5-HTR1D signaling also promote the proliferation and viability of pancreatic cancer cells (PaCa). 5-HTR1B and 5-HTR1D also promotes the invasion and metastasis, and inhibits clonogenicity of PaCa pancreatic cancer cells [116]. Serotonin promotes EMT by upregulating TCF8/ZEB1 and snail proteins that promote EMT. 5-HTR1B and 5-HTR1D signaling promote metastasis by regulating the uPA/MMP2 axis [116]. This combinatorial action of 5-HTR1B and 5-HTR1D signaling is mediated through Src-focal adhesion kinase (FAK) and the transglutaminase 2 - NF-κB signaling axis [116].
Ovarian cancer
5-HTR1A, 5-HTR1B, 5-HTR2B, and 5-HTR4 are expressed in the normal ovary as well as in ovarian malignancies. Henriksen et al. found that these receptors are overexpressed in benign and non-invasive form of tumors, whereas their expression is of these receptors were downregulated in more invasive forms of ovarian tumors [118]. In addition to tumor sections, different ovarian cancer cell lines (SKOV3, HEYA8, 2774, ES2, TOV112D, OV90, SW626, UWB1.298, and CaOV3) also show overexpression of 5-HTR1A, 5-HTR1B, 5-HTR1D, and 5-HTR2A. However, only the SW626, UWB1.298, and CaOV3 cell lines show overexpression of 5-HTR2B, as compared to normal ovarian cells [119].
Christensen et al showed that treatment with both serotonin and 5-HTR2A agonist increases the proliferation and survival of SKOV3, CP20, and ES2 ovarian cancer cell lines. They also showed that injection of serotonin and the SSRI, sertraline, increases the tumor weight and Ki67 expression in the SKOV3 tumor model in athymic nude mice [119]. These finding collectively indicate that serotonin signaling promotes ovarian cancer development.
Melanoma
The abnormal overexpression of the transcript encoding 5-HTR2B in uveal melanoma is considered as a predictive marker for the formation of liver lesions in the metastatic form of uveal melanoma [120]. Le-Bel et al. revealed that this deregulated expression of 5-HTR2B in the metastatic uveal melanoma cell line is mainly due to the inability of the proteasome to degrade 5-HTR2B in the cells [120].
The human skin melanoma cell line IPC-298 shows a constitutive expression of 5-HTR2A, 5-HTR2B, and 5-HTR2C. In contrast to other studies, Muller et al. showed that serotonin enhances radiation-induced inhibition of melanoma cell proliferation. This was likely due to the release of serotonin from mast cells following their exposure to ionizing radiation [121].
Liu et al. showed that the use of the 5-HTR4 agonists, tegaserod, significantly reduces melanoma cell growth and induces apoptosis in these cells. Tegaserod also reduces the tumor volume in the mouse melanoma model. However, tegaserod mediates this action through a serotonin-independent signaling mechanism, where it blunts the phosphorylation of S6 through inhibition of the PI3/Akt/mTOR pathway [122].
Glioblastoma
Glioblastoma, a cancer of glial cells in the brain, and it is one of the deadliest forms of cancer. Depression and psychological distress are associated with worse outcomes with respect to glioblastoma progression. Otto-Meyer et al. showed that the treatment of glioblastoma patients with SSRI, which is often prescribed to treat psychological illness in these patients, does not significantly affect the overall survival rate of those patients [123].
Glioblastoma cell lines (U-373 MG, U-138MG, U-87 MG, DBTRG-05MG, T98G, H4, CCF-STTG1 and Hs 683) express the 5-HTR7 receptor subtype [124]. Glioma tissues also express the 5-HTR5A subtype, but its expression of 5-HTR5A is lower in high-grade glioma than in low-grade glioma [125]. Lieb et al. showed that serotonin promotes IL-6 secretion by the U-373 MG astrocytoma cell line through activation of the 5-HTR7 signaling mediated p38 MAPK and protein kinase Cє pathways. This mechanism may facilitate tumor progression by promoting a pro-inflammatory microenvironment [126]. Lu et al. showed that agonizing 5-HTR5A through valerenic acid inhibits the growth of glioblastoma cells both in vitro and in vivo, by promoting the elevation of intracellular ROS levels and activation of AMPK. Through this mechanism, valerenic acid also inhibits the EMT and subsequent metastasis in glioblastoma patients [125].
Urinary bladder cancer
Different subtypes of serotonin receptors are found to be expressed in the human urinary bladder cancer cell line HT1376 and human bladder cancer tissues. Serotonin exerts a mitogenic effect and increases the proliferative potential of HT1376 cells via 5-HTR1A, and more potently through 5-HTR1B [127]. Hence, this study suggests using 5-HTR1B antagonists as a therapeutic alternative for use in urinary bladder cancer [127].
Placental cancer or choriocarcinoma
The expression of 5-HTR2A is apparent on the trophoblast cancer cell lines JEG3 and BeWo, which show an enhanced proliferation by altering the cell cycle, when treated with serotonin treatment [128]. This action of serotonin is mediated via the MEK-ERK1/2 and JAK/STAT3 pathways, through 5-HTR2A signaling [128].
Gastric cancer
El-Salhy showed that serotonin, in combination with octreotide (a mimic of somatostatin) and galanin (a neuropeptide encoded by the GAL gene, and expressed in the brain, spinal cord and gut), reduces viability and increases apoptosis of in the human gastric cancer cell line (AGS). This combination was also result in reduced the tumor growth in vivo [129].
Role of serotonin in tumor immunity
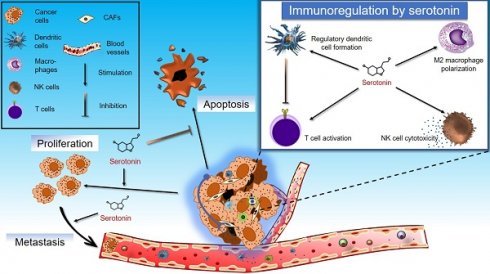
The various effects of serotonin signaling on immune cells has made it a target for studies vis-à-vis tumor immunity. For example, it has been reported that 5-HTR2B and 5-HTR7 signaling promotes the development of anti-inflammatory M2 macrophages, and the latter promotes tumor development [30, 31]. Nocito et al. found that serotonin can also downregulate the expression of matrix metalloproteinase 12 (MMP12) in macrophages, and promote tumor progression by increasing angiogenesis in the mouse model of colon cancer [101]. MMP12 has an anti-angiogenic effect as it cleaves plasminogen to the angiogenesis suppressor, angiostatin. Furthermore, 5-HTR2B is over-expressed on M2 TAMs adjacent to VE-cadherin-positive endothelial cells in the tumor. This indicates that serotonin may promote angiogenesis by modulating of blood vessel formation, assisted by adjacent macrophages through 5-HTR2B signaling [30]. Serotonin promotes maturation and chemotaxis of BMDCs [39, 40]. Serotonin signaling also promotes anti-inflammatory DC development, which in turn has the potential to skew T cell polarization towards the regulatory phenotype [41]. Regulatory T cells normally suppress cytotoxic T cell activity, and in turn, promote tumor development [58]. Duerschmied et al. have shown that platelet serotonin also inhibits the infiltration of innate immune cells like neutrophils and monocytes in the site of inflammation, and leukocyte rolling on endothelial cells is faster in the absence of peripheral serotonin [130].
On the other hand, serotonin has been found to enhance the cytotoxic potential of NK cells [48, 49], and as well as serotonin signaling through 5-HTR3A and 5-HTR7 has been reported to promote T cell activation [66]. However, the detailed mechanism of action of serotonin on NK cells, and of T cells polarization after serotonin signaling mediated activation is still obscure.
Taken together, most of the ways in which serotonin signaling influences immune cells indicates that it facilitates tumor development via suppression of anti-tumor immunity. Therefore, the use of different antipsychotic drugs and chemicals that have the potential to modulate the serotonergic system, could have important implications in modulation of tumor immunity.
Influence of application of agonists and antagonists of serotonin receptors on antitumor immunity
Serotonin has a total of 15 receptor subtypes and through which it is found to modulate a wide array of functions on both immune cells, stromal cells, and cancer cells. The importance of these receptors in tumor and immune modulation have been studied using different chemicals that either stimulate or inhibit signaling via the different 5-HTRs. These chemical agonists and antagonists of serotonin receptors are immensely important in explaining the role of serotonin in tumor immunity effected through the different receptor subtypes. The use of various chemical agonists and antagonists in immunity and cancer research, and their effect on various immune cells and cancer cells are listed in Table 1 and Table 2, respectively. Some of these agonists and antagonists are broad-spectrum, which means that they act on more than one receptor subtypes. Among different agonists, the 5-HTR1A/7 agonist 8-OH-DPAT, the 5-HTR3 agonist 2-methylserotonin, and the 5-HTR4 agonists, 2-MHT and 5-CI, enhanced the release of IL-8 and IL-1β from DCs [38]. Other agonists like the 5-HTR1B agonist AnHcl, 5-HTR1E/1F agonist BRL5443, and the 5-HTR2 agonist DOI also showed a stimulatory effect towards DCs [38]. The 5-HTR2B agonist BW-723C86, and the 5-HTR7 agonist AS19 enhanced the anti-inflammatory property of macrophages [13, 31]. The 5-HTR3 agonist 2-methyserotonin as well as the 5-HTR7 agonist AS19 helped in T cell activation and further proliferation [65, 66].
Among different antagonists, SB-269970 and SB-258729 that block serotonin signaling through 5-HTR7, have been widely studied. These antagonists decrease the differentiation of anti-inflammatory M2 macrophages [13, 30], modulates the cytokine release from DCs [38, 40], and inhibit ERK signaling in T cells [66]. The broad-spectrum antagonists of serotonin receptors, methylsergide and ketanserin inhibited the activation of DCs [38] and abolished the ability of T cells to generate the delayed type of hypersensitivity [67]. Among the other antagonists used, the 5-HTR1A antagonist SB-216641 showed an effect on T cells similar to SB-269970 and SB-258729 [66].
It is clear from these studies that chemical agonists and antagonists of serotonin receptors are promising modulators of immune cells, and thereby have a strong potential for application in therapeutics for diseases like cancer.
Conclusion and future perspective
We have discussed here the complex interactions of serotonin with its receptors expressed on immune cells and cancer cells. Until recently, it was thought that serotonin is mainly confined to regulating body functions through concomitant neurons in the CNS and the peripheral nervous system. But it is now evident that other cells like immune cells and cancer cells also synthesize, release, and respond to serotonin. Though contradictory roles of serotonin in regulating the functions of different immune cells have been reported, most studies support that serotonin plays a role in enhancing their anti-inflammatory functions, as evident from the rise in anti-inflammatory cytokine secretion by some cells of the innate immune system. This suggests that serotonin may have a pro-tumorigenic effect by enhancing tumor immune evasion via the generation of an anti-inflammatory microenvironment. Gaining further insight into the cellular and molecular mechanisms of 5-HT/5-HTRs axis-mediated alterations in the tumor progression could have promising implications in clinical prognosis and therapeutics.
Serotonin promotes cancer progression by directly affecting cancer cells through various mechanisms like promotion of proliferation through cell cycle progression, autophagy, and suppression of apoptosis. This underscores the need to give due consideration to the role of serotonin in controlling tumor growth and in promoting antitumor immunity. This could pave the way to explore the potential of molecules that modulate the serotonergic system in improving or creating new therapeutic interventions for cancer.
Acknowledgements
GL received grants from the Department of Biotechnology, (Grant numbers: BT/PR15533/MED/30/1616/2015; BT/PR14156/BRB/10/1515/2016), Swarna Jayanti Fellowship (DST/SJF/LSA-01/2017-18), from Department of Science and Technology, and Science and Engineering Research Board (EMR/2016/007108), Ministry of Science and Technology, Government of India. SK received Junior Research Fellowship from Council of Scientific and Industrial Research, Government of India. Authors thanks Dr. Jyoti Rao and Dr. Padmashastry for critical reading, edits, suggestions and comments.
Author's contribution
SK and GL wrote the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H. et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:1078197
2. Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velázquez MA, Garcés-Alvarez ME, Hurtado-Alvarado G. et al. Immunomodulatory effects mediated by serotonin. J Immunol Res. 2015 354957: 19
3. Mondanelli G, Volpi C. The double life of serotonin metabolites: in the mood for joining neuronal and immune systems. Curr Opin Immunol. 2020;70:1-6
4. Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nature neuroscience. 2017;20:145-55
5. Veenstra-VanderWeele J, Anderson GM, Cook EH Jr. Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol. 2000;410:165-81
6. Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176:1243-51
7. Zamani A, Qu Z. Serotonin activates angiogenic phosphorylation signaling in human endothelial cells. FEBS Lett. 2012;586:2360-5
8. Yadav VK, Balaji S, Suresh PS, Liu XS, Lu X, Li Z. et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308-12
9. Martin AM, Young RL, Leong L, Rogers GB, Spencer NJ, Jessup CF. et al. The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology. 2017;158:1049-63
10. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-66
11. Nebigil CG, Launay J-M, Hickel P, Tournois C, Maroteaux L. 5-Hydroxytryptamine 2B receptor regulates cell-cycle progression: Cross-talk with tyrosine kinase pathways. Proceedings of the National Academy of Sciences. 2000;97:2591-6
12. Herr N, Bode C, Duerschmied D. The Effects of Serotonin in Immune Cells. Frontiers in Cardiovascular Medicine. 2017 4
13. Domínguez-Soto Á, Usategui A, Casas-Engel ML, Simón-Fuentes M, Nieto C, Cuevas VD. et al. Serotonin drives the acquisition of a profibrotic and anti-inflammatory gene profile through the 5-HT7R-PKA signaling axis. Sci Rep. 2017;7:017-15348
14. Masson J, Emerit MB, Hamon M, Darmon M. Serotonergic signaling: multiple effectors and pleiotropic effects. Wiley Interdisciplinary Reviews: Membrane Transport and Signaling. 2012;1:685-713
15. Dizeyi N, Hedlund P, Bjartell A, Tinzl M, Austild-Taskén K, Abrahamsson P-A. Serotonin activates MAP kinase and PI3K/Akt signaling pathways in prostate cancer cell lines. Urologic oncology. 2011;29:436-45
16. Soll C, Riener MO, Oberkofler CE, Hellerbrand C, Wild PJ, DeOliveira ML. et al. Expression of serotonin receptors in human hepatocellular cancer. Clin Cancer Res. 2012;18:5902-10
17. Quintero-Villegas A, Valdés-Ferrer SI. Role of 5-HT(7) receptors in the immune system in health and disease. Mol Med. 2019;26:019-0126
18. Farrelly LA, Thompson RE, Zhao S, Lepack AE, Lyu Y, Bhanu NV. et al. Histone serotonylation is a permissive modification that enhances TFIID binding to H3K4me3. Nature. 2019;567:535-9
19. Jiang SH, Hu LP, Wang X, Li J, Zhang ZG. Neurotransmitters: emerging targets in cancer. Oncogene. 2020;39:503-15
20. Huh JR, Veiga-Fernandes H. Neuroimmune circuits in inter-organ communication. Nat Rev Immunol. 2020;20:217-28
21. Wu H, Denna TH, Storkersen JN, Gerriets VA. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol Res. 2019;140:100-14
22. Wan M, Ding L, Wang D, Han J, Gao P. Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Frontiers in immunology. 2020;11:186
23. Chen Z, Luo J, Li J, Kim G, Stewart A, Urban JF Jr. et al. Interleukin-33 Promotes Serotonin Release from Enterochromaffin Cells for Intestinal Homeostasis. Immunity. 2020;54(1):151-163
24. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445-55
25. Li X, Liu R, Su X, Pan Y, Han X, Shao C. et al. Harnessing tumor-associated macrophages as aids for cancer immunotherapy. Mol Cancer. 2019;18:019-1102
26. Paul S, Chhatar S, Mishra A, Lal G. Natural killer T cell activation increases iNOS(+)CD206(-) M1 macrophage and controls the growth of solid tumor. J Immunother Cancer. 2019;7:208
27. Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM. et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS pathogens. 2009;5:e1000371
28. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369-82
29. Jackson JC, Walker RF, Brooks WH, Roszman TL. Specific uptake of serotonin by murine macrophages. Life Sciences. 1988;42:1641-50
30. de las Casas-Engel M, Dominguez-Soto A, Sierra-Filardi E, Bragado R, Nieto C, Puig-Kroger A. et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J Immunol. 2013;190:2301-10
31. Nieto C, Rayo I, de Las Casas-Engel M, Izquierdo E, Alonso B, Béchade C. et al. Serotonin (5-HT) Shapes the Macrophage Gene Profile through the 5-HT(2B)-Dependent Activation of the Aryl Hydrocarbon Receptor. J Immunol. 2020;204:2808-17
32. Vašíček O, Lojek A, Číž M. Serotonin and its metabolites reduce oxidative stress in murine RAW264.7 macrophages and prevent inflammation. Journal of physiology and biochemistry. 2020;76:49-60
33. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7-24
34. Wylie B, Macri C, Mintern JD, Waithman J. Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers. 2019;11(4):521
35. Lin JH, Huffman AP, Wattenberg MM, Walter DM, Carpenter EL, Feldser DM. et al. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J Exp Med. 2020;217:20190673
36. Maier B, Leader AM, Chen ST, Tung N, Chang C, LeBerichel J. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580:257-62
37. Zhou H, Li XM, Meinkoth J, Pittman RN. Akt regulates cell survival and apoptosis at a postmitochondrial level. J Cell Biol. 2000;151:483-94
38. Idzko M, Panther E, Stratz C, Müller T, Bayer H, Zissel G. et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. Journal of immunology (Baltimore, Md: 1950). 2004;172:6011-9
39. Szabo A, Gogolak P, Koncz G, Foldvari Z, Pazmandi K, Miltner N. et al. Immunomodulatory capacity of the serotonin receptor 5-HT2B in a subset of human dendritic cells. Sci Rep. 2018;8:018-20173
40. Holst K, Guseva D, Schindler S, Sixt M, Braun A, Chopra H. et al. The serotonin receptor 5-HT7R regulates the morphology and migratory properties of dendritic cells. Journal of Cell Science. 2015;128:2866-80
41. O'Connell PJ, Wang X, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107:1010-7
42. Paul S, Lal G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Frontiers in immunology. 2017;8:1124
43. Paul S, Kulkarni N. et al. Intratumoral natural killer cells show reduced effector and cytolytic properties and control the differentiation of effector Th1 cells. Oncoimmunology. 2016;5:e1235106
44. Ben-Shmuel A, Biber G, Barda-Saad M. Unleashing Natural Killer Cells in the Tumor Microenvironment-The Next Generation of Immunotherapy? Frontiers in Immunology. 2020;11:275
45. Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19:200-18
46. Capellino S, Claus M, Watzl C. Regulation of natural killer cell activity by glucocorticoids, serotonin, dopamine, and epinephrine. Cellular & molecular immunology. 2020;17:705-11
47. Hernandez ME, Martinez-Fong D, Perez-Tapia M, Estrada-Garcia I, Estrada-Parra S, Pavón L. Evaluation of the effect of selective serotonin-reuptake inhibitors on lymphocyte subsets in patients with a major depressive disorder. Eur Neuropsychopharmacol. 2010;20:88-95
48. Hellstrand K, Hermodsson S. Role of serotonin in the regulation of human natural killer cell cytotoxicity. The Journal of Immunology. 1987;139:869-75
49. Hellstrand K, Czerkinsky C, Ricksten A, Jansson B, Asea A, Kylefjord H. et al. Role of serotonin in the regulation of interferon-gamma production by human natural killer cells. J Interferon Res. 1993;13:33-8
50. Oláh T, Ocsovszki I, Mándi Y, Pusztai R, Bakay M, Balint E. Opposite effects of serotonin and interferon-alpha on the membrane potential and function of human natural killer cells. In vitro Cell Dev Biol Anim. 2005;41:165-70
51. Evans DL, Lynch KG, Benton T, Dubé B, Gettes DR, Tustin NB. et al. Selective serotonin reuptake inhibitor and substance P antagonist enhancement of natural killer cell innate immunity in human immunodeficiency virus/acquired immunodeficiency syndrome. Biol Psychiatry. 2008;63:899-905
52. Hellstrand K, Hermodsson S. Enhancement of human natural killer cell cytotoxicity by serotonin: Role of non-T/CD16+ NK cells, accessory monocytes, and 5-HT1A receptors. Cellular Immunology. 1990;127:199-214
53. Hansson M, Asea A, Ersson U, Hermodsson S, Hellstrand K. Induction of apoptosis in NK cells by monocyte-derived reactive oxygen metabolites. Journal of immunology (Baltimore, Md: 1950). 1996;156:42-7
54. Betten Å, Dahlgren C, Hermodsson S, Hellstrand K. Serotonin protects NK cells against oxidatively induced functional inhibition and apoptosis. Journal of Leukocyte Biology. 2001;70:65-72
55. Betten A, Dahlgren C, Mellqvist UH, Hermodsson S, Hellstrand K. Oxygen radical-induced natural killer cell dysfunction: role of myeloperoxidase and regulation by serotonin. J Leukoc Biol. 2004;75:1111-5
56. Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18:635-47
57. Sethi A, Kulkarni N, Sonar S, Lal G. Role of miRNAs in CD4 T cell plasticity during inflammation and tolerance. Frontiers in genetics. 2013;4:8
58. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651-668
59. Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O. et al. Pro- and anti-inflammatory cytokine balance in major depression: effect of sertraline therapy. Clin Dev Immunol. 2007;76396:76396
60. Cloëz-Tayarani I, Petit-Bertron AF, Venters HD, Cavaillon JM. Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: involvement of 5-hydroxytryptamine2A receptors. Int Immunol. 2003;15:233-40
61. Kubera M, Maes M. Serotonin-Immune Interactions in Major Depression. Berlin, Heidelberg: Springer Berlin Heidelberg. 2000 p:79-87
62. Chen Y, Leon-Ponte M, Pingle SC, O'Connell PJ, Ahern GP. T lymphocytes possess the machinery for 5-HT synthesis, storage, degradation and release. Acta physiologica (Oxford, England). 2015;213:860-7
63. Chen Y, Leon-Ponte M, Pingle SC, O'Connell PJ, Ahern GP. T lymphocytes possess the machinery for 5-HT synthesis, storage, degradation and release. Acta Physiol (Oxf). 2015;213:860-7
64. Wu H, Herr D, MacIver NJ, Rathmell JC, Gerriets VA. CD4 T cells differentially express cellular machinery for serotonin signaling, synthesis, and metabolism. International immunopharmacology. 2020;88:106922
65. Khan NA, Poisson JP. 5-HT3 receptor-channels coupled with Na+ influx in human T cells: role in T cell activation. J Neuroimmunol. 1999;99:53-60
66. León-Ponte M, Ahern GP, O'Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109:3139-46
67. Ameisen JC, Meade R, Askenase PW. A new interpretation of the involvement of serotonin in delayed-type hypersensitivity. Serotonin-2 receptor antagonists inhibit contact sensitivity by an effect on T cells. J Immunol. 1989;142:3171-9
68. Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH. et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281-92
69. Iglesia MD, Vincent BG, Parker JS, Hoadley KA, Carey LA, Perou CM. et al. Prognostic B-cell Signatures Using mRNA-Seq in Patients with Subtype-Specific Breast and Ovarian Cancer. Clinical Cancer Research. 2014;20:3818-29
70. Rubtsov AV, Rubtsova K, Kappler JW, Jacobelli J, Friedman RS, Marrack P. CD11c-Expressing B Cells Are Located at the T Cell/B Cell Border in Spleen and Are Potent APCs. J Immunol. 2015;195:71-9
71. Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20:294-307
72. Gilbert AE, Karagiannis P, Dodev T, Koers A, Lacy K, Josephs DH. et al. Monitoring the systemic human memory B cell compartment of melanoma patients for anti-tumor IgG antibodies. PLoS One. 2011;6:0019330
73. Rinaldi A, Chiaravalli AM, Mian M, Zucca E, Tibiletti MG, Capella C. et al. Serotonin receptor 3A expression in normal and neoplastic B cells. Pathobiology: journal of immunopathology, molecular and cellular biology. 2010;77:129-35
74. Meredith EJ, Holder MJ, Chamba A, Challa A, Drake-Lee A, Bunce CM. et al. The serotonin transporter (SLC6A4) is present in B-cell clones of diverse malignant origin: probing a potential anti-tumor target for psychotropics. Faseb J. 2005;19:1187-9
75. Iken K, Chheng S, Fargin A, Goulet AC, Kouassi E. Serotonin upregulates mitogen-stimulated B lymphocyte proliferation through 5-HT1A receptors. Cell Immunol. 1995;163:1-9
76. Serafeim A, Grafton G, Chamba A, Gregory CD, Blakely RD, Bowery NG. et al. 5-Hydroxytryptamine drives apoptosis in biopsylike Burkitt lymphoma cells: reversal by selective serotonin reuptake inhibitors. Blood. 2002;99:2545-53
77. Shu B, Zhai M, Miao X, He C, Deng C, Fang Y. et al. Serotonin and YAP/VGLL4 Balance Correlated with Progression and Poor Prognosis of Hepatocellular Carcinoma. Scientific Reports. 2018;8:9739
78. Abdel-Hamid NM, Shehata DE, Abdel-Ghany AA, Ragaa A, Wahid A. Serum serotonin as unexpected potential marker for staging of experimental hepatocellular carcinoma. Biomed Pharmacother. 2016;83:407-11
79. Liang C, Chen W, Zhi X, Ma T, Xia X, Liu H. et al. Serotonin promotes the proliferation of serum-deprived hepatocellular carcinoma cells via upregulation of FOXO3a. Mol Cancer. 2013;12:1476-4598
80. Soll C, Jang JH, Riener MO, Moritz W, Wild PJ, Graf R. et al. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology. 2010;51:1244-54
81. Yun CW, Lee SH. The Roles of Autophagy in Cancer. Int J Mol Sci. 2018;19(11):3466
82. Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S. et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100-5
83. Lee YK, Lee JA. Role of the mammalian ATG8/LC3 family in autophagy: differential and compensatory roles in the spatiotemporal regulation of autophagy. BMB Rep. 2016;49:424-30
84. Rusten TE, Stenmark H. p62, an autophagy hero or culprit? Nat Cell Biol. 2010;12:207-9
85. Niture S, Gyamfi MA, Kedir H, Arthur E, Ressom H, Deep G. et al. Serotonin induced hepatic steatosis is associated with modulation of autophagy and notch signaling pathway. Cell Communication and Signaling. 2018;16:78
86. Liu S, Miao R, Zhai M, Pang Q, Deng Y, Liu S. et al. Effects and related mechanisms of serotonin on malignant biological behavior of hepatocellular carcinoma via regulation of Yap. Oncotarget. 2017;8:47412-24
87. Zhang L, Yang S, Chen X, Stauffer S, Yu F, Lele SM. et al. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol Cell Biol. 2015;35:1350-62
88. Deng X, Fang L. VGLL4 is a transcriptional cofactor acting as a novel tumor suppressor via interacting with TEADs. Am J Cancer Res. 2018;8:932-43
89. Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, Cross N. et al. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate. 2004;59:328-36
90. Siddiqui EJ, Shabbir M, Mikhailidis DP, Thompson CS, Mumtaz FH. The role of serotonin (5-hydroxytryptamine1A and 1B) receptors in prostate cancer cell proliferation. J Urol. 2006;176:1648-53
91. Dizeyi N, Bjartell A, Hedlund P, Taskén KA, Gadaleanu V, Abrahamsson PA. Expression of serotonin receptors 2B and 4 in human prostate cancer tissue and effects of their antagonists on prostate cancer cell lines. Eur Urol. 2005;47:895-900
92. Del Bello F, Bonifazi A, Giorgioni G, Quaglia W, Amantini C, Morelli MB. et al. Chemical manipulations on the 1,4-dioxane ring of 5-HT(1A) receptor agonists lead to antagonists endowed with antitumor activity in prostate cancer cells. Eur J Med Chem. 2019;168:461-73
93. Siddiqui EJ, Thompson CS, Mikhailidis DP, Mumtaz FH. The role of serotonin in tumour growth (Review). Oncol Rep. 2005;14:1593-7
94. Pfeil K, Eder IE, Putz T, Ramoner R, Culig Z, Ueberall F. et al. Long-term androgen-ablation causes increased resistance to PI3K/Akt pathway inhibition in prostate cancer cells. The Prostate. 2004;58:259-68
95. Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology. 2005;41:5-15
96. Alpini G, Invernizzi P, Gaudio E, Venter J, Kopriva S, Bernuzzi F. et al. Serotonin metabolism is dysregulated in cholangiocarcinoma, which has implications for tumor growth. Cancer Res. 2008;68:9184-93
97. Dowling P, Hughes DJ, Larkin AM, Meiller J, Henry M, Meleady P. et al. Elevated levels of 14-3-3 proteins, serotonin, gamma enolase and pyruvate kinase identified in clinical samples from patients diagnosed with colorectal cancer. Clin Chim Acta. 2015;441:133-41
98. Ataee R, Ajdary S, Zarrindast M, Rezayat M, Hayatbakhsh MR. Anti-mitogenic and apoptotic effects of 5-HT1B receptor antagonist on HT29 colorectal cancer cell line. Journal of cancer research and clinical oncology. 2010;136:1461-9
99. Ataee R, Ajdary S, Rezayat M, Shokrgozar MA, Shahriari S, Zarrindast MR. Study of 5HT3 and HT4 receptor expression in HT29 cell line and human colon adenocarcinoma tissues. Arch Iran Med. 2010;13:120-5
100. Ataee R, Ajdary S, Zarrindast M, Rezayat M, Shokrgozar MA, Ataee A. Y25130 hydrochloride, a selective 5HT3 receptor antagonist has potent antimitogenic and apoptotic effect on HT29 colorectal cancer cell line. Eur J Cancer Prev. 2010;19:138-43
101. Nocito A, Dahm F, Jochum W, Jang JH, Georgiev P, Bader M. et al. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 2008;68:5152-8
102. Kannen V, Hintzsche H, Zanette DL, Silva WA Jr, Garcia SB, Waaga-Gasser AM. et al. Antiproliferative effects of fluoxetine on colon cancer cells and in a colonic carcinogen mouse model. PLoS One. 2012;7:27
103. Kannen V, Marini T, Turatti A, Carvalho MC, Brandão ML, Jabor VA. et al. Fluoxetine induces preventive and complex effects against colon cancer development in epithelial and stromal areas in rats. Toxicol Lett. 2011;204:134-40
104. Kannen V, Garcia SB, Silva WA Jr, Gasser M, Mönch R, Alho EJ. et al. Oncostatic effects of fluoxetine in experimental colon cancer models. Cell Signal. 2015;27:1781-8
105. Liu Y, Zhang H, Wang Z, Wu P, Gong W. 5-Hydroxytryptamine1a receptors on tumour cells induce immune evasion in lung adenocarcinoma patients with depression via autophagy/pSTAT3. European journal of cancer (Oxford, England: 1990). 2019;114:8-24
106. Tone M, Tahara S, Nojima S, Motooka D, Okuzaki D, Morii E. HTR3A is correlated with unfavorable histology and promotes proliferation through ERK phosphorylation in lung adenocarcinoma. Cancer science. 2020;111:3953-61
107. Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009;11:10
108. Hejazi SH, Ahangari G, Deezagi A. Alternative Viewpoint Against Breast Cancer Based on Selective Serotonin Receptors 5HTR3A and 5HTR2A Antagonists that can Mediate Apoptosis in MCF-7 Cell Line. Curr Drug Discov Technol. 2015;12:240-9
109. Olfati Z, Rigi G, Vaseghi H, Zamanzadeh Z, Sohrabi M, Hejazi SH. Evaluation of serotonin receptors (5HTR2A and 5HTR3A) mRNA expression changes in tumor of breast cancer patients. Med J Islam Repub Iran. 2020;34:99
110. Kopparapu PK, Tinzl M, Anagnostaki L, Persson JL, Dizeyi N. Expression and localization of serotonin receptors in human breast cancer. Anticancer Res. 2013;33:363-70
111. Sonier B, Arseneault M, Lavigne C, Ouellette RJ, Vaillancourt C. The 5-HT2A serotoninergic receptor is expressed in the MCF-7 human breast cancer cell line and reveals a mitogenic effect of serotonin. Biochem Biophys Res Commun. 2006;343:1053-9
112. Gwynne WD, Hallett RM, Girgis-Gabardo A, Bojovic B, Dvorkin-Gheva A, Aarts C. et al. Serotonergic system antagonists target breast tumor initiating cells and synergize with chemotherapy to shrink human breast tumor xenografts. Oncotarget. 2017;8:32101-16
113. Gwynne WD, Shakeel MS, Girgis-Gabardo A, Kim KH, Ford E, Dvorkin-Gheva A. et al. Antagonists of the serotonin receptor 5A target human breast tumor initiating cells. BMC Cancer. 2020;20:724
114. Sola-Penna M, Paixão LP, Branco JR, Ochioni AC, Albanese JM, Mundim DM. et al. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br J Cancer. 2020;122:194-208
115. Jiang SH, Li J, Dong FY, Yang JY, Liu DJ, Yang XM. et al. Increased Serotonin Signaling Contributes to the Warburg Effect in Pancreatic Tumor Cells Under Metabolic Stress and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology. 2017;153:277-91.e19
116. Gurbuz N, Ashour AA, Alpay SN, Ozpolat B. Down-regulation of 5-HT1B and 5-HT1D receptors inhibits proliferation, clonogenicity and invasion of human pancreatic cancer cells. PLoS One. 2014;9:e105245
117. Xiao GG. Targeting Serotonin System in Pancreatic Cancer. Pancreas. 2020;49(1):e1
118. Henriksen R, Dizeyi N, Abrahamsson PA. Expression of serotonin receptors 5-HT1A, 5-HT1B, 5-HT2B and 5-HT4 in ovary and in ovarian tumours. Anticancer Res. 2012;32:1361-6
119. Christensen DK, Armaiz-Pena GN, Ramirez E, Matsuo K, Zimmerman B, Zand B. et al. SSRI use and clinical outcomes in epithelial ovarian cancer. Oncotarget. 2016;7:33179-91
120. Le-Bel G, Benhassine M, Landreville S, Guerin SL. Analysis of the proteasome activity and the turnover of the serotonin receptor 2B (HTR2B) in human uveal melanoma. Exp Eye Res. 2019;184:72-7
121. Muller K, Gilbertz KP, Meineke V. Serotonin and ionizing radiation synergistically affect proliferation and adhesion molecule expression of malignant melanoma cells. J Dermatol Sci. 2012;68:89-98
122. Liu W, Stachura P, Xu HC, Umesh Ganesh N, Cox F, Wang R. et al. Repurposing the serotonin agonist Tegaserod as an anticancer agent in melanoma: molecular mechanisms and clinical implications. J Exp Clin Cancer Res. 2020;39(1):38
123. Otto-Meyer S, DeFaccio R, Dussold C, Ladomersky E, Zhai L, Lauing KL. et al. A retrospective survival analysis of Glioblastoma patients treated with selective serotonin reuptake inhibitors. Brain Behav Immun Health. 2020;2:100025
124. Mahe C, Bernhard M, Bobirnac I, Keser C, Loetscher E, Feuerbach D. et al. Functional expression of the serotonin 5-HT7 receptor in human glioblastoma cell lines. Br J Pharmacol. 2004;143:404-10
125. Lu Q, Ding Y, Li Y, Lu Q. 5-HT receptor agonist Valerenic Acid enhances the innate immunity signal and suppresses glioblastoma cell growth and invasion. Int J Biol Sci. 2020;16:2104-15
126. Lieb K, Biersack L, Waschbisch A, Orlikowski S, Akundi RS, Candelario-Jalil E. et al. Serotonin via 5-HT7 receptors activates p38 mitogen-activated protein kinase and protein kinase C epsilon resulting in interleukin-6 synthesis in human U373 MG astrocytoma cells. J Neurochem. 2005;93:549-59
127. Siddiqui EJ, Shabbir MA, Mikhailidis DP, Mumtaz FH, Thompson CS. The effect of serotonin and serotonin antagonists on bladder cancer cell proliferation. BJU Int. 2006;97:634-9
128. Oufkir T, Arseneault M, Sanderson JT, Vaillancourt C. The 5-HT 2A serotonin receptor enhances cell viability, affects cell cycle progression and activates MEK-ERK1/2 and JAK2-STAT3 signalling pathways in human choriocarcinoma cell lines. Placenta. 2010;31:439-47
129. El-Salhy M. Effects of triple therapy with octreotide, galanin and serotonin on a human colon cancer cell line. Oncol Rep. 2005;13:45-9
130. Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A. et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood. 2013;121:1008-15
131. Gautam J, Banskota S, Regmi SC, Ahn S, Jeon YH, Jeong H. et al. Tryptophan hydroxylase 1 and 5-HT(7) receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol Cancer. 2016;15:016-0559
132. Peters MAM, Meijer C, Fehrmann RSN, Walenkamp AME, Kema IP, de Vries EGE. et al. Serotonin and Dopamine Receptor Expression in Solid Tumours Including Rare Cancers. Pathol Oncol Res. 2020;26:1539-47
133. Aune TM, McGrath KM, Sarr T, Bombara MP, Kelley KA. Expression of 5HT1a receptors on activated human T cells. Regulation of cyclic AMP levels and T cell proliferation by 5-hydroxytryptamine. J Immunol. 1993;151:1175-83
134. Abdouh M, Storring JM, Riad M, Paquette Y, Albert PR, Drobetsky E. et al. Transcriptional mechanisms for induction of 5-HT1A receptor mRNA and protein in activated B and T lymphocytes. J Biol Chem. 2001;276:4382-8
Author contact
![]() Corresponding author: Girdhari Lal, Ph.D. Scientist, National Centre for Cell Science, NCCS Complex, Ganeshkhind, Pune, MH-411007, India. E-mail: glalres.in; Phone: +91-20-2570-8292; Fax: +91-20-2568-2259.
Corresponding author: Girdhari Lal, Ph.D. Scientist, National Centre for Cell Science, NCCS Complex, Ganeshkhind, Pune, MH-411007, India. E-mail: glalres.in; Phone: +91-20-2570-8292; Fax: +91-20-2568-2259.
 Global reach, higher impact
Global reach, higher impact