13.3
Impact Factor
Theranostics 2021; 11(10):4825-4838. doi:10.7150/thno.55330 This issue Cite
Research Paper
SIRT2 ablation inhibits glucose-stimulated insulin secretion through decreasing glycolytic flux
1. Department of Endocrine and Metabolic Diseases/ Shanghai institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China.
2. Center for Reproductive Medicine, Shandong University, Jinan 250000, China.
3. Department of VIP Clinic, Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200123, China.
* These authors contributed equally to this study
Abstract
Rationale: Sirtuins are NAD+-dependent protein deacylases known to have protective effects against age-related diseases such as diabetes, cancer, and neurodegenerative disease. SIRT2 is the only primarily cytoplasmic isoform and its overall role in glucose homeostasis remains uncertain.
Methods: SIRT2-knockout (KO) rats were constructed to evaluate the role of SIRT2 in glucose homeostasis. The effect of SIRT2 on β-cell function was detected by investigating the morphology, insulin secretion, and metabolomic state of islets. The deacetylation and stabilization of GKRP in β-cells by SIRT2 were determined by western blot, adenoviral infection, and immunoprecipitation.
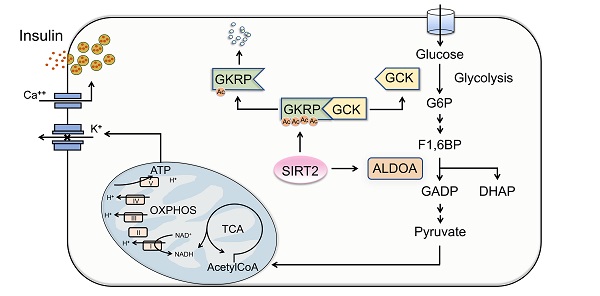
Results: SIRT2-KO rats exhibited impaired glucose tolerance and glucose-stimulated insulin secretion (GSIS), without change in insulin sensitivity. SIRT2 deficiency or inhibition by AGK2 decreased GSIS in isolated rat islets, with lowered oxygen consumption rate. Adenovirus-mediated overexpression of SIRT2 enhanced insulin secretion from rat islets. Metabolomics analysis revealed a decrease in metabolites of glycolysis and tricarboxylic acid cycle in SIRT2-KO islets compared with control islets. Our study further demonstrated that glucokinase regulatory protein (GKRP), an endogenous inhibitor of glucokinase (GCK), was expressed in rat islets. SIRT2 overexpression deacetylated GKRP in INS-1 β-cells. SIRT2 knockout or inhibition elevated GKRP protein stability in islet β-cells, leading to an increase in the interaction of GKRP and GCK. On the contrary, SIRT2 inhibition promoted the protein degradation of ALDOA, a glycolytic enzyme.
Conclusions: SIRT2 ablation inhibits GSIS through blocking GKRP protein degradation and promoting ALDOA protein degradation, resulting in a decrease in glycolytic flux.
Keywords: SIRT2, islets, acetylation, glucokinase regulatory protein, glucose-stimulated insulin secretion, glycolysis.
 Global reach, higher impact
Global reach, higher impact