13.3
Impact Factor
Theranostics 2021; 11(10):4770-4789. doi:10.7150/thno.54235 This issue Cite
Research Paper
Vertebral-specific activation of the CX3CL1/ICAM-1 signaling network mediates non-small-cell lung cancer spinal metastasis by engaging tumor cell-vertebral bone marrow endothelial cell interactions
1. Department of Orthopaedic Surgery, Zhongshan Hospital, Fudan University, Shanghai 200032, China.
2. JMS Burn and Reconstruction Center, Jackson, Mississippi, USA.
3. Department of Orthopaedic Surgery, Zhongshan Hospital Wusong Branch, Fudan University, Shanghai 200940, China.
4. Department of Thoracic Surgery, Zhongshan Hospital, Fudan University, Shanghai 200032, China.
* Ketao Wang, Libo Jiang and Annan Hu contributed equally to this work.
Abstract
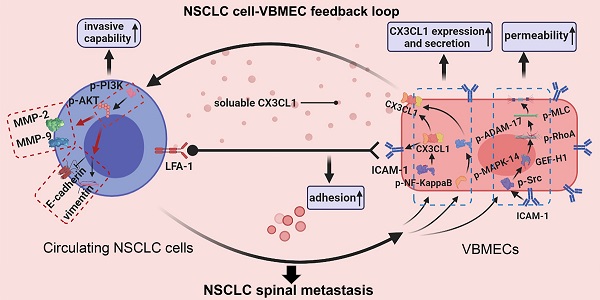
Rationale: The spine is one of the most common metastatic sites of non-small cell lung cancer (NSCLC), and NSCLC spinal metastasis results in serious consequences. Metastatic extravasation of disseminated cancer cells including increased invasiveness, adhesion and transendothelial migration is crucial for tumor metastasis. This study aimed to investigate the mechanisms underlying NSCLC spinal metastasis based on the C-X3-C motif chemokine ligand 1- (CX3CL1) and intercellular adhesion molecule-1- (ICAM-1) mediated signaling network.
Methods: Immunohistochemistry, western blotting, and reverse transcription-quantitative PCR were conducted to detect the distribution of CX3CL1/ICAM-1 in different organs. Transwell, adhesion, and transendothelial migration assays were performed to evaluate the regulatory effects of CX3CL1/ICAM-1 on NSCLC cell invasion, adhesion, and transendothelial migration in vitro. A spontaneous spinal metastasis mouse model was established via injection of NSCLC cells into the left cardiac ventricle of NOD/SCID mice. The effects of CX3CL1/ICAM-1 on NSCLC spinal metastasis in vivo were validated using bioluminescent, micro-computerized tomography, immunohistochemistry and histological analyses.
Results: CX3CL1 expression was specifically higher in vertebral bone compared with limb bones and lung tissue, and was associated with NSCLC spinal metastasis. Mechanically, vertebral bone marrow endothelial cells (VBMECs) enhanced NSCLC cell invasion via CX3CL1 signaling-mediated activation of the PI3K/AKT pathway. Furthermore, we found that VBMECs effectively induced ICAM-1-dependent NSCLC cell adhesion in coordination with platelets through the CX3CL1/ICAM-1/LFA-1 pathway. Meanwhile, CX3CL1 enhanced NSCLC cell transendothelial migration by increasing permeability of VBMECs via ICAM-1-dependent activation of the Src/GEF-H1 pathway. Interestingly, NSCLC cells were indicated to promote CX3CL1 secretion of VBMECs through MAPK14/ADMA17-dependent CX3CL1 release and NF-κB-dependent CX3CL1 synthesis. Based on these findings, we revealed a novel feedback cycle between circulating NSCLC cells and VBMECs mediated by CX3CL1/ICAM-1 signaling. Further disengagement of the CX3CL1/ICAM-1-mediated feedback cycle in vivo significantly restricted metastasis and prolonged mouse survival.
Conclusions: Our results indicated a unique feedback cycle between circulating NSCLC cells and VBMECs mediated by CX3CL1/ICAM-1 signaling, which is necessary for NSCLC spinal metastasis. This work provides a new perspective for underlying the mechanisms of NSCLC spinal metastasis and indicates potential novel targets for the prevention of NSCLC spinal metastasis.
Keywords: Non-small cell lung cancer, spinal metastasis, CX3CL1, ICAM-1, extravasation.
 Global reach, higher impact
Global reach, higher impact