13.3
Impact Factor
Theranostics 2021; 11(10):4567-4584. doi:10.7150/thno.56995 This issue Cite
Research Paper
Monovalent antibody-conjugated lipid-polymer nanohybrids for active targeting to desmoglein 3 of keratinocytes to attenuate psoriasiform inflammation
1. Graduate Institute of Biomedical Sciences, Chang Gung University, Kweishan, Taoyuan, Taiwan.
2. Graduate Institute of Natural Products, Chang Gung University, Kweishan, Taoyuan, Taiwan.
3. Chinese Herbal Medicine Research Team, Healthy Aging Research Center, Chang Gung University, Kweishan, Taoyuan, Taiwan.
4. Research Center for Food and Cosmetic Safety and Research Center for Chinese Herbal Medicine, Chang Gung University of Science and Technology, Kweishan, Taoyuan, Taiwan.
5. Department of Chemical Engineering, Ming Chi University of Technology, New Taipei City, Taiwan.
6. Department of Anesthesiology, Chang Gung Memorial Hospital, Kweishan, Taoyuan, Taiwan.
7. Department of Traditional Chinese Medicine, Chang Gung Memorial Hospital, Keelung, Taiwan.
8. School of Traditional Chinese Medicine, Chang Gung University, Kweishan, Taoyuan, Taiwan.
9. School of Nursing, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan.
10. Laboratory of Pharmaceutical Engineering, Gifu Pharmaceutical University, Gifu, Japan.
11. Institute of Macromolecular Chemistry, Czech Academy of Sciences, Prague, Czech Republic.
Abstract
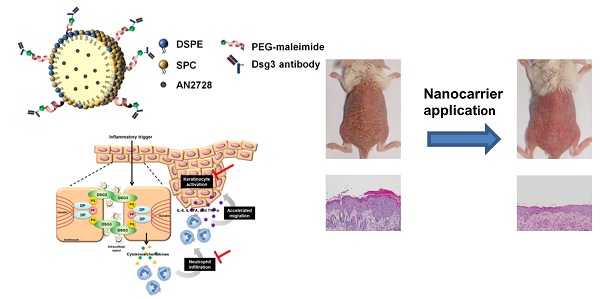
To improve the treatment of psoriasiform inflammation, we developed actively targeted nanocarriers loaded with the phosphodiesterase 4 inhibitor AN2728.
Methods: Phospholipid-poly(lactic-co-glycolic acid) nanohybrids were prepared and conjugated with monovalent anti-desmoglein 3 antibody to bind keratinocytes.
Results: The actively targeted nanohybrids were 229 nm in mean size with a nearly neutral surface charge. Flow cytometry and confocal microscopy showed a 9-fold increase in keratinocyte uptake of targeted nanohybrids relative to non-targeted nanoparticles. The nanoparticles localized mainly in lysosomes after internalization. AN2728-loaded antibody-conjugated nanocarriers inhibited cytokine/chemokine overexpression in activated keratinocytes without affecting cell viability. The targeted nanohybrids also suppressed neutrophil migration by reducing CXCL1 and CXCL2 release from keratinocytes. Following subcutaneous administration in mice, the nanohybrids distributed to the epidermis and hair follicles. In a psoriasis-like skin mouse model, the actively targeted nanoparticles were superior to free drug and non-targeted nanoparticles in mitigating skin inflammation. Intervention with the targeted nanosystem reduced the epidermal thickness of the psoriasiform lesion from 191 to 42 µm, decreased the Psoriasis Area Severity Index by 74%, restored barrier function, and returned chemokine levels to baseline.
Conclusions: Our developed nanosystem was safe and demonstrated efficient targeting properties for the treatment of cutaneous inflammation.
Keywords: desmoglein 3, keratinocyte, psoriasis, lipid-polymer nanohybrid, active targeting, monovalent antibody
 Global reach, higher impact
Global reach, higher impact