13.3
Impact Factor
Theranostics 2021; 11(6):2966-2986. doi:10.7150/thno.48699 This issue Cite
Research Paper
Cytoplasmic SHMT2 drives the progression and metastasis of colorectal cancer by inhibiting β-catenin degradation
1. State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, West China Medical School, and Collaborative Innovation Center for Biotherapy, Sichuan University, Chengdu 610041, China
2. National Chengdu Center for Safety Evaluation of Drugs, State Key Laboratory of Biotherapy, West China Hospital, West China Medical School, and Collaborative Innovation Center for Biotherapy, Sichuan University, Chengdu 610041, China
3. Analytical & Testing Center, Sichuan University, Chengdu 610041, China
4. Department of Gastroenterology, West China Hospital, West China Medical School, Sichuan University, Chengdu 610041, China
5. Department of Gastrointestinal Surgery, West China Hospital, West China Medical School, Sichuan University, Chengdu 610041, China
* Equal contributor
Abstract
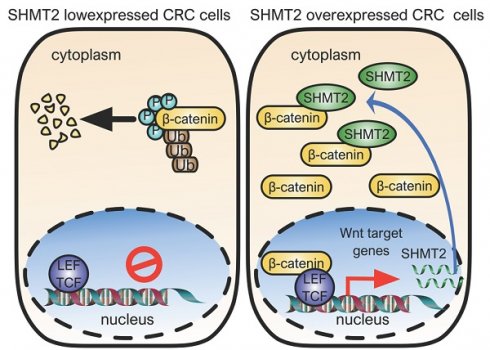
Introduction: Serine hydroxymethyltransferase 2 (SHMT2) plays a critical role in serine-glycine metabolism to drive cancer cell proliferation. However, the nonmetabolic function of SHMT2 in tumorigenesis, especially in human colorectal cancer (CRC) progression, remains largely unclear.
Methods: SHMT2 expression in human CRC cells was identified by western blot and immunofluorescence assay. The CRC cell proliferation, migration, and invasion after SHMT2 knockdown or overexpression were explored through in vitro and in vivo assays. Immunofluorescence, mRNA-seq, co-immunoprecipitation, chromatin immunoprecipitation-qPCR and immunohistochemistry assays were used to investigate the underlying mechanisms behind the SHMT2 nonmetabolic function.
Results: We demonstrated that SHMT2 was distributed in the cytoplasm and nucleus of human CRC cells. SHMT2 knockdown resulted in the significant inhibition of CRC cell proliferation, which was not restored by serine, glycine, or formate supplementation. The invasion and migration of CRC cells were suppressed after SHMT2 knockdown. Mechanistically, SHMT2 interacted with β-catenin in the cytoplasm. This interaction inhibited the ubiquitylation-mediated degradation of β-catenin and subsequently modulated the expression of its target genes, leading to the promotion of CRC cell proliferation and metastasis. Notably, the lysine 64 residue on SHMT2 (SHMT2K64) mediated its interaction with β-catenin. Moreover, transcription factor TCF4 interacted with β-catenin, which in turn increased SHMT2 expression, forming an SHMT2/β-catenin positive feedback loop. In vivo xenograft experiments confirmed that SHMT2 promoted the growth and metastasis of CRC cells. Finally, the level of SHMT2 was found to be significantly increased in human CRC tissues. The SHMT2 level was correlated with an increased level of β-catenin, associated with CRC progression and predicted poor patient survival.
Conclusion: Taken together, our findings reveal a novel nonmetabolic function of SHMT2 in which it stabilizes β-catenin to prevent its ubiquitylation-mediated degradation and provide a potential therapeutic strategy for CRC therapy.
Keywords: cytoplasmic SHMT2, colorectal cancer, β-catenin, nonmetabolic function, ubiquitylation-mediated degradation
 Global reach, higher impact
Global reach, higher impact