13.3
Impact Factor
Theranostics 2021; 11(6):2917-2931. doi:10.7150/thno.50825 This issue Cite
Research Paper
Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair
1. Department of Radiology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510120, China.
2. Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Medical Research Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510120, China.
3. PCFM Lab, GD HPPC Lab, School of Chemistry, Sun Yat-sen University, Guangzhou, Guangdong 510275, China.
4. Guangdong Functional Biomaterials Engineering Technology Research Center, School of Materials Science and Engineering, Sun Yat-sen University, Guangzhou, Guangdong 510275, China.
# These authors contributed equally to this work.
Abstract
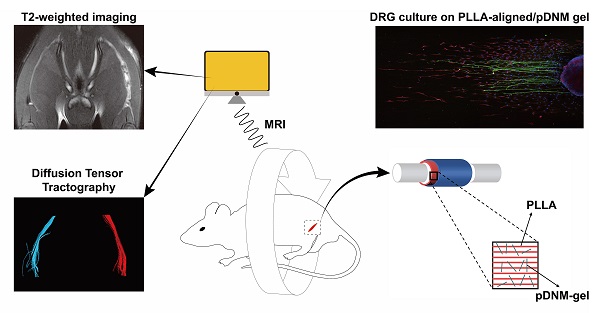
Rationale: Peripheral nerve injury (PNI) is a great challenge for regenerative medicine. Nerve autograft is the gold standard for clinical PNI repair. Due to its significant drawbacks, artificial nerve guidance conduits (NGCs) have drawn much attention as replacement therapies. We developed a combinatorial NGC consisting of longitudinally aligned electrospun nanofibers and porcine decellularized nerve matrix hydrogel (pDNM gel). The in vivo capacity for facilitating nerve tissue regeneration and functional recovery was evaluated in a rat sciatic nerve defect model.
Methods: Poly (L-lactic acid) (PLLA) was electrospun into randomly oriented (PLLA-random) and longitudinally aligned (PLLA-aligned) nanofibers. PLLA-aligned were further coated with pDNM gel at concentrations of 0.25% (PLLA-aligned/0.25% pDNM gel) and 1% (PLLA-aligned/1% pDNM gel). Axonal extension and Schwann cells migration were evaluated by immunofluorescence staining of dorsal root ganglia cultured on the scaffolds. To fabricate implantable NGCs, the nanofibrous scaffolds were rolled and covered with an electrospun protection tube. The fabricated NGCs were then implanted into a 5 mm sciatic nerve defect model in adult male Sprague-Dawley rats. Nerves treated with NGCs were compared to contralateral uninjured nerves (control group), injured but untreated nerves (unstitched group), and autografted nerves. Nerve regeneration was monitored by an established set of assays, including T2 values and diffusion tensor imaging (DTI) derived from multiparametric magnetic resonance imaging (MRI), histological assessments, and immunostaining. Nerve functional recovery was evaluated by walking track analysis.
Results: PLLA-aligned/0.25% pDNM gel scaffold exhibited the best performance in facilitating directed axonal extension and Schwann cells migration in vitro due to the combined effects of the topological cues provided by the aligned nanofibers and the biochemical cues retained in the pDNM gel. Consistent results were obtained in animal experiments with the fabricated NGCs. Both the T2 and fractional anisotropy values of the PLLA-aligned/0.25% pDNM gel group were the closest to those of the autografted group, and returned to normal much faster than those of the other NGCs groups. Histological assessment indicated that the implanted PLLA-aligned/0.25% pDNM gel NGC resulted in the largest number of axons and the most extensive myelination among all fabricated NGCs. Further, the PLLA-aligned/0.25% pDNM gel group exhibited the highest sciatic nerve function index, which was comparable to that of the autografted group, at 8 weeks post-surgery.
Conclusions: NGCs composed of aligned PLLA nanofibers decorated with 0.25% pDNM gel provided both topological and biochemical guidance for directing and promoting axonal extension, nerve fiber myelination, and functional recovery. Moreover, T2-mapping and DTI metrics were found to be useful non-invasive monitoring techniques for PNI treatment.
Keywords: decellularized nerve matrix hydrogel, peripheral nerve injury, nerve guidance conduit, magnetic resonance imaging, electrospinning
 Global reach, higher impact
Global reach, higher impact