13.3
Impact Factor
Theranostics 2021; 11(6):2670-2690. doi:10.7150/thno.53083 This issue Cite
Research Paper
Visualized podocyte-targeting and focused ultrasound responsive glucocorticoid nano-delivery system against immune-associated nephropathy without glucocorticoid side effect
1. Department of Nephrology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China.
2. Institute of Ultrasound Imaging, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China.
3. Department of Nephrology, Santai County People's Hospital (Affiliated Hospital of North Sichuan Medical College in Santai County), Mianyang 621100, China.
4. State Key Laboratory of Ultrasound in Medicine and Engineering, Chongqing Medical University, Chongqing 400016, China.
5. Radiation Oncology Center, Chongqing University Cancer Hospital, Chongqing University, Chongqing 400030, China.
6. Department of Respiratory Medicine, The Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China.
#These authors contributed equally to this work.
Abstract
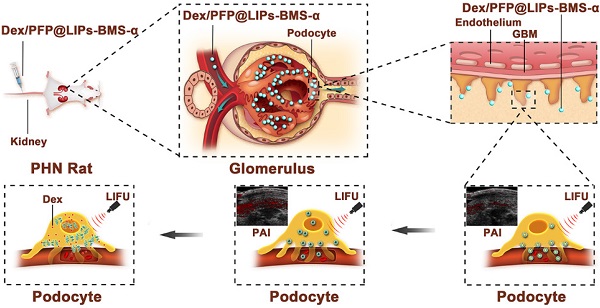
Glucocorticoids are widely used in the treatment of nephritis, however, its dose-dependent side effects, such as the increased risk of infection and metabolic disturbances, hamper its clinical use. This study reports a visualized podocyte-targeting and focused ultrasound responsive glucocorticoid nano-delivery system (named as Dex/PFP@LIPs-BMS-α), which specific delivers dexamethasone (Dex) to podocyte targets and reduces systemic side effects.
Methods: The glucocorticoid nano-delivery system was synthesized by a lipid thin film and a simple facile acoustic-emulsification method. This glucocorticoid nano-delivery system used BMS-470539 (BMS-α), a synthetic compound, as a “navigator” to specifically identify and target the melanocortin-1 receptor (MC-1R) on podocytes. The loaded perfluoropentane (PFP) realizes the directed "explosion effect" through ultrasound-targeted microbubble destruction (UTMD) technology under the coordination of low intensity focused ultrasound (LIFU) to completely release Dex.
Results: Both in vitro and in vivo experiments have demonstrated that Dex/PFP@LIPs-BMs-α accurately gathered to podocyte targets and improved podocyte morphology. Moreover, in vivo, proteinuria and serum creatinine levels were significantly reduced in the group treated with Dex/PFP@LIPs-BMS-α, and no severe side effects were detected. Furthermore, Dex/PFP@LIPs-BMS-α, with capabilities of ultrasound, photoacoustic and fluorescence imaging, provided individualized visual guidance and the monitoring of treatment.
Conclusion: This study provides a promising strategy of Dex/PFP@LIPs-BMS-α as effective and safe against immune-associated nephropathy.
Keywords: immune-associated nephropathy, podocyte, dexamethasone, nano-delivery system, side effect
 Global reach, higher impact
Global reach, higher impact