13.3
Impact Factor
Theranostics 2021; 11(5):2263-2277. doi:10.7150/thno.51571 This issue Cite
Review
Targeted delivery of extracellular vesicles in heart injury
1. Laboratory of Heart Center and Department of Cardiology, Zhujiang Hospital, Southern Medical University, Guangzhou, China.
2. Guangdong Provincial Biomedical Engineering Technology Research Center for Cardiovascular Diseases, Guangzhou, China.
3. Laboratory of Heart Center, Sino-Japanese Cooperation Platform for Translational Research in Heart Failure, Guangzhou, China.
4. Biomaterials Research Center, School of Biomedical Engineering, Southern Medical University, Guangzhou 510515, China.
Received 2020-8-5; Accepted 2020-11-18; Published 2021-1-1
Abstract
Extracellular vesicles (EVs) are nanoscale extracellular vesicles derived from endocytosis that are crucial to intercellular communication. EVs possess natural biocompatibility and stability that allow them to cross biological membranes and that protect them from degradation. Recent studies have shown that EVs-mediated crosstalk between different cell types in the heart could play important roles in the maintenance of cardiac homeostasis and the pathogenesis of heart diseases. In particular, EVs secreted by different types of stem cells exhibit cardioprotective effects. However, numerous studies have shown that intravenously injected EVs are quickly cleared by macrophages of the mononuclear phagocyte system (MPS) and preferentially accumulate in MPS organs such as the liver, spleen, and lung. In this review, we discuss exosome biogenesis, the role of EVs in heart diseases, and challenges in delivering EVs to the heart. Furthermore, we extensively discuss the targeted delivery of EVs for treating ischemic heart disease. These understandings will aid in the development of effective treatment strategies for heart diseases.
Keywords: Extracellular Vesicles, Biogenesis, Heart Injury, Challenges, Targeted Delivery
Introduction
Heart diseases remain the leading causes of death or disability in the world [1, 2]. Stem cell transplantation may be an effective way to improve and treat acute and chronic ischemic heart disease [3, 4]. However, the therapeutic potential of transplanted stem cells is limited by their low survival rate following transplantation into damaged myocardium. Also, arrhythmias and myocardial hypertrophy are prone to occur after stem cell transplantation, and there is a risk of cancer development [5, 6].
Many studies have shown that stem cell-derived extracellular vesicles (EVs) have the same myocardial repair function as transplanted stem cells [7-9]. EVs are nanoscale vesicles secreted by almost all cells in the body with a lipid double-layer membrane [10]. EVs are widely distributed in blood, cerebrospinal fluid, saliva, amniotic fluid, urine, and other body fluids [11, 12]. In 1987, Johnstone and colleagues [13, 14] identified the maturation process of red blood cells (RBCs) in sheep. They showed that the transferrin receptor located on the immature RBC membrane is transferred from the cell membrane to the membrane of EVs secreted by erythrocytes during RBC maturation. In recent years, studies have shown that EVs carry some essential signaling molecules, such as DNAs, proteins, lipids, mRNAs, miRNAs, and siRNAs [15], and that they can mediate signal transmission between cells [16-18]. Moreover, EVs play an important role in cardiovascular pathophysiology [19, 20]. EVs have the following advantages over stem cells in the treatment of heart diseases: 1) EVs lack self-replicating entities; therefore, they have no tumorigenic potential [21-23]; 2) EVs preserve their contents and their functions are relatively stable [24]; 3) EVs can cross biological barriers, so it is easier for them to reach the area of ischemic injury [25]; 4) EVs can be easily modified and stored [26]; 5) EVs have the biological characteristics of their source cells and can carry a variety of bioactive molecules to act on receptor cells [27]; and, 6) EVs can help cells clear misfolded prion proteins [28]. EVs also play essential roles in the interactions between different cell types in the microenvironment of heart diseases. Bang et al. [29] investigated the potential paracrine miRNA crosstalk between cardiac fibroblasts and cardiomyocytes and found that cardiac fibroblasts secrete miRNA-enriched EVs. Through confocal imaging and co-culture experiments, they determined that miR-21 derived from fibroblast EVs is an effective paracrine RNA molecule that can induce cardiomyocyte hypertrophy. Following cardiac injury, EVs are rapidly released in a remarkable quantity to the local microenvironment. Cheng et al. [30] found that EVs secreted by ischemic cardiomyocytes are enriched in miR-1 and miR-133, which affect action potentials and cardiac conduction through targeting of Ca2+/calmodulin-dependent protein kinase II [31]. In the cardiomyopathy associated with type 2 diabetes, Wang et al. [32] indicated that cardiomyocytes exert an anti-angiogenic function in type 2 diabetic rats through EVs-mediated transfer of miR-320 into endothelial cells. Non-stem cells-derived EVs can also mediate intercellular communication and provide cardioprotective effects. For example, under glucose deprivation conditions, EVs derived from cardiomyocytes were found to promote angiogenesis of endothelial cells [33].
EVs have a tremendous potential to replace stem cells and tissue-engineered therapies. However, the main limitation of this application is engraftment of EVs at the target site. Delivering a therapeutic dosage of EVs to the target site, particularly via systemic injection, can be challenging. Therefore, it is of paramount importance to explore strategies for targeted therapeutic delivery of EVs to the heart in order to devise more unique and valid treatment strategies for heart diseases.
Biogenesis of Extracellular Vesicles
EVs are nanoscale bubble-like membranous structures secreted by cells [34, 35]. Exosomes, with an average diameter of 30-120 nm, are a subset of EVs [36]. Exosomes carry a variety of biologically active substances (e.g., proteins, lipids, nucleic acids) that can affect the biological function of recipient cells and so exosomes are a way for cells to interact. Exosome formation is a complex process [37]. It begins with the formation of early-sorting endosomes (ESE), which are created by fusion of primary endocytic vesicles [38-40]. The trans-Golgi endoplasmic reticulum promotes the formation of ESE [41-43]. ESE can return to the plasma membrane as the “recycling endosome” or mature to generate a late endosome (LSE)/multivesicular body (MVB) [18, 39]. The formation of MVB requires assistance from the endosomal sorting complex required for transport (ESCRT). ESCRT is a protein complex located on the cytoplasmic side of the endosome and its main role is to sort specific components into ILVs, which in turn constitute the precursors of exosomes [44]. The ESCRT apparatus comprises four types of complexes (ESCRT- 0, I, II, and III) and accessory proteins (VPS4, VTA1, ALIX, etc.), each playing diverse regulatory roles. ESCRT-0 is responsible for the recognition and separation of ubiquitin-labeled endosomal transmembrane proteins [45-47]. ESCRT-I can be connected to ESCRT-II, and the two entities germinate inward to promote absorption of corresponding secretions by the endocytic membrane [48]. Subsequently, ESCRT-III binds to the corresponding complex and communicates with the cell membrane to release buds, which then enter endosomes in the cell [49]. If the cargo are deubiquitinated by deubiquitinating enzymes (DUBs), the goal is delivery to lysosomes for degradation [50]. The Rab family are small GTPase proteins that control the transport processes of intracellular vesicles, such as movement of vesicles through the cytoskeleton and positioning of vesicles on the plasma membrane [51]. Studies have shown that Rab11, Rab35, Rab27A/B, and Rab9 are related to the secretion of exosomes [48, 52-54]. Soluble N-ethyl maleimide (NEM)-sensitive factor attachment protein receptor (SNARE) is a protein complex that can fuse plasma membranes that are in contact with each other and also promote the fusion of vesicle membranes and cell plasma membranes [55, 56]. Cells can also generate LVs and MVBs without relying on the ESCRT pathway, and assist in the generation of lipids, ceramides, tetraspanins, and heat shock proteins [57] (Figure 1A). Microvesicles are released into the extracellular space through outward sprouting and fission of plasma membrane [58].
EVs contain proteins, lipids, and nucleic acids [40] that are closely related to the donor cell and powerfully influence the recipient cell [59, 60]. Depending on the origin of exosomes, protein components include typical transmembrane proteins that cross the membrane such as LAMP1/2, CD13, PGRL, and trafficking membrane proteins such as annexin and RABs. The lipid bilayer membrane of EVs also has a complex structure and mainly contains adhesion molecules such as ICAM-1 and other proteins such as LBPA, flotillins, cholesterol, tetraspanins, and stomatin that can affect lipid rafts [61]. CD9, CD63, CD81, and CD82, which are present on the membrane, are commonly used as markers of exosomes. Additionally, the EVs membrane surface is assembled with immunomodulatory molecules such as MHC-I/II [62, 63]. In the exosome cavity, several proteins have been found that stabilize and preserve the “informative” exosome cargo: HSP, cytoskeletal protein, metabolic enzymes (ATPase, GAPDH, elongation factors, and pgk1), and cytoskeletal proteins (tublin, actin, vimentin, cofilin, moesin, and talin) [64, 65] (Figure 3A).
Intracellular biogenesis and secretion of extracellular vesicles. A: The process of exosome formation begins with early-sorting endosomes (ESE) formed by endocytosis on the surface of plasma membranes. Subsequently, ESE matures to generate a late endosome (LSE)/multivesicular body (MVB) and exosomes are released into the extracellular space by fusion of MVBs. B: Exosomes interact with target cells via receptors, fusion with plasma membrane, endocytosis, or release of their cargo.
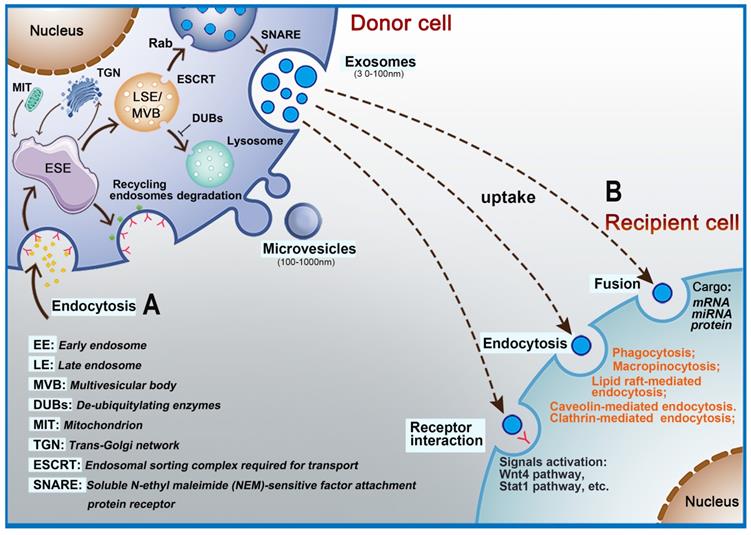
The contents of EVs that can be transferred from donor cells to target cells in the microenvironment include RNAs and a large number of proteins depending on the recipient cells [40, 66, 67]. EVs have also been shown to contribute to intercellular communication by passing signal molecules or by surface-expressed ligands. Interactions between EVs and recipient cells are highly intriguing. Depending on their contents and the type of recipient cell, EVs transfer their cargo to recipient cells by fusing with the plasma membrane through receptors or by endocytosis (Figure 1B) [68, 69]. For example, EVs (especially exosomes) promote communication between neural stem/precursor cells and the microenvironment through receptor-ligand interactions [70]. Many key proteins that promote EVs uptake have been discovered, including the tetraspanin membrane proteins CD9 and CD81 and intercellular adhesion molecule (ICAM)-1 [71, 72]. However, accumulating data show that the contents, size, and membrane composition of EVs are highly heterogeneous and dynamic and depend on the cellular source, state, and environmental conditions [73]. Thus, exosome biogenesis plays an important role in its ability to transfer contents to recipient cells. At the same time, EVs enter recipient cells via a variety of mechanisms, which adds to their diversity. According to the biological context, the relative importance of the various uptake pathways differs greatly [74], which indicates that the key protein needed for transfer of contents is different for EVs originating from different cell types. Numerous studies have shown that endocytosis is the primary method for EVs uptake. Endocytosis pathways involved include clathrin- and caveolin-dependent endocytosis, clathrin-dependent endocytosis, micropinocytosis, phagocytosis and micropinocytosis, and lipid raft-mediated endocytosis [75]. In addition, the surface proteins on EVs can bind and activate receptors on recipient cells. For example, EVs-associated interferon gamma receptor 1 (IFNGR-1) binds free interferon (IFN)-γ via the Stat1 pathway in recipient cells to activate signal transduction [76]. Other reports have also shown that EVs can directly fuse with the recipient cell membrane to deliver their cargoes into cells [77].
The Roles of Extracellular Vesicles in Heart Diseases
Extracellular Vesicles are Involved in Physiological and Pathological processes of Heart Diseases
Extracellular Vesicles in Atherosclerosis
Atherosclerosis, a chronic inflammatory disease of blood vessels, involves multiple processes such as lipid penetration, endothelial dysfunction, inflammatory response, and cell proliferation. Hutcheson et al. [78] reported for the first time that EVs mediate the occurrence and development of microcalcification in atherosclerotic plaques. EVs can increase the expression of adhesion molecule receptors in monocytes, which is conducive to the adhesion of monocytes to endothelial cells. At the same time, accumulation of EVs can aggravate the formation of calcifications and promote vasoactive responses. Development of atherosclerosis is initiated by endothelial dysfunction, which is mainly due to local disturbances in blood flow along endothelial cells. Zhang et al. [79] found exosomes-mediated miRNA-155 induces endothelial injury and promotes atherosclerosis. Platelet-derived EVs mediate the atherosclerotic interaction of platelets with endothelial cells and monocytes. A recent study showed that activated platelet-derived exosomes can rapidly decrease the expression of type II scavenger receptor CD36 in platelets by enhancing CD36 ubiquitination and proteasome degradation, thereby reducing platelet aggregation and collagen adhesion in the body [80].
Extracellular Vesicles in Myocardial Infarction
Rupture of atherosclerotic plaques and subsequent hemorrhage lead to acute myocardial infarction (AMI) [81]. During AMI, cardiomyocytes increase secretion of EVs containing heart-specific non-coding RNA, which have a significant protective effect on the heart. For example, miRNA-133 has anti-fibrosis effects, miR-1 has specific antioxidant effects, and miRNA-499 has anti-apoptotic properties [82, 83]. Among them, hypoxia-treated cardiac progenitor cell (CPC)-derived EVs can promote angiogenesis after AMI [84].
Extracellular Vesicles in Heart Failure
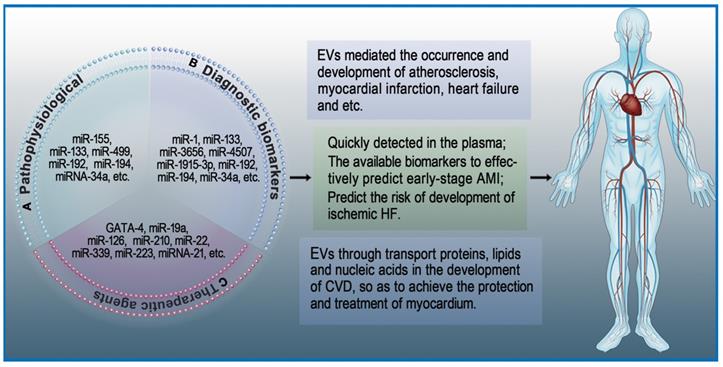
Continuous damage of numerous cardiomyocytes is an important reason why MI develops into heart failure and eventually leads to death [85]. Many studies have shown that EVs secreted from cardiomyocytes are involved in the process of heart failure [86]. Matsumoto et al. [87] found that circulating exosomal miRNAs (miRNA-192, miRNA-194 and miRNA-34a) are significantly correlated with heart failure after AMI. In another study, Liu et al. [88] showed that overexpression of miRNA-132 can protect against apoptosis and oxidative stress in heart failure (Figure 2A).
Roles of extracellular vesicles in heart diseases. EVs play an important role in the maintenance of cardiac homeostasis and the pathogenesis of heart diseases. A: The role of EVs in pathophysiological processes. B: EVs as diagnostic biomarkers in heart diseases. C: EVs as therapeutic agents in heart diseases.
Extracellular Vesicles as Diagnostic Biomarkers in Heart Diseases
Cardiac troponins and creatine kinase-MB are classical biomarkers in the diagnosis of AMI. Among these, the 'gold standard' for AMI diagnosis is commonly believed to be cardiac troponins [89]. However, this has not stopped the exploration of new biomarkers with higher sensitivity and specificity for diagnosis of early AMI [90]. EVs isolated from cardiac cells are partially internalized by neighboring cells, while most of the remaining EVs are released into body fluids. Studies on heart diseases have revealed that EVs isolated from the serum of patients with AMI contain specific mRNA and miRNA [91]. For example, the serum levels of miRNA-1 and miRNA-133 in patients with acute coronary syndrome (ACS) are elevated. These elevated miRNA-1 and miRNA-133 have been proven to be derived from damaged myocardium, and they are likely to be stored in EVs [92]. Deddens et al. [93] demonstrated that heart- and muscle-specific miRNAs are transported by EVs and can be quickly detected in the plasma. Since these EVs are rich in released miRNAs and their detection precedes the expression of traditional damage markers, they have a strong possibility to become early biomarkers of AMI. Su et al. suggested that serum exosomal miRNAs (has-miRNA-3656, has-miRNA-4507, and has-miRNA-1915-3p) can be used for the prediction of AMI at an early stage [94]. Additionally, Matsumoto and colleagues found that circulating exosomal miRNAs (has-miRNA-192, has-miRNA-194, and has-miRNA-34a) can predict the risk of developing ischemic heart failure after AMI [95]. Similarly, EVs were found to mediate the occurrence and development of microcalcification in atherosclerotic plaques [78]. In a study of 488 consecutive patients with various coronary heart disease (CHD) risks, Nozaki et al. [96] suggested that endothelial exosomal CD144+ could be an independent predictor of future cardiovascular events and contribute to risk stratification of CHD. Altogether, these studies indicate that exosomal miRNAs can become potential biomarkers of heart diseases (Figure 2B).
Extracellular Vesicles as Therapeutic Agents in Heart Diseases
The adult mammalian heart is a terminally differentiated organ, meaning cardiomyocyte injury is difficult to repair [97]. As an important transmission system in vivo, EVs regulate gene expression in target cells through transport proteins, lipids, and nucleic acids during the development of heart diseases, thereby protecting and treating myocardium [98]. Early research showed that overexpression of GATA-4 increases mesenchymal stem cells (MSCs) differentiation into cardiac cell phenotypes as well as promotes the survival of MSCs in ischemic environments [99]. Yu et al. [72] suggested that EVs secreted from GATA-4-overexpressing MSCs could deliver miRNA-19a into myocardium and produce a greater cardioprotective effect. Hypoxia-inducible factor 1 (HIF-1) is an oxygen-sensitive transcription factor that has great significance in angiogenesis [100]. EVs secreted from HIF-1-overexpressing CPCs have been shown to deliver miRNA-126/miRNA-210 and increase angiogenic responses in the hypoxic environment [101]. EVs derived from stem cells encapsulate various molecules such as mRNA, miRNA, and proteins that have cardioprotective effects similar to stem cell transplantation. For example, EVs derived from MSCs were found to be enriched with miRNA-22, which directed targeting to methyl CpG binding protein 2 (MECP2) and reduced apoptosis of cardiomyocytes due to ischemia [102].
Cardioprotective effects can also be derived from endogenous EVs in vivo. During oxidative stress, cardiomyocytes increase the synthesis and secretion of EVs. Garcia et al. suggested that EVs secreted by cardiomyocytes can stimulate endothelial cells to produce vessels under glucose-deprived culture conditions [33]. Platelet-derived EVs can also affect endothelial cells and platelet function in CVD. Tan et al. [103] showed that active platelet-derived EVs delivering miRNA-339, miRNA-223, and miRNA-21 inhibit the expression of platelet-derived growth factor receptor-beta (PDGFRβ) in vascular smooth muscle cells (SMCs) and increase the number of capillaries in ischemic myocardium. Li and colleagues [104] indicated that anti-IL-1 platelet-derived EVs remove cytotoxic IL-1 and repair ischemic myocardium during AMI. Li et al. suggested that coronary serum exosomes in patients with MI regulate angiogenesis through miR-939-mediated nitric oxide signaling pathway [105]. Another interesting new research direction is cardio-renal exosome-derived miRNA-1956, which regulates the activation of paracrine VEGF signaling in adipose-derived MSCs after AMI [106] (Figure 2C).
Challenges in the Delivery of Extracellular Vesicles to the Heart
Due to their unique advantages, EVs can be positioned as efficient drug carriers [107]. The special structure of EVs can protect their contents from degradation in the extracellular environment for a long time [73], and their surfaces contain special lipids and proteins that are conducive to fusion with recipient cells and subsequent release of cargo including drugs [41, 108, 109]. EVs containing short hairpin RNA plasmids and interfering RNA inserted by conventional mass electroporation have shown higher therapeutic efficacy in inhibiting targets than synthetic nanocarriers in conventional preclinical studies. However, insertion of large amounts of RNA into EVs is still technically challenging and may be limited to specific cell types. Recently, Yang et al. [110] discovered a type of cellular nanoperforation (CNP) that can effectively integrate high levels of mRNA into EVs for targeted transcriptional operations and therapy. Compared to mass electroporation and other EV production methods, CNP can produce up to 50-fold more EVs from cells with low basal secretion levels, and mRNA transcripts can be increased by more than 103-fold. However, Kooijmans et al. [111] stated that electroporation disrupts EVs integrity and siRNA loading is accompanied by substantial siRNA aggregate formation, which may lead to overestimation of the amount of siRNA actually loaded into EVs. Similar to EVs, liposomes are synthetic vesicles with phospholipid bilayer structures that can be loaded with a variety of proteins, nucleic acids, and drug molecules. A comparison of liposomes and EVs as drug delivery vehicles is provided in Table 1. Current studies have shown that EVs provide potentially superior drug delivery than liposomes [112].
Advantages and disadvantages of liposomes and EVs.
| Liposomes | EVs | |
|---|---|---|
| Origin | Artificially produced from a wide variety of phospholipids | Secreted by cells |
| Shape and size | Phospholipid bilayer structures; ~100 nm and homogenous | Phospholipid bilayer structures including many proteins (e.g., tetraspanins); 30-120 nm |
| Manufacture | Many manufacturing possibilities | No current manufacturing methods |
| Contents | Single component | Complex components (e.g., DNA, RNA, proteins, small molecule drugs) |
| Drug loading capacity | High loading of hydrophilic drugs | Low drug loading |
| Half-life | 10-55 h | 70-80 min |
| Targeted delivery | Poor and dependent on the enhanced permeability and retention (EPR) effect | Strong with ability to cross biological membranes and high endogenous targeting potential |
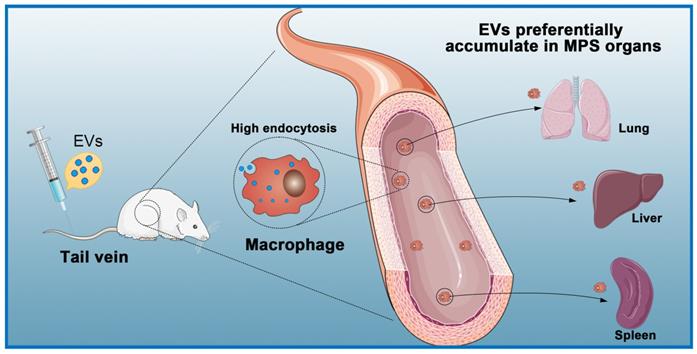
The expected biological effects of EVs are mostly produced from internalization by recipient cells through endocytosis pathways [113]. Studies have shown that EVs administered intraperitoneally, subcutaneously, and intravenously are quickly cleared from the blood circulation and subsequently accumulate in the lung, spleen, liver, and gastrointestinal tract [114, 115] (Figure 4). Regardless of the delivery route and cell source, most systemically injected EVs are rapidly absorbed by macrophages in the reticuloendothelial system and excreted from the body [116-118]. In a previous study, to counteract non-specific delivery, the authors used approximately ten times the normal dose used for intramyocardial injections [119]. Another study used intracoronary and intramyocardial injections and showed that intramyocardial delivery was more effective [120]. Although intramuscular injections can be performed in animal studies, such a situation is more complicated in a clinical setting and requires a physician to perform the catheterization [121].
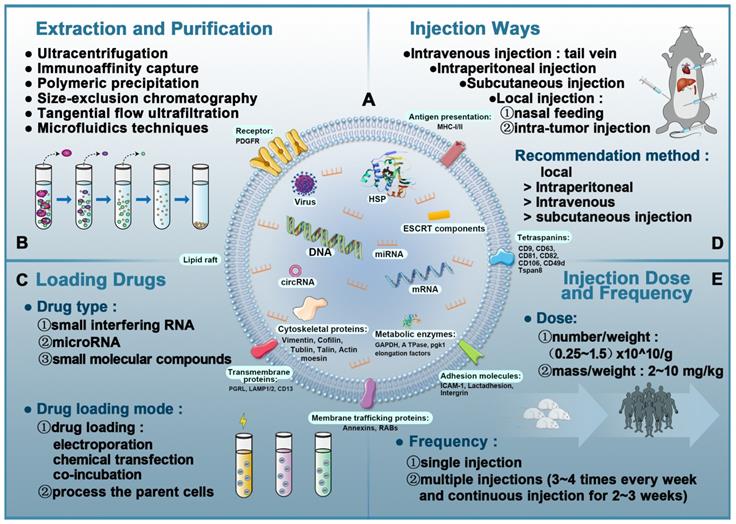
EVs extraction and purification do not have universally recommended techniques. Currently, there are six major methods: ultracentrifugation, immunoaffinity capture, polymeric precipitation, tangential flow ultrafiltration (TFU), microfluidics techniques, and size-exclusion chromatography (SEC) (Figure 3B). Based on investigations by the International Society for Extracellular Vesicles (ISEV) in 2015, ultracentrifugation is the most widely adopted and reliable method and considered to be the gold standard for EVs extraction. Each technique has a unique set of advantages and disadvantages, as shown in Table 2. Another pertinent question is the cost of extracting EVs. EVs are secreted by cells, so their production depends on the ability to produce large numbers of cells without changing their phenotype [122]. Moreover, it is not only difficult to produce large quantities of EVs, it is also challenging to produce EVs with high purity and stable quality.
The typical yield of EVs isolated from 1 mL of culture medium could be less than 1 µg of EV protein. As such, therapeutic doses of EVs (~10-100 µg of protein) can be achieved in mouse models [123, 124]. For humans, the effective dose is an order of magnitude more than the dose used in mouse models to compensate for the rapid elimination of EVs from the body. Owing to the high incidence rate of heart diseases, there is an urgent need to improve the specificity of EVs delivery to cardiomyocytes to reduce consumption by non-specific delivery [125]. Figure 3C-E shows the details of EV-based nanotherapeutics including drug loading techniques, administration routes, and injection dose and frequency.
Targeted Delivery of Therapeutic Extracellular Vesicles in Heart Injury
Local Delivery of Hydrogels Encapsulating Extracellular Vesicles
Hydrogels have been widely used to create drug delivery systems with ideal therapeutic effects [126]. Hydrogels are suitable for biological applications due to their large water content and biocompatibility. They typically have excellent malleability and are similar to the natural extracellular matrix (ECM). Moreover, the physical properties of hydrogels can be controlled to adjust the rate of matrix degradation to release encapsulated cargo. In the past few decades, a lot of research has focused on hydrogels, and significant progress has been made in their design, synthesis, and use in many biological and biomedical applications [127] including delivery of EVs [128]. Hydrogels can provide practical options for delivering large numbers of EVs to target sites. Qin et al. [129] first described the concept of encapsulating EVs in hydrogels and confirmed that the delivery system can significantly enhance bone formation in vivo.
Comparison of EVs extraction and purification techniques.
| Strategy | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Ultracentrifugation | Density, size, and shape | Low cost Low risk of pollution Fast Scalable | Low purity Damages membrane integrity Time consuming Labor intensive |
| Immunoaffinity capture | Specific recognition of exosome markers by corresponding immobilized antibodies | Suitable for separating exosomes of specific origin High purity | High cost Low yield Damages membrane integrity |
| Polymeric precipitation | High hydrophilic water-excluding polymers | Low cost Scalable Simple | Low purity Protein aggregates remain |
| Tangential flow ultrafiltration | Size and shape | High purity Fast Scalable Simple | Low purity Membrane-fouling High cost |
| Microfluidics techniques | Immunoaffinity, size, and density | Low cost Fast Simple Easily automated and integrated with diagnosis | Low sensitivity and specificity Small sample size |
| Size-exclusion chromatography | Size | High purity Fast Maintains membrane integrity | High cost Needs specialized equipment and filler |
Overview of extracellular vesicles, their composition, isolation, and analysis in vivo. A: The composition of exosomes (including proteins, lipids, and nucleic acids). B: Exosomal isolation and purification techniques. C: Drug-loading techniques to produce EVs-based nanotherapeutics. D: Administration routes in EVs-based nanotherapeutics. E: Injection dose and frequency in EVs-based nanotherapeutics.
Macrophages eliminate circulating extracellular vesicles. Injected EVs are quickly cleared by macrophages of the mononuclear phagocyte system (MPS) and preferentially accumulate in MPS organs (e.g., liver, spleen, lung).
Hydrogels used to encapsulate EVs for treatment of myocardial infarction.
| Type of Hydrogel | Materials | Cell-derived EVs | Function | EVs Preservation in Heart | Advantages | Disadvantages | Ref |
|---|---|---|---|---|---|---|---|
| Shear-thinning (STG) | adamantane- and b-cyclodextrin-modified hyaluronic acid | Endothelial progenitor cells | Improve angiogenesis and promote function | 21 days; Slow release | Prolonged therapeutic duration, slow elution of EVs, and high local concentrations; Translation to the clinical setting | Increased inflammation in the ischemic border | Chen et al. (2018) |
| PA-GHRPS (PGN) | Peptide amphiphile, cardiac protective peptides, matrix metalloprotease-2 | Human umbilical cord mesenchymal stem cells | Promote cardiac repair | 21 days; Enhanced retention and stability | Non-immunogenic and relatively small pore size; Easily tunable | Difficult to control release | Han et al. (2019) |
| Nanocomposite (nSi) | gelatin, Laponite | Human adipose-derived stem cells | Repair injured cardiac tissue | 21 days; Controlled release of growth factors present in EVs | High surface-to-volume ratio and discoidal charged surface; Biocompatible | Biodegradation in myocardial tissue is unknown | Waters et al. (2017) |
| Hydrogel patch | Rat tail collagen type I, Gelfoam mesh | Induced pluripotent stem cells | Promote recovery of the heart | 21 days; Sustained release | Well-defined neutral material | Larger pore size; More invasive injury | Liu et al. (2018) |
In heart diseases, the ability to release EVs over a long duration may be more practical than repeated implantation of fresh hydrogels into the heart with release of EVs over a short duration. Table 3 summarizes studies on the duration of EV release from hydrogels in the heart. Chen et al. [130] found that endothelial progenitor cells (EPCs)-derived EVs encapsulated in shear-thinning hydrogel (STG) could be injected into the ischemic myocardium, where they promoted angiogenesis and improved myocardial hemodynamics in a rat MI model. Additionally, STG improved therapeutic efficiency and the efficacy of EVs-mediated myocardial protection. Hydrogel encapsulation localized the EVs to the myocardial ischemic border area, where they could be observed for 21 days. In a separate study by Han et al. [131], the authors loaded EVs secreted by human umbilical cord MSCs (HUC-MSCs) into a peptide-based hydrogel called PGN. The authors showed that the PGN hydrogel ensured stable and sustained release of EVs at the myocardial ischemic border area over 21 days. Additionally, the EV-PGN hydrogel treatment better improved cardiac function than EVs alone and also reduced fibrosis, apoptosis, and inflammation. Laponite® is a smectite nanoclay composed of discoidal nanoparticles that can solve the biocompatibility problems associated with carbon-based nanoparticles. Owing to its high surface area-to-volume ratio and disk-shaped charged surface, Laponite® possess good loading capacity for growth factors [132, 133]. Waters et al. [134] created a hydrogel of Laponite® and gelatin (nSi) as a vehicle for the delivery of EVs. The authors showed that the nSi hydrogel can sustain high strain and reduced EVs effusion out of the therapeutic site for 21 days after injection. In another study, Liu et al. [135] engineered a hydrogel patch to slowly release iPSC-derived cardiomyocyte EVs, which have been shown to promote recovery within 24 h after MI and reduce arrhythmia in humans. The hydrogel-delivered EVs recovered the normal physiological activity of cardiomyocytes and reduced the infarct size 4 weeks after MI implantation in diseased rats. Furthermore, Huang et al. [136] developed an off-the-shelf therapeutic cardiac patch composed of a decellularized porcine myocardial extracellular matrix scaffold and synthetic cardiac stromal cells (synCSCs) generated by encapsulating secreted factors from isolated human cardiac stromal cells. The transplanted artCP promoted cardiac recovery by reducing scarring, promoting angiogenesis, and boosting cardiac function in both rat and porcine models of AMI. Interestingly, these cases indicate that some hydrogels alone have therapeutic effects. For example, the PGN hydrogel described above is a functional peptide hydrogel based on a growth hormone-releasing peptide (His-DTrp-Ala-Trp-DPhe-Lys-NH2) that activates pro-survival pathways and inhibits inflammation and fibrosis [131]. In addition, Chen et al. [130] found that STG treatment alone significantly improved the end-systolic pressure volume relationship 4 weeks after MI compared with PBS control. This effect was due to the hyaluronic acid (HA) composition of STG, as HA is a biologically active pro-angiogenic molecule that can affect the proliferation, migration, and tubule formation of endothelial cells through CD44- and HA-mediated cell movement signal receptors.
In short, biodegradable and highly porous hydrogels can provide continuous treatment of heart tissue with a matrix of vesicles. By placing hydrogels loaded with EVs directly at or around the target site, hydrogels also prevent the disappearance of EVs from the target site. With hydrogels, only a small number of EVs are required to achieve therapeutic effects. In comparison, a large number of EVs must be injected intravenously to counter the poor systemic bioavailability of EVs [137-144].
Genetic Engineering of Extracellular Vesicles for Therapeutic Delivery
Compared with other gene delivery vectors, EVs are non-mutagenic, less immunogenic, and non-cytotoxic. These characteristics indicate that EVs can become an ideal therapeutic carrier [145]. In addition, genetic engineering can modify EVs to improve their therapeutic efficiency and targeting ability by displaying homing peptides or ligands on their surface. Although genetic engineering of EVs does not change their biological distribution time, it does shorten the time required by EVs to reach their therapeutic target and significantly reduces off-target effects, thereby improving the therapeutic effect [146]. Transmembrane proteins have been shown to accumulate in EV compartments, and targeting EVs to specific sites is achieved by displaying ligands/homologous peptides that can be fused to EV surface proteins [147]. There are ample opportunities to explore the potential uses of EVs in targeted therapies because phage display and in vivo biological screening techniques can target specific sites on many peptides [148]. Zhu et al. [8] modified EVs secreted from hypoxia-conditioned MSCs with an ischemic myocardium-targeting peptide (CSTSMLKAC), thereby preferentially targeting ischemic injured cardiomyocytes and minimizing off-target effects. In another study, Vandergriff et al. [149] showed that modification with CSTSMLKAC, here called cardiac homing peptide (CHP), improved the efficacy and reduced the effective dose of EVs delivered intravenously. To generate an effective EVs delivery strategy that can target cardiomyocytes, Mentkowski et al. [150] designed cardiomyocyte-derived cells (CDC) that express LAMP2B (an EV-addressed membrane protein) fused with a cardiomyocyte-specific peptide (CMP; WLSEAGPVVTVRALRGTGSW). EVs isolated from the engineered CDCs expressed CMP on their surface and maintained their true physical properties. Compared to non-targeted EVs, the targeted EVs reduced cardiomyocyte apoptosis after cardiomyocyte injection, increased cardiomyocyte uptake, and improved cardiac retention. In another study, Zhang et al. [151] enhanced the efficiency of EVs transport in ischemia-injured myocardium by engineering EVs with monocyte mimics (monocyte/macrophage membrane vesicles) using membrane fusion. An intriguing new study [152] suggests that platelet-derived EVs fused with cardiac stem cells (CSCs) can selectively bind collagen-coated surfaces and endothelium-denuded aortas. Therefore, these CSCs have natural targeting and repair capabilities to the damaged area of MI. In addition, Yim et al. [153] developed a strategy called “exosomes for protein loading via optically reversible protein-protein interactions (EXPLORs)” for intracellular delivery of target proteins. By integrating a reversible protein-protein interaction module controlled by blue light with the endogenous process of exosome biogenesis, the authors were able to successfully load cargo proteins into newly generated exosomes, which offered efficient intracellular delivery of soluble proteins into recipient cells. The authors demonstrated transfer of mCherry, Bax, super-repressor IκB protein, and Cre enzyme as functional proteins into target cells in vitro and into brain parenchymal cells in vivo. Increasing data show that soluble proteins play critical roles in the therapeutic effects of EVs.
Altogether, these EV engineering strategies might offer better ways to assess the effects of EVs and provide novel technologies to help clinicians better manage regenerative therapeutics for heart diseases.
Two-step Extracellular Vesicles Delivery Strategy
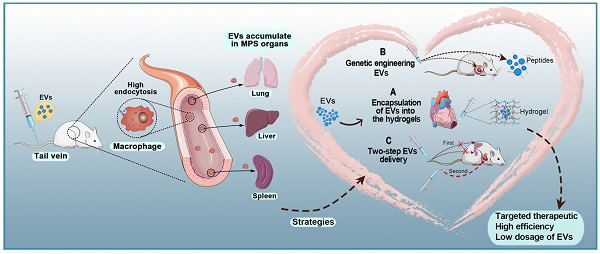
Even though EVs have various advantages compared to existing delivery systems, such as lower immunogenicity and higher affinity, their distribution to the heart is limited by rapid clearance from the blood by the MPS and subsequent accumulation in the liver and spleen [154]. The plasma half-life of EVs is only 70-80 min [155, 156]. In one study, fluorescently labeled EVS injected into the tail vein of mice were mainly captured by the liver, spleen, and lung, as well as kidney, bone marrow, and other organs. Additionally, 4 h after intravenous injection of EVs in mice, 28%, 7%, and 1.6% of fluorescence activity was detected in the liver, lung, and spleen, respectively [155]. However, EVs were mainly absorbed by macrophages in the liver and spleen. There are multiple processes by which macrophages take up EVs, such as micropinocytosis, endocytosis, phagocytosis, and plasma membrane fusion [157]. Whether endocytosis or phagocytosis is the dominant process in EVs uptake by macrophages remains controversial. Clathrin has great significance in the formation of vesicles. Clathrin is a hexamer of proteins, three light and three heavy chains, that can assemble into a basket-like lattice spontaneously to promote the budding process of endocytosis [158]. The CLTC gene encodes clathrin heavy chain 1 [159]. Wan and colleagues were the first to show that CLTC plays a significant role in EVs uptake by the MPS. Inhibition of CLTC with siRNA was shown to significantly block endocytosis mediated by the MPS in the spleen and liver, thereby increasing the delivery of intravenously injected EVs in the heart [117]. In the therapeutic anticancer area, Belhadj et al. [160] exploited a combined “eat me/don't eat me” tactic to reduce endocytosis of macrophages. For the “eat me” component, EVs extracted from DC2.4 cells were modified with cationic mannan to saturate the MPS. For the “don't eat me” component, CD47-enriched exosomes from human serum were fused to nanocarriers to avoid MPS phagocytosis. This combined tactic reduced endocytosis of EVs by macrophages, extended their circulation time, and increased tumor accumulation of EVs by 123.53% in comparison with conventional nanocarriers.
Conclusions and Perspective
Various types of cells secrete EVs, which can act as vehicles for regulating intercellular communication and gene delivery [161, 162]. As naturally derived nanovesicles, EVs have increased stability and biocompatibility, as well as lower toxicity and immunogenicity than synthetic nanocarriers [17]. However, multiple studies have shown that EVs injected intravenously are rapidly removed by macrophages of the MPS and accumulate in organs such as the liver, spleen, and lungs [154]. Such challenges in the efficient delivery of EVs to target sites are yet to be solved. To date, three strategies for the targeted delivery of therapeutic EVs to the heart have been reported: 1) encapsulation of EVs in hydrogels, 2) genetic engineering of EVs, and 3) two-step EVs delivery (Figure 5). Each strategy has a unique set of advantages and disadvantages (Table 4). Hydrogels have been widely used in biomedical research for drug delivery to tissues, as well as cell-based therapies and tissue engineering. Hydrogels have emerged as attractive biomaterials due to their excellent biodegradability and biocompatibility. The most important advantage of hydrogels is their ability to provide an excellent condition for maintaining the integrity of EVs. Recently, various forms of hydrogels such as chitosan hydrogel, imine cross-linked hydrogel, and chitosan/silk hydrogel have been used to improve the therapeutic efficacy of EVs in different medical fields [143, 163, 164]. However, this strategy also has challenges; for example, it is difficult to identify cross-linking agents that do not participate in intracellular chemical reactions and at the same time produce effective hydrogels. Alternatively, surface modification methods (aka “EV engineering”) can be used to enhance the specific binding of EVs to receptors. This method can be used to link heart-targeting peptides to EVs to help reduce the effective dose for intravenous administration. However, since the liver and spleen are the main receptors and target organs of EVs, they are very vulnerable to adverse effects. Therefore, it is necessary to avoid non-specific accumulation of EVs in the spleen and liver so as to improve the delivery efficiency of EVs to the target sites. The two-step EVs delivery strategy is a promising gene therapy method. Macrophage saturation with EVs in advance can successfully block subsequent endocytosis of therapeutic EVs and effectively improve their distribution in the heart. However, the effects of this method are not yet clear and further theoretical and experimental studies are required. Although EV-based theragnostic treatments have made revolutionary progress over the past few decades, there are still unresolved challenges in the field. In summary, strategies for the targeted delivery of EVs to the heart will effectively shorten the arrival time of EVs to the target site, prolong the survival time of EVs, and improve their therapeutic effects.
Targeted therapeutic delivery of extracellular vesicles in heart diseases. Three strategies for targeted delivery of therapeutic EVs to the heart. A: Encapsulation of EVs in hydrogels. B: Genetic engineering of EVs. C: Two-step EVs delivery.
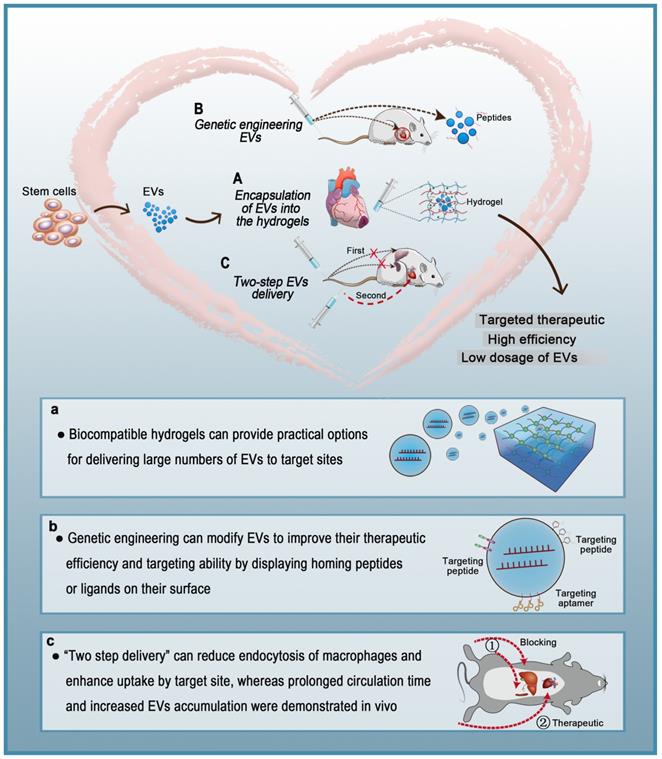
Current strategies for targeted delivery of therapeutic EVs to the heart.
| Strategy | Principles | Advantages | Disadvantages |
|---|---|---|---|
| Encapsulation of EVs in hydrogels | Injectable hydrogel to localize EVs in the myocardial ischemic border | Low dosage of EVs Low cost Avoids repeated implantation Efficient heart-targeted delivery of EVs Stable and sustained release of EVs Hydrogels have a therapeutic effect on cardiovascular diseases Easy to use | Epicardial injection Risk of angiemphraxis Potential toxicity of residual unreacted cross-linkers Uncertain release profiles in vivo |
| Genetic engineering of EVs | Surface modification of EVs with homing peptides or ligands that target the heart | Low dosage of EVs Avoids repeated implantation Efficient heart-targeted delivery of EVs Intravenous administration | High-tech equipment required Labor intensive High cost Unstable release of EVs Low efficiency EVs with a shorter duration |
| Two-step EVs delivery | Blockage of MPS uptake followed by delivery of therapeutic EVs | Avoids repeated implantation Localizes EVs to the target site Intravenous administration Efficient heart-targeted delivery of EVs Blocks the endocytic function of the MPS in the spleen and liver | Time consuming Labor intensive Unstable release of EVs Uncertain efficiency EVs with a shorter duration |
Abbreviations
EVs: extracellular vesicles; AMI: acute myocardial infarction; CHD: coronary heart disease; HIF-1: hypoxia-inducible factor 1; CDC: cardiomyocyte-derived cells; CHP: cardiac homing peptide; CMP: cardiomyocyte-specific peptide; CNP: cellular nanoperforation; DUBs: deubiquitinating enzymes; ECM: extracellular matrix; EPCs: endothelial progenitor cells; ESCRT: endosomal sorting complexes required for transport; ESEs: early-sorting endosomes; iCMs: induced cardiomyocytes; ILV: intra-luminal vesicles; MPS: mononuclear phagocyte system; MVB: multivesicular bodies; NEM: N-ethylmaleimide; STG: shear-thinning hydrogel.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81971765, 31771060, 31671025 and 81871504).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hernesniemi JA, Lyytikainen LP, Oksala N, Seppala I, Kleber ME, Mononen N. et al. Predicting sudden cardiac death using common genetic risk variants for coronary artery disease. Eur Heart J. 2015;36:1669-75
2. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB. et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2018;72:e91-e220
3. Higuchi A, Ku N-J, Tseng Y-C, Pan C-H, Li H-F, Kumar SS. et al. Stem cell therapies for myocardial infarction in clinical trials: bioengineering and biomaterial aspects. Lab Invest. 2017;97:1167-79
4. Péault B, Levente Ume K, Alotti N, Alejandro Lerman D. Cardiac Repair and Regeneration: The Value of Cell Therapies. Eur Cardiol. 2016;11:43
5. Balbi C, Bollini S. Fetal and perinatal stem cells in cardiac regeneration: Moving forward to the paracrine era. Placenta. 2017;59:96-106
6. Heallen TR, Martin JF. Heart repair via cardiomyocyte-secreted vesicles. Nat Biomed Eng. 2018;2:271-2
7. Gong XH, Liu H, Wang SJ, Liang SW, Wang GG. Exosomes derived from SDF1-overexpressing mesenchymal stem cells inhibit ischemic myocardial cell apoptosis and promote cardiac endothelial microvascular regeneration in mice with myocardial infarction. J Cell Physiol. 2019;234:13878-93
8. Zhu LP, Tian T, Wang JY, He JN, Chen T, Pan M. et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163-77
9. Zhu J, Lu K, Zhang N, Zhao Y, Ma Q, Shen J. et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells Nanomed Biotechnol. 2018;46:1659-70
10. Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual review of cell and developmental biology. 2014;30:255-89
11. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells. 2019;8:307
12. Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom Rev. 2015;34:474-90
13. Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74(5):1844-51
14. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-20
15. Vader P, Breakefield XO, Wood MJA. Extracellular vesicles: emerging targets for cancer therapy. Trends Mol Med. 2014;20:385-93
16. Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415-21
17. Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871-81
18. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-28
19. Ibrahim A, Marban E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu Rev Physiol. 2016;78:67-83
20. Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333-44
21. Stahl PD, Barbieri MA. Multivesicular Bodies and Multivesicular Endosomes: The "Ins and Outs" of Endosomal Traffic. Sci STKE. 2002;2002:pe32
22. Sun L, Zhu W, Zhao P, Wang Q, Fan B, Zhu Y. et al. Long noncoding RNA UCA1 from hypoxia-conditioned hMSC-derived exosomes: a novel molecular target for cardioprotection through miR-873-5p/XIAP axis. Cell Death Dis. 2020;11:696
23. Laggner M, Gugerell A, Bachmann C, Hofbauer H, Vorstandlechner V, Seibold M. et al. Reproducibility of GMP-compliant production of therapeutic stressed peripheral blood mononuclear cell-derived secretomes, a novel class of biological medicinal products. Stem Cell Res Ther. 2020;11:9
24. Im H, Lee K, Weissleder R, Lee H, Castro CM. Novel nanosensing technologies for exosome detection and profiling. Lab on a chip. 2017;17:2892-8
25. Kooijmans SAA, Schiffelers RM, Zarovni N, Vago R. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol Res. 2016;111:487-500
26. Casado-Diaz A, Quesada-Gomez JM, Dorado G. Extracellular Vesicles Derived From Mesenchymal Stem Cells (MSC) in Regenerative Medicine: Applications in Skin Wound Healing. Front Bioeng Biotechnol. 2020;8:146
27. Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL. et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14:195-204
28. Cheng L, Zhao W, Hill AF. Exosomes and their role in the intercellular trafficking of normal and disease associated prion proteins. Mol Aspects Med. 2018;60:62-8
29. Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A. et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136-46
30. Cheng M, Yang J, Zhao X, Zhang E, Zeng Q, Yu Y. et al. Circulating myocardial microRNAs from infarcted hearts are carried in exosomes and mobilise bone marrow progenitor cells. Nat Commun. 2019;10:959
31. Belevych AE, Sansom SE, Terentyeva R, Ho H-T, Nishijima Y, Martin MM. et al. MicroRNA-1 and -133 increase arrhythmogenesis in heart failure by dissociating phosphatase activity from RyR2 complex. PLoS One. 2011;6:e28324
32. Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y. et al. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139-50
33. Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res. 2016;109:397-408
34. Eitan E, Suire C, Zhang S, Mattson MP. Impact of lysosome status on extracellular vesicle content and release. Ageing Res Rev. 2016;32:65-74
35. Choi D, Montermini L, Kim DK, Meehan B, Roth FP, Rak J. The Impact of Oncogenic EGFRvIII on the Proteome of Extracellular Vesicles Released from Glioblastoma Cells. Mol Cell Proteomics. 2018;17:1948-64
36. Palazzolo S, Bayda S, Hadla M, Caligiuri I, Corona G, Toffoli G. et al. The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes. Curr Med Chem. 2018;25:4224-68
37. Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W. et al. Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol Cancer. 2019;18:116
38. Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol. 2020 219
39. Vietri M, Radulovic M, Stenmark H. The many functions of ESCRTs. Nat Rev Mol Cell Biol. 2020;21:25-42
40. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977
41. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17
42. McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019;18:52
43. Kumari N, Saxena S, Agrawal U. Exosomal protein interactors as emerging therapeutic targets in urothelial bladder cancer. J Egypt Natl Canc Inst. 2015;27:51-8
44. Frydrychowicz M, Kolecka-Bednarczyk A, Madejczyk M, Yasar S, Dworacki G. Exosomes - Structure, Biogenesis and Biological Role in Non-Small-Cell Lung Cancer. Scand J Immunol. 2015;81:2-10
45. van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13-21
46. Ren X, Hurley JH. VHS domains of ESCRT-0 cooperate in high-avidity binding to polyubiquitinated cargo. EMBO J. 2010;29:1045-54
47. Kobayashi H, Tanaka N, Asao H, Miura S, Kyuuma M, Semura K. et al. Hrs, a mammalian master molecule in vesicular transport and protein sorting, suppresses the degradation of ESCRT proteins signal transducing adaptor molecule 1 and 2. J Biol Chem. 2005;280:10468-77
48. Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A. et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677-85
49. Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172-7
50. Yeates EF, Tesco G. The Endosome-associated Deubiquitinating Enzyme USP8 Regulates BACE1 Enzyme Ubiquitination and Degradation. J Biol Chem. 2016;291:15753-66
51. Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513-25
52. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19-30 1-13
53. Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS. et al. Neurotransmitter-Triggered Transfer of Exosomes Mediates Oligodendrocyte-Neuron Communication. PLoS Biol. 2013;11:e1001604
54. Abrami L, Brandi L, Moayeri M, Brown Michael J, Krantz Bryan A, Leppla Stephen H. et al. Hijacking Multivesicular Bodies Enables Long-Term and Exosome-Mediated Long-Distance Action of Anthrax Toxin. Cell Rep. 2013;5:986-96
55. Kennedy MJ, Ehlers MD. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69:856-75
56. Zylbersztejn K, Galli T. Vesicular traffic in cell navigation. FEBS J. 2011;278:4497-505
57. Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular Endosome Biogenesis in the Absence of ESCRTs. Traffic. 2009;10:925-37
58. Van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-28
59. Yokoi A, Villar-Prados A, Oliphint PA, Zhang J, Song X, De Hoff P. et al. Mechanisms of nuclear content loading to exosomes. Sci Adv. 2019;5:eaax8849
60. Rana S, Zöller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011;39:559-62
61. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581-93
62. Graner MW, Cumming RI, Bigner DD. The Heat Shock Response and Chaperones/Heat Shock Proteins in Brain Tumors: Surface Expression, Release, and Possible Immune Consequences. J Neurosci. 2007;27:11214
63. Petersen SH, Odintsova E, Haigh TA, Rickinson AB, Taylor GS, Berditchevski F. The role of tetraspanin CD63 in antigen presentation via MHC class II. Eur J Immunol. 2011;41:2556-61
64. Wang Z, Zoller M. Exosomes, metastases, and the miracle of cancer stem cell markers. Cancer Metastasis Rev. 2019;38:259-95
65. Mathivanan S, Ji H, Simpson RJ. Exosomes: Extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907-20
66. Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44:11-9
67. Simpson RJ, Lim JWE, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267-83
68. Choi D, Lee TH, Spinelli C, Chennakrishnaiah S, D'Asti E, Rak J. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin Cell Dev Biol. 2017;67:11-22
69. Ogorevc E, Kralj-Iglic V, Veranic P. The role of extracellular vesicles in phenotypic cancer transformation. Radiol Oncol. 2013;47:197-205
70. Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D. et al. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell. 2014;56:193-204
71. Segura E, Nicco C, Lombard Brr, Véron P, Raposo Ga, Batteux Fdr. et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216-23
72. Yu B, Kim HW, Gong M, Wang J, Millard RW, Wang Y. et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015;182:349-60
73. Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI. et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066 -
74. Ibrahim A, Marbán E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu Rev Physiol. 2016;78:67-83
75. Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:10.3402 /jev.v3.24641
76. Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W. et al. Circulating Exosomes Induced by Cardiac Pressure Overload Contain Functional Angiotensin II Type 1 Receptors. Circulation. 2015;131:2120-30
77. He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: Biology and Translational Medicine. Theranostics. 2018;8:237-55
78. Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W. et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15:335-43
79. Zheng B, Yin W-N, Suzuki T, Zhang X-H, Zhang Y, Song L-L. et al. Exosome-Mediated miR-155 Transfer from Smooth Muscle Cells to Endothelial Cells Induces Endothelial Injury and Promotes Atherosclerosis. Mol Ther. 2017;25:1279-94
80. Goetzl EJ, Goetzl L, Karliner JS, Tang N, Pulliam L. Human plasma platelet-derived exosomes: effects of aspirin. FASEB J. 2016;30:2058-63
81. Anderson JL, Morrow DA. Acute Myocardial Infarction. N Engl J Med. 2017;376:2053-64
82. Yu Y, Liu H, Yang D, He F, Yuan Y, Guo J. et al. Aloe-emodin attenuates myocardial infarction and apoptosis via up-regulating miR-133 expression. Pharmacol Res. 2019;146:104315
83. Xu C, Hu Y, Hou L, Ju J, Li X, Du N. et al. β-Blocker carvedilol protects cardiomyocytes against oxidative stress-induced apoptosis by up-regulating miR-133 expression. J Mol Cell Cardiol. 2014;75:111-21
84. Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO. et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116:255-63
85. McMurray JJV, Pfeffer MA. Heart failure. The Lancet. 2005;365:1877-89
86. Wang L, Liu J, Xu B, Liu Y-L, Liu Z. Reduced exosome miR-425 and miR-744 in the plasma represents the progression of fibrosis and heart failure. Kaohsiung J Med Sci. 2018;34:626-33
87. Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M. et al. Circulating p53-Responsive MicroRNAs Are Predictive Indicators of Heart Failure After Acute Myocardial Infarction. Circ Res. 2013;113:322-6
88. Liu X, Tong Z, Chen K, Hu X, Jin H, Hou M. The Role of miRNA-132 against Apoptosis and Oxidative Stress in Heart Failure. Biomed Res Int. 2018;2018:3452748
89. White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570-84
90. Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J. et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659-66
91. Sluijter JPG, Davidson SM, Boulanger CM, Buzás EI, de Kleijn DPV, Engel FB. et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2018;114:19-34
92. D'Alessandra Y, Devanna P, Limana F, Straino S, Di Carlo A, Brambilla PG. et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31:2765-73
93. Deddens JC, Vrijsen KR, Colijn JM, Oerlemans MI, Metz CH, van der Vlist EJ. et al. Circulating Extracellular Vesicles Contain miRNAs and are Released as Early Biomarkers for Cardiac Injury. J Cardiovasc Transl Res. 2016;9:291-301
94. Su J, Li J, Yu Q, Wang J, Li X, Yang J. et al. Exosomal miRNAs as potential biomarkers for acute myocardial infarction. IUBMB life. 2020;72:384-400
95. Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M. et al. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res. 2013;113:322-6
96. Nozaki T, Sugiyama S, Koga H, Sugamura K, Ohba K, Matsuzawa Y. et al. Significance of a Multiple Biomarkers Strategy Including Endothelial Dysfunction to Improve Risk Stratification for Cardiovascular Events in Patients at High Risk for Coronary Heart Disease. J Am Coll Cardiol. 2009;54:601-8
97. Kishore R, Khan M. Cardiac cell-derived exosomes: changing face of regenerative biology. Eur Heart J. 2017;38:212-5
98. Jung J-H, Fu X, Yang PC. Exosomes Generated From iPSC-Derivatives: New Direction for Stem Cell Therapy in Human Heart Diseases. Circ Res. 2017;120:407-17
99. Li H, Zuo S, Pasha Z, Yu B, He Z, Wang Y. et al. GATA-4 promotes myocardial transdifferentiation of mesenchymal stromal cells via up-regulating IGFBP-4. Cytotherapy. 2011;13:1057-65
100. Ong S-G, Hausenloy DJ. Hypoxia-inducible factor as a therapeutic target for cardioprotection. Pharmacol Ther. 2012;136:69-81
101. Ong S-G, Lee WH, Huang M, Dey D, Kodo K, Sanchez-Freire V. et al. Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer. Circulation. 2014;130:S60-S9
102. Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9:e88685
103. Tan M, Yan HB, Li JN, Li WK, Fu YY, Chen W. et al. Thrombin Stimulated Platelet-Derived Exosomes Inhibit Platelet-Derived Growth Factor Receptor-Beta Expression in Vascular Smooth Muscle Cells. Cell Physiol Biochem. 2016;38:2348-65
104. Li Z, Hu S, Huang K, Su T, Cores J, Cheng K. Targeted anti-IL-1β platelet microparticles for cardiac detoxing and repair. Sci Adv. 2020;6:eaay0589
105. Li H, Liao Y, Gao L, Zhuang T, Huang Z, Zhu H. et al. Coronary Serum Exosomes Derived from Patients with Myocardial Ischemia Regulate Angiogenesis through the miR-939-mediated Nitric Oxide Signaling Pathway. Theranostics. 2018;8:2079-93
106. Gao L, Mei S, Zhang S, Qin Q, Li H, Liao Y. et al. Cardio-renal Exosomes in Myocardial Infarction Serum Regulate Proangiogenic Paracrine Signaling in Adipose Mesenchymal Stem Cells. Theranostics. 2020;10:1060-73
107. Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396-405
108. Pitt JM, Kroemer G, Zitvogel L. Extracellular vesicles: masters of intercellular communication and potential clinical interventions. J Clin Invest. 2016;126:1139-43
109. El-Andaloussi S, Lee Y, Lakhal-Littleton S, Li J, Seow Y, Gardiner C. et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc. 2012;7:2112-26
110. Yang Z, Shi J, Xie J, Wang Y, Sun J, Liu T. et al. Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nat Biomed Eng. 2020;4:69-83
111. Kooijmans SAA, Stremersch S, Braeckmans K, de Smedt SC, Hendrix A, Wood MJA. et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release. 2013;172:229-38
112. Johnsen KB, Gudbergsson JM, Duroux M, Moos T, Andresen TL, Simonsen JB. On the use of liposome controls in studies investigating the clinical potential of extracellular vesicle-based drug delivery systems - A commentary. J Control Release. 2018;269:10-4
113. Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014 3
114. Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145-55
115. Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T. et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77-84
116. Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I. et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316
117. Wan Z, Zhao L, Lu F, Gao X, Dong Y, Zhao Y. et al. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics. 2020;10:218-30
118. Verweij FJ, Revenu C, Arras G, Dingli F, Loew D, Pegtel DM. et al. Live Tracking of Inter-organ Communication by Endogenous Exosomes In Vivo. Dev Cell. 2019;48:573-89.e4
119. Vandergriff AC, de Andrade JB, Tang J, Hensley MT, Piedrahita JA, Caranasos TG. et al. Intravenous Cardiac Stem Cell-Derived Exosomes Ameliorate Cardiac Dysfunction in Doxorubicin Induced Dilated Cardiomyopathy. Stem Cells Int. 2015;2015:960926
120. Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R. et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J. 2017;38:201-11
121. Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheter-based delivery of cells to the heart. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S57-64
122. Yamashita T, Takahashi Y, Takakura Y. Possibility of Exosome-Based Therapeutics and Challenges in Production of Exosomes Eligible for Therapeutic Application. Biol Pharm Bull. 2018;41:835-42
123. Willis GR, Kourembanas S, Mitsialis SA. Toward Exosome-Based Therapeutics: Isolation, Heterogeneity, and Fit-for-Purpose Potency. Front Cardiovasc Med. 2017;4:63
124. Yamashita T, Takahashi Y, Nishikawa M, Takakura Y. Effect of exosome isolation methods on physicochemical properties of exosomes and clearance of exosomes from the blood circulation. Eur J Pharm Biopharm. 2016;98:1-8
125. Riau AK, Ong HS, Yam GHF, Mehta JS. Sustained Delivery System for Stem Cell-Derived Exosomes. Front Pharmacol. 2019;10:1368
126. Lee KY, Peters MC, Mooney DJ. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J Control Release. 2003;87:49-56
127. Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv Mater. 2006;18:1345-60
128. Annabi N, Tamayol A, Uquillas JA, Akbari M, Bertassoni LE, Cha C. et al. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater. 2014;26:85-123
129. Qin Y, Wang L, Gao Z, Chen G, Zhang C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961
130. Chen CW, Wang LL, Zaman S, Gordon J, Arisi MF, Venkataraman CM. et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc Res. 2018;114:1029-40
131. Han C, Zhou J, Liang C, Liu B, Pan X, Zhang Y. et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater Sci. 2019;7:2920-33
132. Waters R, Pacelli S, Maloney R, Medhi I, Ahmed RP, Paul A. Stem cell secretome-rich nanoclay hydrogel: a dual action therapy for cardiovascular regeneration. Nanoscale. 2016;8:7371-6
133. Krishna KV, Ménard-Moyon C, Verma S, Bianco A. Graphene-based nanomaterials for nanobiotechnology and biomedical applications. Nanomedicine. 2013;8:1669-88
134. Waters R, Alam P, Pacelli S, Chakravarti AR, Ahmed RPH, Paul A. Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater. 2018;69:95-106
135. Liu B, Lee BW, Nakanishi K, Villasante A, Williamson R, Metz J. et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat Biomed Eng. 2018;2:293-303
136. Huang K, Ozpinar EW, Su T, Tang J, Shen D, Qiao L. et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci Transl Med. 2020;12:eaat9683
137. Zhang K, Zhao X, Chen X, Wei Y, Du W, Wang Y. et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl Mater Interfaces. 2018;10:30081-91
138. Han C, Zhou J, Liu B, Liang C, Pan X, Zhang Y. et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater Sci Eng C Mater Biol Appl. 2019;99:322-32
139. Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W. et al. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics. 2019;9:65-76
140. Shafei S, Khanmohammadi M, Heidari R, Ghanbari H, Taghdiri Nooshabadi V, Farzamfar S. et al. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J Biomed Mater Res A. 2019;108:545-56
141. Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H. et al. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front Physiol. 2017;8:904
142. Mol EA, Lei Z, Roefs MT, Bakker MH, Goumans MJ, Doevendans PA. et al. Injectable Supramolecular Ureidopyrimidinone Hydrogels Provide Sustained Release of Extracellular Vesicle Therapeutics. Adv Healthc Mater. 2019;8:e1900847
143. Liu X, Yang Y, Li Y, Niu X, Zhao B, Wang Y. et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 2017;9:4430-8
144. Xu N, Wang L, Guan J, Tang C, He N, Zhang W. et al. Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol. 2018;117:102-7
145. Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N. et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185-91
146. Hartman ZC, Wei J, Glass OK, Guo H, Lei G, Yang XY. et al. Increasing vaccine potency through exosome antigen targeting. Vaccine. 2011;29:9361-7
147. Xitong D, Xiaorong Z. Targeted therapeutic delivery using engineered exosomes and its applications in cardiovascular diseases. Gene. 2016;575:377-84
148. Zahid M, Lu X, Mi Z, Robbins PD. Cationic and Tissue-Specific Protein Transduction Domains: Identification, Characterization, and Therapeutic Application. Adv Genet. 2010;69:83-95
149. Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG. et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. 2018;8:1869-78
150. Mentkowski KI, Lang JK. Exosomes Engineered to Express a Cardiomyocyte Binding Peptide Demonstrate Improved Cardiac Retention in Vivo. Sci Rep. 2019;9:10041
151. Zhang N, Song Y, Huang Z, Chen J, Tan H, Yang H. et al. Monocyte mimics improve mesenchymal stem cell-derived extracellular vesicle homing in a mouse MI/RI model. Biomaterials. 2020;255:120168
152. Tang J, Su T, Huang K, Dinh P-U, Wang Z, Vandergriff A. et al. Targeted repair of heart injury by stem cells fused with platelet nanovesicles. Nat Biomed Eng. 2018;2:17-26
153. Yim N, Ryu S-W, Choi K, Lee KR, Lee S, Choi H. et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. 2016;7:12277
154. Qiu X, Li Z, Han X, Zhen L, Luo C, Liu M. et al. Tumor-derived nanovesicles promote lung distribution of the therapeutic nanovector through repression of Kupffer cell-mediated phagocytosis. Theranostics. 2019;9:2618-36
155. Charoenviriyakul C, Takahashi Y, Morishita M, Matsumoto A, Nishikawa M, Takakura Y. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: Yield, physicochemical properties, and pharmacokinetics. Eur J Pharm Sci. 2017;96:316-22
156. Wang AL, Lukas TJ, Yuan M, Du N, Tso MO, Neufeld AH. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PLoS One. 2009;4:e4160
157. Shibaguchi K, Tamura A, Terauchi M, Matsumura M, Miura H, Yui N. Mannosylated Polyrotaxanes for Increasing Cellular Uptake Efficiency in Macrophages through Receptor-Mediated Endocytosis. Molecules. 2019 24
158. Zhang S, Gao H, Bao G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano. 2015;9:8655-71
159. Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857-902
160. Belhadj Z, He B, Deng H, Song S, Zhang H, Wang X. et al. A combined "eat me/don't eat me" strategy based on extracellular vesicles for anticancer nanomedicine. J Extracell Vesicles. 2020;9:1806444
161. Kourembanas S. Exosomes: Vehicles of Intercellular Signaling, Biomarkers, and Vectors of Cell Therapy. Annu Rev Physiol. 2015;77:13-27
162. Cervio E, Barile L, Moccetti T, Vassalli G. Exosomes for Intramyocardial Intercellular Communication. Stem Cells Int. 2015;2015:482171
163. Au - Pape ACH, Au - Bakker MH, Au - Tseng CCS, Au - Bastings MMC, Au - Koudstaal S, Au - Agostoni P. et al. An Injectable and Drug-loaded Supramolecular Hydrogel for Local Catheter Injection into the Pig Heart. J Vis Exp. 2015: e52450.
164. Wang W, Tan B, Chen J, Bao R, Zhang X, Liang S, Shang Y, Liang W, Cui Y, Fan G, Jia H, Liu W. An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials. 2018;160:69-81
Author contact
![]() Corresponding authors: Caiwen Ou, oucaiwenedu.cn; Minsheng Chen, minshengsmucom
Corresponding authors: Caiwen Ou, oucaiwenedu.cn; Minsheng Chen, minshengsmucom
 Global reach, higher impact
Global reach, higher impact