13.3
Impact Factor
Theranostics 2021; 11(5):2247-2262. doi:10.7150/thno.51666 This issue Cite
Research Paper
Activation of N-methyl-D-aspartate receptor regulates insulin sensitivity and lipid metabolism
1. Xiangya Nursing School, Central South University, Changsha, Hunan, China
2. Department of Orthopedics, Xiangya Hospital, Central South University, Changsha, Hunan, China
3. Department of Physiology, School of Basic Medicine Science, Central South University, Changsha, Hunan, China
Abstract
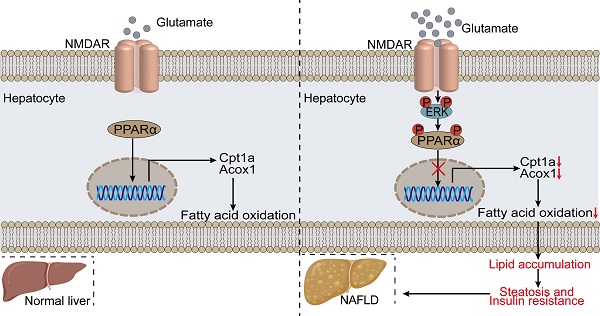
Rationale: Although significant progress has been made in understanding the mechanisms of steatosis and insulin resistance, the physiological functions of regulators in these processes remain largely elusive. Evidence has suggested that the glutamate/N-methyl-D-aspartic acid receptor (NMDAR) axis contributes to acute lung injury, pulmonary arterial hypertension, and diabetes, but the specific metabolic contribution of the glutamate/NMDAR axis is not clear. Here we provide data at the animal, cellular, and molecular levels to support the role of the glutamate/NMDAR axis as a therapeutic target for metabolic syndrome in obesity.
Methods: We examined the glutamate level in the obese mouse induced by a high-fat diet (HFD) for 12 weeks. To assess the role of NMDAR in insulin sensitivity and lipid metabolism, we tested the effects of Memantine (an NMDAR antagonist) and NMDA (an NMDAR agonist) on mice fed with HFD or standard chow diet. The in vitros NMDAR roles were analyzed in hepatocytes and potential mechanisms involved in regulating lipid metabolism were investigated.
Results: Glutamate was increased in the serum of HFD-treated mice. The NMDAR blockade by Memantine decreased the susceptibility to insulin resistance and hepatic steatosis in obese mice. NMDA treatment for 6 months induced obesity in mice, characterized by hyperglycemia, hyperlipidemia, insulin resistance, and pathological changes in the liver. We provided in vitro evidence demonstrating that NMDAR activation facilitated metabolic syndrome in obesity through promoting lipid accumulation. NMDAR inhibition attenuated lipid accumulation induced by palmitic acid. Mechanistically, NMDAR activation impaired fatty acid oxidation by reducing PPARα phosphorylation and activity. The PPARα activity reduction induced by NMDAR activation was reversibly mediated by ERK1/2 signaling.
Conclusion: These findings revealed that targeting NMDAR might be a promising therapeutic strategy for metabolic syndrome in obesity.
Keywords: Obesity, Insulin resistance, Nonalcoholic fatty liver disease, Glutamate, N-methyl-D-aspartate receptor, PPARα
 Global reach, higher impact
Global reach, higher impact