13.3
Impact Factor
Theranostics 2021; 11(5):2218-2231. doi:10.7150/thno.53056 This issue Cite
Review
Cancer photo-immunotherapy: from bench to bedside
1. School of Biomedical Engineering, Hainan University, Haikou, 570228, China
2. College of Physics and Optoelectronic Engineering, Shenzhen University, Shenzhen, 518060, China
3. Department of Oncology, the First Affiliated Hospital of Chinese PLA General Hospital, Beijing 100048, China
4. Stephenson School of Biomedical Engineering, The University of Oklahoma, Norman, OK 73019, USA
5. Baylor Scott & White Healthcare, Waco, Texas 76712, USA
6. Immunophotonics, Inc., 4320 Forest Park Ave., #303 (BAL), St. Louis, MO 63108, USA
Received 2020-9-9; Accepted 2020-11-18; Published 2021-1-1
Abstract
Targeted therapy and immunotherapy in combination is considered the ideal strategy for treating metastatic cancer, as it can eliminate the primary tumors and induce host immunity to control distant metastases. Phototherapy, a promising targeted therapy, eradicates primary tumors using an appropriate dosage of focal light irradiation, while initiating antitumor immune responses through induced immunogenic tumor cell death. Recently, phototherapy has been employed to improve the efficacy of immunotherapies such as chimeric antigen receptor T-cell therapy and immune checkpoint inhibitors. Phototherapy and immunoadjuvant therapy have been used in combination clinically, wherein the induced immunogenic cell death and enhanced antigen presentation synergy, inducing a systemic antitumor immune response to control residual tumor cells at the treatment site and distant metastases. This review summarizes studies on photo-immunotherapy, the combination of phototherapy and immunotherapy, especially focusing on the development and progress of this unique combination from a benchtop project to a promising clinical therapy for metastatic cancer.
Keywords: Cancer, phototherapy, immunotherapy, photo-immunotherapy, combination therapy
Introduction
With developments in diagnosis and treatment techniques, tumor control at the early stages of cancer has been improved. However, late-stage cancers, especially those with metastasis, are still a major cause of treatment failure and death for patients with cancer [1]. Cancer immunotherapy can activate and/or enhance the immune system to track and destroy tumor cells. Therefore, many immunotherapies have been used in clinical studies for treatment of metastatic cancer, including monoclonal antibody (mAb) therapy, cytokine therapy, vaccination, checkpoint inhibition, and chimeric antigen receptor (CAR) T-cell therapy [2, 3]. In particular, the discoveries of dendritic cell (DC)-based vaccines and checkpoint inhibitors were recognized with Nobel Prizes in 2011 and 2018. Although cancer immunotherapies have achieved promising results in preclinical and clinical studies, control of metastases remains a big challenge [2, 3]. In particular, systemic immunity is required to fight against metastases and prevent recurrence [4].
Cancer vaccines are designed to treat existing cancers by enhancing the natural defensive ability of the human body. They are usually made from substances taken from tumor cells or immune cells sensitized with tumor cells [5]. The DC vaccine Provenge (sipuleucel-T) was the first active immunotherapy drug approved by the US FDA in 2011 for treatment of patients with prostate cancer [6]. In a preclinical trial, neoantigen vaccines that minimize potential induction of central and peripheral tolerance, as well as reduce the risk of autoimmunity, were developed as personalized tumor vaccines for treatment of “cold tumors” [7, 8]. However, the preparation of effective vaccines still faces challenges, particularly in the identification of immunogenic neoepitopes on different cancer cells.
A novel strategy for in situ cancer vaccination uses the patient's own tumor antigens that are produced by a local treatment such as phototherapy or radiotherapy. Phototherapy provides an elegant solution for ablating primary tumors due to its high specificity in light delivery, low level of trauma, and effectiveness in destroying target tumors [9]. Because of the deep penetrability of near-infrared (NIR) light in biological tissues, NIR light can be used for phototherapy with corresponding in situ administered or natural absorbance agents [10]. These photoagents convert absorbed light energy into heat for photothermal effects, as in photothermal therapy (PTT), or into reactive oxygen species (ROS) for photochemical effects, as in photodynamic therapy (PDT). A strategy called photoimmunotherapy (PIT) uses an NIR-absorbing photoagent conjugated to a mAb to target and destroy tumor cells under light irradiation. Phototherapies with appropriate photoagents and light doses have been found to induce immunogenic cell death (ICD) in target tumors with the release of tumor-associated antigens (TAAs) and damaged-associated molecular patterns (DAMPs), which may trigger a T helper 1 (Th1)-biased immune response [11-13]. Additionally, PDT may cause necrosis and apoptosis in target cells and surrounding non-target cells, inducing an inflammatory response [14]. Therefore, phototherapy provides sources of tumor antigens and DAMPs locally, creating a potential for generating in situ autologous tumor vaccines to prevent tumor progression and metastasis.
Photoagents should possess strong optical absorption at a therapeutic wavelength, high photothermal/photochemical conversion efficiency, and good biocompatibility. Many photosensitizers have been used in the clinic for PDT including porphyrins, indocyanine green, methylene blue, and Rose Bengal. However, a limited number of photothermal agents have been used in the clinic for PTT [15]. Nanoparticles composed of metals, polymers, carbon, and lipids are considered ideal photothermal candidates due to their strong optical absorption and easily modulated structures [16, 17]. Some nanoparticles have been developed for imaging-guided phototherapy, such as MoSe2/Bi2Se3 for high-contrast computed tomography (CT) imaging-guided PTT [18], and a biocompatible titanium nitride (TiN) nanoplatform for NIR-II laser-excited photoacoustic (PA) imaging-guided PTT [19, 20]. Yang et al. synthesized a gadolinium ion-loaded thermally sensitive polymer nanoplatform for PA, magnetic resonance (MR), and positron emission tomography (PET) multimodal imaging-guided chemo-photothermal combination therapy [21]. AuroShells are tiny silica spheres with a thin outer shell of gold that were developed for treatment of patients with prostate cancer. A recent feasibility study revealed that 13 of 15 prostate cancer patients evidenced no detectable signs of cancer a year after PTT with AuroShells [22]. As the first clinical study of a nanoparticle-based PTT, this study showed great potential for further clinical applications.
Targeted approaches usually aim to inhibit tumor growth directly, whereas immunotherapies attempt to relieve immunoregulatory suppression or stimulate host immunity to achieve long-lived tumor control [23]. Therefore, a combination of targeted therapy and immunotherapy is the ideal strategy to eliminate primary tumors while triggering systemic immunity to control residual tumors and distant metastases. Based on their synergistic thermal-immuno effects, combinations of photothermal agents and immunoadjuvants (e.g., LPS, CpG, R848) or cytokines (e.g., GM-CSF, G-CSF) as endogenous vaccinations have been developed in recent years [24-27]. In addition, the introduction of checkpoint inhibitors (e.g., antibodies against PD‐L1 (programmed cell death-ligand 1), antibodies against CTLA-4 (cytotoxic T lymphocyte-associated antigen-4), small molecule IDO inhibitors (indoleamine 2,3-dioxygenase)) after phototherapy has been shown to markedly improve treatment efficacy by blocking the immunosuppressive receptors on the cell surface, thereby restoring the cytotoxic function of tumor-specific T-cells [28, 29].
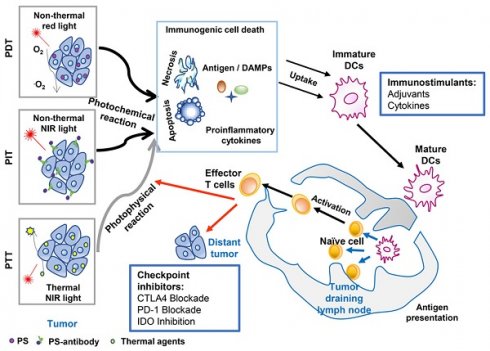
The combination strategy of phototherapy and immunotherapy (photo-immunotherapy) has been found to achieve synergistic effects in the treatment of metastatic cancer, with an enhanced systemic immunostimulatory response (Figure 1) [30, 31]. Phototherapy provides the first line of defense against the tumor, whether it is the original or recurrent tumor, either the same or mutated. More importantly, phototherapy releases antigens, DAMPs, and other tumor components, providing a source for activating immune system. Therefore, photo-immunotherapy can overcome the challenges of tumor heterogeneity, tumor mutation, tumor immune editing, and escape. In particular, phototherapy combined with immunoadjuvant has been utilized to treat patients with advanced cancer [32-35]. It is expected that photo-immunotherapy will experience continuous progress both in basic and clinical research. This review summarizes the development of cancer treatment using this unique combination and introduces its progress in clinical studies.
Photothermal immunotherapy
Photothermal immunotherapy, a novel concept combining PTT with immunotherapy, can lead to a synergistic thermal-immune effect and trigger a specific antitumor immunity [36]. PTT can directly target and destroy the primary tumor by increasing the temperature of the tumor tissue, a strategy that has been used for ablation of glioma, hepatocellular carcinoma, lung cancer et al. [37, 38]. PTT induces a temperature gradient within the tumor tissue that leads to various biological responses from cell stress to cell death [39, 40]. An appropriate temperature gradient can cause a high level of cell death with antigen exposure and DAMPs release, including the exposure/release of HSPs (heat shock proteins), ATP (adenosine triphosphate), and HMGB1 (high-mobility group box 1) [39, 40]. This type of cell death is called immunogenic cell death (ICD) and it provides potential for enhancing APCs activation and antigen presentation. However, high temperature would cause tumor eradication with denatured proteins, and simultaneously attack surrounding normal tissues and inhibit the host immunoreaction [41]. PTT inducing ICD has been developed in combination with immunotherapies such as CAR T-cell therapy, checkpoint inhibitors, and immunoadjuvants to enhance control of both primary tumors and metastases. With nearly two decades of research, photothermal immunotherapy has been developed from a concept to a benchtop project and is finally a promising clinical therapy for metastatic cancer [32, 42].
An overview of cancer treatment using the combination of phototherapy and immunotherapy. Agents absorbed energy from light to kill tumor cells, by photochemical reaction in the case of PDT (Photodynamic therapy) and PIT (Photoimmunotherapy), or photophysical reaction in the case of PTT (Photothermal therapy). Induced tumor cell death with the release of antigens, DAMPs, and proinflammatory cytokines, can provide in situ autologous cancer vaccines. Immunoadjuvants or cytokines can enhance the antigen capture and presentation by APCs, which will amplify the subsequent systemic immune response, resisting the residual tumor cells in the primary sites while allowing the host to establish a long-term defense against homologous cancer. Checkpoint inhibitors (antibodies against PD‐L1, antibodies against CTLA-4, or small molecule IDO inhibitors) can further improve the treatment efficacy by blocking the immunosuppressive receptors on the cell surface, restoring the cytotoxic function of tumor-specific T-cells.
Preclinical studies of photothermal immunotherapy
Combination of PTT with immunoadjuvant
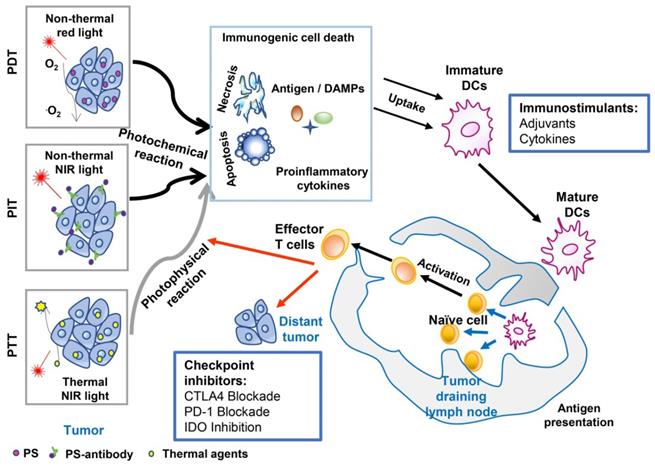
The combination of target PTT and in situ administration of an immunoadjuvant, formally referred to as laser immunotherapy (LIT), was first proposed in 1997 [43]. LIT was found to induce a host antitumor immunity that eliminated primary residual tumor cells and distant metastases [44]. In this early study of LIT, non-invasive PTT was performed using an 805 nm diode laser and the photoagent indocyanine green (ICG). N-dihydrogalactochitosan (GC), a functionalized soluble chitosan, was used as the immunoadjuvant. The therapeutic effects of LIT were first studied in a metastatic rat mammary tumor model, DMBA-4. LIT treatment (laser-ICG-GC) resulted in a 33% tumor-free survival rate versus 0% survival in the control group [44]. It is noteworthy that some of the surviving rats had metastases at an early stage that gradually subsided after LIT. In addition, all the cured rats showed complete resistance to 10-fold tumor cell rechallenge (Figure 2) [45].
To further confirm the immune-stimulating ability of GC, the effects of four different immunostimulants combined with laser-ICG were evaluated in the DMBA-4 model: 1% GC aqueous solution, 50% complete Freund's (CF) adjuvant, 50% incomplete Freund's (IF) adjuvant, and Corynebacterium parvum (CP) [46, 47]. Compared with the control group, all immunostimulants (CF, IF, and CP) significantly improved the survival rate of animals, increasing the cure rate from 0% to 18%, 7% and 9%, respectively. In contrast, the survival rate of rats induced by GC was 29%.
Recently, non-invasive interstitial PTT without administered photoagents has been achieved by directly delivering laser energy into the tumor tissue with an optical fiber. GC combined with PTT using a 980 nm laser resulted in a cure rate of 70% in mice bearing subcutaneous EMT6 mammary tumors and resulted in a cure rate of 75% in mice bearing subcutaneous Panc02-H7 pancreatic tumors [48, 49]. In particular, LIT treatment of orthotopic pancreatic cancer in mice destroyed primary tumors, slowed metastases, and prolonged survival [49]. During this treatment, the process of the immune response was investigated. Exposed/released DAMPs were detected in primary tumor treated by PTT, which initiated infiltration of APCs and a Th1 immunization. The combined regimen of GC-amplified Th1 immunization increased the quantity of cytotoxic T lymphocytes (CTLs) to control residual tumor cells. Simultaneously, the collaborative strategy also led to tumor-specific immune memory, inhibiting tumor recurrence and producing memory T-cells [49]. In another study, intravital imaging of CXCR6-GFP mice bearing CFP-B16 melanoma showed that infiltration of tumor-infiltrating lymphocytes (TILs) was increased with highly active motility at recurrent sites in mice treated with PTT+GC (Figure 3) [50].
Tumor rechallenge of rats cured by LIT. Left table is the resistance to tumor challenge after laser immunotherapy treatment. The table is adapted with permission from [45], copyright 2001 American Association for Cancer Research. Right figure is the schematic of anti-tumor immunity induced by laser immunotherapy in tumor rechallenge. The figure is adapted with permission from [36], copyright 2015 Elsevier Ireland Ltd.
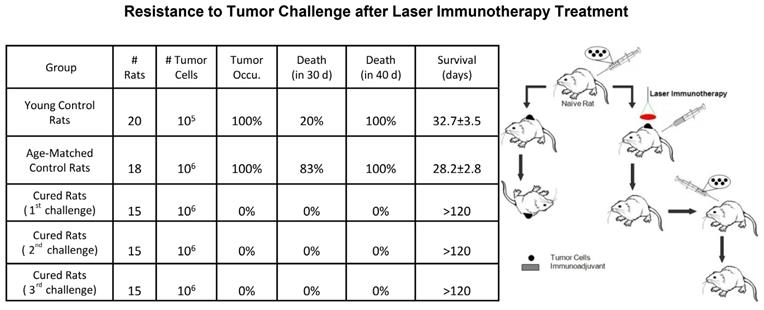
LIT (PTT+GC) induced tumor-specific immune memory. In vivo time-lapse images showing migration of endogenous GFP+ TILs in the CFP-B16 tumor area of CXCR6-GFP mice treated by LIT and rechallenged with CFP-B16. Scale bar: 70 μm. The figure is adapted with permission from [50], copyright 2020 Ivyspring International Publisher.
Due to their strong light absorption and carrier capabilities, nanoparticles have been employed for photothermal immunotherapy. Zhou et al. developed single-walled carbon nanotubes (SWNTs) to deliver GC intracellularly. With the synergized photothermal and immunostimulation effects, CTL response was amplified to control remote EMT6 tumor and memory immunity was triggered to resist tumor rechallenge [48]. Guo et al. constructed chitosan-coated hollow CuS nanoparticles (HCuSNPs) with oligodeoxynucleotides (ODNs) containing cytosine-guanine (CpG) motifs as immunoadjuvants for PTT. In an EMT6 mouse model, synergized photo-immune effects destroyed the tumor and controlled metastasis [51]. In another study, the immunoadjuvant Resiquimod R848 was loaded into polydopamine nanoparticles with co-loaded carbon dots. PTT with this nanoconstruct eliminated primary 4T1 breast tumors and triggered infiltration of CTLs into distant tumors [27]. Many novel synergized nanosystems have been designed using similar strategies for cancer therapy [52].
Combination of PTT with checkpoint inhibitors
Immunological checkpoint inhibitors break tumor immune tolerance by blocking immunosuppressive receptors on the cell surface, which restores the cytotoxic function of tumor-specific T-cells [53]. Currently, the main immunological checkpoint inhibitors include CTLA-4 mAb and PD-1/PD-L1 mAb. CTLA-4 is a cell surface transmembrane receptor that is induced by T-cell receptor binding and acts as a negative regulator of antigen-specific T-cell activation. Binding of anti-CTLA-4 antibodies to CTLA-4 receptors on Treg cells induces evasion of this immune checkpoint to reduce T-cell-activated immunosuppression. Yervoy (Ipilimumab) was the first immunological checkpoint inhibitor approved by the FDA in 2011 for treatment of metastatic melanoma. Yervoy acts by blocking the activity of the “brake” protein CTLA-4 on T-cells and restoring the immune system's ability to fight cancer [54]. PD-L1, a ligand of PD-1 expressed in many types of tumors, has been shown to inhibit T-cell receptor-mediated activation of positive signals. Two PD‐1 pathway inhibitors, Pembrolizumab and Nivolumab (biological mAbs), have been approved by the FDA [55]. However, infiltrating T-cells are the prerequisite for effective anti-CTLA-4/PD-1 therapy. In addition, not all tumors express ligands binding to CTLA-4 and PD-1, and anti-CTLA-4 and anti-PD-1 mAbs are relatively less effective at ablating solid primary tumors.
Based on the systemic antitumor immune response induced by PTT or LIT, checkpoint inhibitors have been included to magnify the effects of CTLs. Li et al. reported that combining laser-SWNT-GC treatment with anti-CTLA-4 promoted synergistic immunomodulatory effects and further prolonged the survival time of 4T1 tumor-bearing mice [56]. Yang et al. reported the construction of a Au@Pt-LMDP nanoplatform with a designed peptide antagonist of PD-L1 (LyP-1-PLGVRG-DPPA-1, LMDP) for cancer photothermal immunotherapy [57]. Zhang et al. synthesized IR820-1MT nanocomposites as photothermal agents for the combination of PTT and IDO inhibitor [29]. Qian et al. linked ICG to imiquimod, an immunoadjuvant, by poly lactic-co-glycolic acid, providing an in situ vaccination for antitumor immune response, which was further enhanced by anti-CTLA-4, a checkpoint inhibitor, to inhibit metastasis [58].
Combination of PTT with other strategies
Currently, CAR T-cell therapy has been combined with PTT to treat melanoma [59]. T-cells with CAR have been used to treat a number of patients with lymphoma and leukemia. However, CAR T-cell therapy has had limited success in the treatment of solid tumors due to the protective microenvironment. A study reported that the combination of photothermal ablation and CAR T-cells inhibited the growth of melanoma tumors in mice for up to 20 days. After receiving the combination therapy, 33% of mice remained tumor free after 20 days [59]. Multimodal therapies have also been developed, such as the synergistic combination of PTT, immunotherapy, and doxorubicin (Dox) chemotherapy in the light-responsive biodegradable nanoparticle Dox/CAT-PDA@M. This nanoparticle delivery strategy resulted in higher accumulation (15.3%) versus the retention effect of conventional nanodrugs (7.5%) and complete tumor elimination in vivo [60].
Clinical studies of photothermal immunotherapy
The encouraging results from animal studies have prompted clinical applications of photothermal immunotherapy. In particular, LIT has been used to treat patients with late-stage cancer who have failed other feasible treatment modalities. Preliminary clinical studies have shown that photothermal immunotherapy can reduce primary tumors, control untreated metastases, and prolong survival of patients. However, these studies were nonrandomized, investigator-driven, and included a limited number of patients [32-34, 42].
Photothermal immunotherapy for advanced breast cancer
The treatment of breast cancer has been significantly improved due to developments in technologies for early diagnosis and targeted therapies [61]. However, advanced breast cancer is still the leading cause of death in women, especially in underdeveloped countries, due to limited access to either earlier diagnosis or advanced therapies. LIT was used in an investigator-driven preliminary clinical study to treat patients with breast cancer in South America using ICG as the photoagent and GC as the immunoadjuvant. The treatment was performed every 4 weeks for a total of 4 times as one cycle. An additional treatment cycle was carried out if viable targeted tumor tissue remained.
In a study conducted in Lima, Peru, ten patients with breast cancer (stage III or stage IV) were enrolled in a photothermal immunotherapy clinical trial [32]. All the patients had failed conventional therapies and had a life expectancy of 3-6 months at the time of enrollment. The patients only experienced minor side effects in the area of treatment, without any grade 3 or 4 side effects. In eight patients available for evaluation 3 years after treatment, there were 2 patients with complete response (CR) and 2 patients with partial response (PR). The overall clinical benefit rate (CR+PR) was 50% (unpublished data). Significant reductions of metastases in lymph nodes, lungs, and livers were observed in 4 patients. All lung metastases disappeared in one complete responder. The overall 3-year survival of evaluable patients was significant, giving the fact that these patients had a life expectancy of 3-6 months prior to the study.
Photothermal immunotherapy for melanoma
Although melanoma accounts for only 4% of skin cancer cases, it leads to 79% of all skin cancer deaths [62]. The prognosis of metastatic melanoma is very poor, with only a 5% long-term survival rate. Photothermal immunotherapy of melanoma using imiquimod, an FDA-approved toll-like receptor agonist, as the immunoadjuvant was previously referred to as in situ photoimmunotherapy (ISPI) [33, 34]. Topical application of imiquimod was found to be effective on advanced melanoma, even when used as a monotherapy [63]. Its immunostimulatory effect makes imiquimod a reasonable candidate for photothermal immunotherapy.
In 2006, Naylor et al. reported initial results from the treatment of two patients using ISPI [34]. Patient 1 had primary tumors and regional metastases on the left arm with pulmonary metastases (AJCC stage IV). Patient 2 had primary melanoma on the head and neck with regional metastases (AJCC Ⅱ IC stage). The second patient failed multiple surgical resections and numerous cycles of high-dose radiotherapy. Both patients received ISPI and eventually cleared all detectable tumors. Both patients had no clinically detectable tumor (including pulmonary metastasis) for more than 60 months. In 2010, Murda et al. reported the use of ISPI for the treatment of a patient with acral lentiginous melanoma with metastases in an inguinal sentinel lymph node and on the left leg [64]. Two subcutaneous nodules were treated on the dorsal side of the left foot. The treatment was well tolerated by the patient with no serious adverse events and only a few minor side effects such as local wound ulcers and local pain. Five weeks after ISPI, one non-targeted adjacent tumor showed clinical remission, while another non-targeted lesion remained unchanged. The recurrence rate was decreased, with only 3 new nodules compared to 12 recurrences prior to ISPI treatment. When a new or recurrent nodule appeared, the patient continued to receive further ISPI to support the initial goal of limb salvage. There was no detectable visceral metastasis 18 months after the initiation of ISPI. These three cases showed that ISPI could eliminate local tumors and induce beneficial systemic reactions. Its side effects were more favorable than other methods in the treatment of advanced melanoma.
Li et al. reported results of a preliminary clinical study to determine the safety and efficacy of ISPI on metastatic melanoma patients [33]. Eleven patients with advanced melanoma (stage III or stage IV) were enrolled in the clinical trial. The most serious side effects usually occurred in the first treatment cycle. Approximately 20% of patients felt obvious pain during laser treatment and usually responded to pre-oral administration of anesthesia, although one patient needed conscious sedation. There were no level 4 adverse events in this study. In ten patients available for evaluation after treatment, 6 showed CR, and 2 showed PR. All lesions in the treatment area responded to local laser irradiation, and 8 cases achieved complete local response (CLR). Notably, 4 cases also achieved CLR of lesions in the nontreatment site, implying that the combination modality may induce an abscopal effect to treat metastatic melanoma. A summary of this study is given in Table 1. The overall 12-month survival rate reached 70%, and the survival distribution was never less than 50%. Another important outcome of this study is that the surviving patients continued to maintain a good quality of life after ISPI treatment.
Clinical characteristics of patients with melanoma and objective response
| No. | Age | Sex | AJCC stage | Primary site | Initial metastatic sites* | Cycles of treatment | Treatment site response | Non-treatment site regional response | Best overall response | Time to response (months) | Response duration (months) | Overall survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 | F | IV M1b | Left forearm | Bilateral lower lobe lung | 3 | CLR | CLR | CR | 1 | 12 | 66.4 |
| 2 | 67 | M | IIIC | Left side of head | - | 3 | CLR | CLR | CR | 4 | 7 | 64.2+ |
| 3 | 46 | F | IV M1c | Back | perihilar region of lungs, liver, uterus | 2 | PLR | N/A | PR | 1 | 2 | 8.5 |
| 4 | 63 | F | IIIB N2c | Left leg | - | 2 | PLR | PLR | PR | 3 | 4 | 44.9+ |
| 5 | 60 | F | IV M1A | Right frontal scalp | Distal skin site | 2 | CLR | CLR | CR | 2 | 4 | 6.6 |
| 6 | 87 | M | IIIC N2c | Right foot (plantar) | - | 6 | PLR | SD | SD | 1 | 0 | 15.5 |
| 7 | 71 | M | IV M1c | Eye | Liver | 1 | CLR | N/A | PD | 1 | 2 | 2.3 |
| 8 | 74 | M | IIIC N3 | Left lower back | - | 1 | CLR | N/A | CR | 8 | 2 | 38.7+ |
| 9 | 84 | M | IIIB N2c | Left foot | - | 1 | CLR | SD | CR | 6 | 1 | 6.0** |
| 10 | 85 | M | IV M1c | Left arm | Anterior mediastinum | 1 | CLR | CLR | CR*** | 1 | 8 | 22.5+ |
| 11 | 69 | M | IV M1c | Right forehead | Bone, lung | 1 | CLR | N/A | CR | 1 | 6 | 20.6+ |
Table 1. All the treatment area lesions of enrolled patients responded to ISPI. Abbreviations: M, male; F, female; AJCC, American Joint Committee on Cancer; CLR, complete local response; PLR, partial local response; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; N/A, not available or not applicable. *In addition to skin, present at the time of study entry. **This patient died of progression of myelodysplasia to leukemia (unrelated tumor death). ***The metastatic lesion was treated with cyberknife. Table 1 is adapted with permission from [33], copyright 2010 Landes Bioscience.
ISPI therapy for advanced melanoma patients stimulates a long-term adoptive immune response against residual primary and metastatic cancer tissues [33, 34], which may increase the quantity of anti-tumor T-cells, setting the stage for enhanced effects with a checkpoint inhibitor such as ipilimumab. Therefore, the combination of ISPI and ipilimumab was developed to treat patients with advanced melanoma [35]. In one particular patient, all melanoma nodules on the head and neck treated by ISPI achieved CLR. However, there were no changes in pulmonary metastases. After the implementation of ipilimumab, all the pulmonary metastases were decreased and finally disappeared. This patient remained tumor free and healthy seven years after treatment. This patient's response supports the hypothesis that ISPI increases the number and quality of T-cells in the tumor microenvironment, making the treatment more effective in combination with ipilimumab and other checkpoint inhibitors.
Dinitrophenyl (DNP), a type of hapten, can lead to contact-delayed hypersensitivity (DTH) due to its strong antigenicity and effective absorption in normal skin. DTH is a T-cell-mediated immune response, which is induced by an allergen, with the function of human cellular immunity evaluation [65]. DNP hapten combined with PTT to treat patients with malignant melanoma significantly increased interferon‑γ production by CD8+ and CD4+ T-cells and reduced secretion of IL‑10, TGF‑β1, and TGF‑β2, resulting in significantly longer overall survival and disease‑free survival [66].
While photothermal immunotherapy has been used in preliminary clinical studies for breast cancer and melanoma, it has limitations. In these applications, non-invasive light delivery was employed, which limits treatment to surface tumors. Interstitial photothermal immunotherapy has been experimented within pre-clinical studies and in limited clinical studies, which can extend the use of photothermal immunotherapy to treat deep-seated tumors or tumors in internal organs. Imaging-guided photothermal immunotherapy and nanotechnology-assisted photothermal immunotherapy will further broaden the applications of photothermal immunotherapy.
Photodynamic immunotherapy
Photodynamic therapy (PDT) is a targeted treatment based on local activation of a photosensitizer (PS) by light at a corresponding wavelength, which leads to ROS generation and induced tumor cell death, which has been used to treat cancer in the clinic for more than 40 years [15]. To achieve higher precision and deeper tissue penetration, interstitial photodynamic therapy (iPDT) has been used to destroy large, deep-seated tumors by directly transferring light into solid tumors through fiber optics inserted under imaging guidance [67]. While PDT has become a mature cancer therapy, it has undesirable side effects such as prolonged phototoxicity due to systemic administration of photosensitizers. In addition, new PSs have been developed that are capable of absorbing two photons of long-wavelength light [68]. At present, due to improvements in second- and third-generation photosensitizers, such as biocompatible BiOI/BiOIO3 heterostructure nanocomposites (BB NCs), and nanoscale metal-organic frameworks (nMOFs), an increasing number of trials are underway to improve the feasibility and efficacy of PDT [16, 69-71]. PDT with appropriate photosensitizers and light doses can enhance antitumor immune responses by releasing antigens and danger signals, supporting the combination of PDT with immunotherapy [72]. PDT-based tumor vaccines have been developed and enhanced by combination with immunostimulants and immunological checkpoint inhibitors [73].
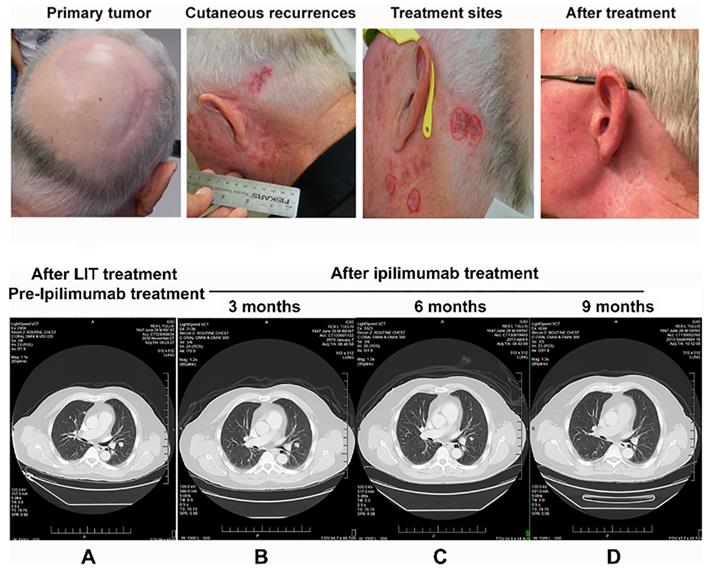
Photographs and CT images of a patient with stage IV melanoma during treatment. Upper: Photographs of the LIT treatment areas around the left ear. A. The primary tumor site with only surgical scars visible. B. The areas of biopsy-proven cutaneous recurrences around 2 surgical scars from the biopsy sites. Scalp hair has been trimmed away from the scalp recurrence site to make superficial laser therapy more effective. Reddish areas around the surgical scars represent melanoma deposits in the superficial dermis. C. Areas circled in red represent the 4 treatment sites selected for LIT. All other areas of regional involvement completely resolved during the 4-week treatment phase, which is typically seen with superficial LIT. D. The treatment area after LIT. Three months later, the LIT treatment completely cleared all cutaneous melanoma around the left ear. Bottom: CT images of the patient taken 3 months apart showing the same level in the thorax. A. The image was taken before ipilimumab treatment (2 months following LIT) and demonstrates the size and location of the pulmonary metastases. B-C. The images show shrinkage of the pulmonary metastases 3 months (B) and 6 months (C) after completion of the combination of LIT and ipilimumab. D. The image shows that pulmonary metastases were completely resolved 9 months after completion of the combination of LIT and ipilimumab. The figure is adapted with permission from [35], copyright 2017 Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Combination of PDT with mAbs
NIR-photoimmunotherapy (NIR-PIT) is a cell-selective cancer therapy that uses cancer-targeting antibodies (mAbs) conjugated to a nontoxic NIR-absorbing PS that can be activated at the tumor site with light of a corresponding wavelength. The antibody-PS conjugate binds to cancer cells that overexpress the targeted cancer-associated antigens. NIR light activates photochemical reactions that make the hydrophilic antibody-PS become hydrophobic, leading to the formation and accumulation of antibody-antigen complexes on the membrane with physical stress, resulting in increased transmembrane water flow, and ultimately causing cell rupture and necrosis [14, 74]. Moreover, PIT-mediated rapid cell death with associated release of tumor-associated antigens and membrane damage signals, induces maturation of local DCs and promotes tumor-specific naïve T-cell activation and proliferation [75].
Preclinical study of PIT
In 1983, the term PIT was used for cancer treatment involving chemical phototoxicity and antibody targeting [76]. Chemically coupled hematoporphyrin and a mAb of DBA/2J myoma M-1 was found to suppress M-1 tumor growth following exposure to incandescent light. A number of photosensitizers (e.g., mTHPC, pheophorbide a (PPa), chlorin e6 (Ce6)) and various mAbs have been used for PIT [77-80]. However, the success was very limited to use in vivo, because these conjugates were trapped and catabolized in the liver due to the hydrophobicity of photosensitizers soon after intravenous injection. In 2011, an EGFR mAb conjugated to a phthalocyanine dye (IRDye 700DX), initially discovered by Kobayashi and coworkers, was found to induce the death of EGFR-expressing cells immediately upon irradiation [81]. The photosensitizer benzoporphyrin derivative (BPD) was conjugated to Cetuximab, an FDA-approved anti-EGFR mAb, for selective treatment and quantitative, longitudinal imaging of micrometastases in vivo in an advanced ovarian cancer model [82].
Since the discovery of PIT, various antibodies have been conjugated to IRDye 700DX to target different cells [83]. For example, the HER2-specific antibody trastuzumab was synthesized with IRDye 700DX and administered to a non-small-cell lung carcinoma mouse model and a HER2-positive model of disseminated peritoneal ovarian cancer. PIT led to significant reductions in tumor volume in both the flank model and the pleural dissemination model [84-89]. Similarly, the delta-like protein 3 (DLL3) mAb rovalpituzumab was conjugated to IRDye 700DX to form rova-IR700, which decreased tumor burden markedly by PIT [90]. An anti-podoplanin antibody, NZ-1, was also synthesized with IRDye 700DX to treat a malignant pleural mesothelioma (MPM) model [74]. Further, anti-PSMA-IR700 was developed by conjugating IRDye 700DX to a full human IgG1 anti-PSMA mAb for the treatment of prostate cancer. PIT with this construct inhibited tumor growth and prolonged survival [91].
Although PIT has been shown to destroy target tumor cells and induce ICD, in most cases, it failed to induce durable antitumor responses against homologous tumor. Therefore, Kobayashi et al. combined CD44-targeting PIT with PD-1 blockade and evaluated its therapeutic effects in multiple syngeneic tumor models [92]. Because PD-1 blockade enhances tumor antigen-specific T-cell response, this combination led to complete rejection of MC38 tumors and untreated distant tumors. In addition, CD25-targeted PIT was developed to selectively clear Tregs. This therapy activated CD8+ T-cells and natural killer cells and restored local antitumor immunity, resulting in regression of treated tumors and separate untreated tumors [93].
Clinical study of PIT
RM-1929 is a parenteral formulation that consists of a chemical conjugate of the dye IR700 with an FDA-approved EGFR receptor-targeting antibody. In July 2015, a Phase I clinical trial was initiated to evaluate the safety and antitumor activity of RM-1929 in patients with terminal head-and-neck cancer (NCT number: NCT02422979). Nine patients were enrolled in three cohorts for dose escalation studies. No dose-limiting toxicities were found. The objective response rate (ORR) was 75% (6/8), with 3 complete responders for the duration of 4-16 months. In 7/8 of patients, the tumor density was decreased, consistent with necrosis after treatment. The disease control rate (DCR) was 100% [94].
Thirty patients with recurrent head-and-neck squamous cell carcinoma (rHNSCC) were enrolled in a Phase 2 study in June 2016 to evaluate the safety and anticancer efficacy of repeated treatment with PIT (four times under the maximum feasible dose of RM-1929 with a fixed dose of red light). Thirteen patients (43.3%) had at least one serious adverse event (SAE). A total of 86% (19/22) of SAEs were not considered to be related to treatment, including three kinds of fatal SAEs. Three kinds of SAEs may have been related to treatment (i.e., site/oral pain, tumor hemorrhage, and airway obstruction). The ORR was 50% (15/30), with 16.7% (5/30) CR and 86.7% (26/30) DCR [95]. For patients with locally recurrent head-and-neck squamous cell carcinoma who failed after at least two treatments, a Phase 3 randomized, double-arm, open-label trial of PIT started in May 2019. In addition, the NIR-PIT was approved in human clinical use by Japanese Agency, in September 2020.
Combination of PDT with other immunotherapies
Various immunoadjuvants have been used in combination with PDT, such as schizophyllan (SPG), GC, Bacille Calmette-Guérin (BCG) and mycobacterium cell wall extract (MCWE) [80]. In the treatment of line 1 lung adenocarcinoma in mice, a poorly immunogenic tumor model, 37% of the tumor-bearing mice were cured by the combination of 1.67% GC solution with noncurative PDT using m-tetra (hydroxyphenyl) chlorin (mTHPC) [47]. Combinations of BCG or MCWE with PDT using different photosensitizers, including Photofrin (porfimer sodium), BPD, zinc phthalocyanine, mTHPC, mono-L-aspartyl-chlorin e6, and lutetium texaphyrin, all resulted in delayed tumor regrowth and an increased cure rate in experimental animals [80, 96].
Synthetic long peptides containing epitopes from tumor antigens were combined with Bremachlorin-based PDT for the treatment of TC-1 and RMA aggressive mouse tumor models. The treatment cured one-third of tumor-bearing mice and cured mice completely resisted tumor rechallenge [97]. The checkpoint inhibitor nivolumab was combined with Redaporfin-mediated PDT for the treatment of a patient with head-and-neck cancer [98]. The patient was diagnosed with SCC of the oral floor and had failed surgery, radiotherapy, and multiple lines of systemic treatment. PDT destroyed all visible tumors, while sequential use of nivolumab produced a sustained complete response.
Using strategies similar to those employed in nanoparticle-based photothermal immunotherapy, numerous nanosystems have been designed for photodynamic immunotherapy, including metal materials or organic nanoparticles as PSs combined with immunoadjuvants or checkpoint inhibitors. Lin et al. designed a novel up-conversion luminescence (UCL)-enhanced spindle-shaped nanocomposite for mitochondrial imaging that is coated with gold nanoparticles for PDT (SPS@Au), and is combined with anti-CTLA-4 mAb for photodynamic immunotherapy [99]. Recently, multifunctional nanoparticles have been developed to synergize PDT, chemotherapy, and immunotherapy, such as a TME-triggered oxygen nanogenerator PTX/ICG-NVs@Au@CAT [100]. In this nanoconstruct, H2O2 is catalyzed to oxygen by CAT to relieve tumor hypoxia, enhancing ROS generation by ICG-PDT, which potentiates the efficacy of immunotherapy.
Clinical studies of photodynamic immunotherapy
Imiquimod has been combined with PDT for the treatment of patients with skin cancer. In one trial, 10 days after two sessions of PDT treatment, patients received imiquimod 5 times a week for 3 weeks, which resulted in clinical clearance and no recurrence at 15 months [101]. Osiecka et al. reported a trial of 10 patients who underwent PDT treatment with 5-aminolevulinic acid (ALA) and 24 patients who underwent ALA-PDT combined with imiquimod. In the first group, 6 cases (60%) were completely cured and 4 cases (40%) had reduced lesions; in the second group, 18 cases (75%) had complete lesion erasure and 6 cases had reduced lesions [102]. Requena et al. developed a trial of recurrent giant basal cell carcinoma of the face, showing excellent efficacy with methyl aminolevulinate (MAL)-based PDT plus imiquimod treatment [103]. Another randomized, prospective, observer-blinded study of photodynamic vaccination in patients with non-melanoma skin cancer (NMSCs) was started in 2013 to determine the enhanced efficacy of PDT in combination with 5% imiquimod in 44 patients (EudraCT ID: 2013-000092-33). MAL‐PDT and imiquimod did not show improved efficacy in reducing growth of new recurrent NMSCs at any follow‐up period compared to MAL-PDT alone. Both therapies were safe and acceptable. Patients preferred MAL‐PDT based on the treatment strategy modalities, response rates, and future choice [104].
The combination of PDT with different immunological approaches provides possibilities to improve treatment efficiency. However, there are few clinical trials including immunotherapy as an adjunctive therapy for PDT. PDT-generated vaccine is a promising tool to induce tumor-specific immunity. With an understanding of the anti-tumor immune response triggered by PDT, combination therapies that can be used with PDT to target and potentiate the immune response will be further developed.
Summary and outlook
Immunotherapy uses the host's immune system against cancer cells. Phototherapy is a promising modality that eradicates tumors through targeted light irradiation. The tumor treated by phototherapy serves as an in situ source of cancer antigens that come from the patients themselves, resulting in an in situ autologous whole-cell cancer vaccination. Therefore, the strategy of photo-immunotherapy is to destroy the tumor directly, induce a tumor-specific host immune response, and eliminate residual tumor cells and distant metastases. Clinical results show the potential of photo-immunotherapy for effective, local, and safe interventions for metastatic cancer.
However, phototherapy still has limitations in the treatment of human cancer, especially the limited tissue penetration of light, which restricts the non-invasive application of phototherapy for tumors in deep organs. Therefore, non-invasive phototherapy is suitable for superficial cancers such as melanoma, osteosarcoma, and squamous cell carcinoma. To circumvent these barriers, interstitial phototherapy was developed. Using interstitial fibers, phototherapy can treat deep-seated tumors and avoid damage to healthy tissue. Recently, MR-guided laser interstitial thermal therapy (LITT) has been used for treatment of brain tumors [71]. Moreover, Hu et al. developed interventional PTT under CT imaging guidance and Bryan et al. developed interventional PDT, both using an orthotopic xenograft model of human pancreatic cancer [73, 74]. Therefore, with the development of interventional and imaging techniques, imaging-guided phototherapy will be used widely for either epidermal tumors or deeper tumors, although further studies are needed for clinical applications.
Summary of studies utilizing phototherapy and immunotherapy combinatorial treatments
| Phototherapy | Phototherapy agent | Immunotherapy | Immunotherapy agent | Cancer model | Therapeutic outcome | Ref. |
|---|---|---|---|---|---|---|
| PTT | CuS | Immunoadjuvant | Lipopolysaccharide (LPS) | CT26 | Complete tumor eradication and prevention of metastasis | [24] |
| PTT | IR-7-lipo | Immunoadjuvant | HA-CpG | CT26 | Inhibited tumor growth | [25] |
| PTT | PDA, CD | Immunoadjuvant | Resiquimod (R848) | 4T1 | Inhibited distant tumor | [27] |
| PTT | Gold, Pt | PD-L1 Blockade | Anti-PD-L1 antibody: LMDP | 4T1 | Suppressed primary and distal tumor growth | [57] |
| PTT | IR820 | IDO inhibition | 1MT | B16F10, 4T1 | Inhibition ratio of 87% | [29] |
| PTT | ICG | Immunoadjuvant | Glycated chitosan | Breast cancer patients | Clinical beneficial response rate was 75% in 1 year | [32] |
| PTT | ICG | Immunoadjuvant | Imiquimod | Melanoma patients | Survival rate reached 70% in 1 year | [33] |
| PTT | ICG | Immunoadjuvant | Imiquimod | Melanoma patients | Eliminated primary tumor and pulmonary metastasis | [34] |
| PTT | ICG; ICG; SWNT; None; Optical fiber | Immunoadjuvant | GC | DMBA-4; DMBA-4; EMT6; Panc02-H7; B16 | 33% tumor-free survival rate; Total resistance to the primary tumors and metastases; Cure rate of 70%; 75% of mice complete tumor regression; The infiltration of TILs increased | [44] [45] [48] [49] [50] |
| PTT | CuS | Immunoadjuvant | CpG | EMT6 | Destroyed treated tumors and inhibited remote untreated tumors | [51] |
| PTT | SWNT | Immunoadjuvant/CTLA-4 Blockade | GC/ anti-CTLA-4 antibody | 4T1 | Prolonged the survival time | [56] |
| PTT | ICG | Immunoadjuvant/CTLA-4 Blockade | Imiquimod/ anti-CTLA-4 antibody | 4T1 | Primary tumors eradication and metastasis prevention | [58] |
| PTT | ICG | Immunoadjuvant | CAR-T cell | WM115 | Inhibition the growth of melanoma | [59] |
| PTT | ICG | Immunoadjuvant | Imiquimod | Melanoma patients | No detectable visceral metastasis in 18 months | [64] |
| PTT | Focal laser | Immunoadjuvant | Dinitrophenyl (DNP) | Melanoma patients | Longer overall survival time | [66] |
| PIT | IRDye-700DX | Monoclonal antibody | NZ-1 | MPM | Reductions in tumor volume | [74] |
| PIT | IRDye-700DX | Monoclonal antibody | Anti-DLL3 rovalpituzumab | SCLC | Tumor shranked | [90] |
| PIT | IRDye-700DX | Monoclonal antibody | Trastuzumab | Ovarian cancer | Reductions in tumor volume | [86] |
| PIT | IRDye-700DX | Monoclonal antibody | Human IgG1 anti-PSMA mAb | PC3 | Prolonged survival time | [91] |
| PIT | BPD | Monoclonal antibody | FDA-approved anti-EGFR mAb | human EOC | Inhibition of early recurrence | [82] |
| PIT | IRDye-700DX | Monoclonal antibody/PD-1 Blockade | Anti-CD44/anti-PD-1 antibody | MC38 | Complete rejection of MC38 tumors and distant tumors | [92] |
| PIT | IRDye-700DX | Monoclonal antibody | FDA-approved anti-EGFR mAb | Terminal head and neck cancer patients | Tumor density was decreased in 7/8, with ORR 75% and DCR 100%. | [94] |
| PIT | IRDye-700DX | Monoclonal antibody | FDA-approved anti-EGFR mAb | rHNSCC (in Phase 2) | The ORR was 50% (15/30), with 16.7% (5/30) CR and 86.7% (26/30) DCR | [95] |
| PDT | mTHPC/ICG | Immunoadjuvant | GC | EMT6/ lung adenocarcinoma | Cured 75%/37% of the tumor-bearing mice | [47] |
| PDT | Gold | CTLA-4 Blockade | Anti-CTLA-4 antibody | 4T1 | Reductions in tumor volume | [99] |
| PDT | Levulan | Immunoadjuvant | Aldara/imiquimod | Basal-cell carcinoma patients | 6 cases, 18 cases were completely cured | [102] |
| PDT | Methyl aminolevulinate (MAL) | Immunoadjuvant | Imiquimod cream (IMIQ) | Basal cell carcinoma patient | No sign of the tumor IN two years | [103] |
| PDT | Methyl aminolevulinate (MAL) | Immunoadjuvant | Imiquimod cream (IMIQ) | NMSC patients | MAL-PDT compared to IMIQ (25/44, 56.81% vs. 19/44, 43.19%) | [104] |
| PTT +PDT | PDA, CAT | Immunoadjuvant | CAT | U14 | Complete eradication | [60] |
| PTT +PDT | Gold, ICG, CAT | Immunoadjuvant | CAT | U14 | Complete eradication | [100] |
For nanomaterial-based phototherapy, another challenge is the removal of nanoparticles from circulation by cells in the liver, spleen, and other parts of the reticuloendothelial system (RES), which may induce toxicity and reduce nanoparticle accumulation in tumor tissue. Therefore, intratumoral administration is commonly used to overcome RES uptake and low tumor targeting efficiency. Furthermore, PEG or zwitterionic polymers are often chosen to modify the surface of nanoparticles to avoid RES cells and increase accumulation in the tumor [105, 106]. In addition, due to the differences and complexities of human tumor microenvironments compared with those of animal models, it is impossible to predict the clinical performance of photo-immunotherapies from in vivo results.
It has been recognized that phototherapy can initiate immune responses when treating tumors. It is further recognized that phototherapy alone usually cannot initiate and sustain curative anti-tumor immune responses. As introduced in this review, a combination of phototherapy and immunotherapy, particularly using immunostimulants, immune-targeting agents, and checkpoint inhibitors, can significantly advance cancer treatment. The effect of such combinations has resulted in promising clinical outcomes using PTT, PDT, and PIT with different immunotherapeutic agents, as shown in Sections 2 and 3.
In summary, with continued development of photo-immunotherapies, expanded preclinical and clinical studies, as well as further investigations of the mechanisms of photo-immunotherapy-induced antitumor immune responses, photo-immunotherapy will attract increasing attention. The potentials of photo-immunotherapy should be achieved for the treatment of patients with late-stage, metastatic cancers.
Acknowledgements
This study was supported by grants from the US National Institutes of Health CA205348 (W.R.C.), Guangdong Province Key Area R&D Program 2019B110233004, and Hainan University R&D Program (KYQD(ZR)20074) (F. Zhou).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30
2. Couzin-Frankel J. Cancer immunotherapy. Science. 2013;342:1432-3
3. Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell. 2018;175:313-26
4. Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM. et al. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168:487-502
5. Van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16:219-33
6. Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17:3520-6
7. Guo Y, Lei K, Tang Li. Neoantigen vaccine delivery for personalized anticancer immunotherapy. Front Immunol. 2018;9:1499
8. Fang Y, Mo F, Shou J, Wang H, Luo K, Zhang S. et al. A pan-cancer clinical study of personalized neoantigen vaccine monotherapy in treating patients with various types of advanced solid tumors. Clin Cancer Res. 2020;26:4511-20
9. Hamblin MR, Huang Y. Handbook of photomedicine. Taylor Francis. 2013
10. Guo XL, Ding ZY, Deng SM, Wen CC, Shen XC, Jiang BP. et al. A novel strategy of transition-metal doping to engineer absorption of carbon dots for near-infrared photothermal/photodynamic therapies. Carbon. 2018;134:519-30
11. Garg AD, Vandenberk L, Koks C, Verschuere T, Boon L, Van Gool SW. et al. Dendritic cell vaccines based on immunogenic cell death elicit danger signals and T cell-driven rejection of high-grade glioma. Sci Transl Med. 2016;8:328ra27
12. Shen Z, Xia J, Ma Q, Zhu W, Gao Z, Han S. et al. Tumor microenvironment-triggered nanosystems as dual-relief tumor hypoxia immunomodulators for enhanced phototherapy. Theranostics. 2020;10:9132-52
13. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685-91
14. Sato K, Ando K, Okuyama S, Moriguchi S, Ogura T, Totoki S. et al. Photoinduced ligand release from a silicon phthalocyanine dye conjugated with monoclonal antibodies: a mechanism of cancer cell cytotoxicity after near-infrared photoimmunotherapy. ACS Central Sci. 2018;4:1559-69
15. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657-74
16. Hou X, Tao Y, Pang Y, Li X, Jiang G, Liu Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer. 2018;143:3050-60
17. Ma Y, Zhang Y, Li X, Zhao Y, Li M, Jiang W. et al. Near-infrared II phototherapy induces deep tissue immunogenic cell death and potentiates cancer immunotherapy. ACS Nano. 2019;13:11967-80
18. Wang Y, Zhao J, Chen Z, Zhang F, Wang Q, Guo W. et al. Construct of MoSe2/Bi2Se3 nanoheterostructure: multimodal CT/PT imaging-guided PTT/PDT/chemotherapy for cancer treating. Biomaterials. 2019;217:119282
19. Xu Y, Wang X, Cheng L, Liu Z, Zhang Q. High-yield synthesis of gold bipyramids for in vivo CT imaging and photothermal cancer therapy with enhanced thermal stability. Chem Eng J. 2019;378:122025
20. Wang C, Dai C, Hu Z, Li H, Yu L, Lin H. et al. Photonic cancer nanomedicine using the near infrared-II biowindow enabled by biocompatible titanium nitride nanoplatforms. Nanoscale Horiz. 2019;4:415-25
21. Yang Z, Dai Y, Shan L, Shen Z, Wang Z, Yung BC. et al. Tumour microenvironment-responsive semiconducting polymer-based self-assembling nanotheranostics. Nanoscale Horiz. 2019;4:426-33
22. Rastinehad AR, Anastos H, Wajswol E, Winoker JS, Sfakianos JP, Doppalapudi SK. et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. P Natl Acad Sci USA. 2019;116:18590-6
23. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237-51
24. Jang B, Xu L, Moorthy MS, Zhang W, Zeng L, Kang M. et al. Lipopolysaccharide-coated CuS nanoparticles promoted anti-cancer and anti-metastatic effect by immuno-photothermal therapy. Oncotarget. 2017;8:105584-95
25. Li L, Yang S, Song L, Zeng Y, He T, Wang N. et al. An endogenous vaccine based on fluorophores and multivalent immunoadjuvants regulates tumor micro-environment for synergistic photothermal and immunotherapy. Theranostics. 2018;8:860-73
26. Pan J, Wang Y, Zhang C, Wang X, Wang H, Wang J. et al. Antigen-directed fabrication of a multifunctional nanovaccine with ultrahigh antigen loading efficiency for tumor photothermal-immunotherapy. Adv Mater. 2018;30:1704408
27. Lu Q, Qi S, Li P, Yang L, Yang S, Wang Y. et al. Photothermally activatable PDA immune nanomedicine combined with PD-L1 checkpoint blockade for antimetastatic cancer photoimmunotherapy. J Mater Chem B. 2019;7:2499-511
28. Liu Y, Pan Y, Cao W, Xia F, Liu B, Niu J. et al. A tumor microenvironment responsive biodegradable CaCO3/MnO2- based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics. 2019;9:6867-84
29. Zhang D, Zhang J, Li Q, Song A, Li Z, Luan Y. Cold to hot: rational design of a minimalist multifunctional photo-immunotherapy nanoplatform toward boosting immunotherapy capability. ACS Appl Mater Inter. 2019;11:32633-46
30. Ng CW, Li J, Pu K. Recent progresses in phototherapy-synergized cancer immunotherapy. Adv Funct Mater. 2018;28:1804688
31. Zhou F, Nordquist RE, Chen WR. Photonics immunotherapy - a novel strategy for cancer treatment. J Innov Opt Heal Sci. 2016;9:1630001
32. Li X, Ferrel GL, Guerra MC, Hode T, Lunn JA, Adalsteinsson O. et al. Preliminary safety and efficacy results of laser immunotherapy for the treatment of metastatic breast cancer patients. Photoch Photobio Sci. 2011;10:817-21
33. Li X, Naylor MF, Le H, Nordquist RE, Teague TK, Howard CA. et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10:1081-7
34. Naylor MF, Chen WR, Teague TK, Perry L, Nordquist RE. In situ photoimmunotherapy: a tumour-directed treatment for melanoma. Brit J Dermatol. 2006;155:1287-92
35. Naylor MF, Zhou F, Geister BV, Nordquist RE, Li X, Chen WR. Treatment of advanced melanoma with laser immunotherapy and ipilimumab. J Biophotonics. 2017;10:618-22
36. Zhou F, Li X, Naylor MF, Hode T, Nordquist RE, Alleruzzo L. et al. In CVAX-a novel strategy for treatment of late-stage, metastatic cancers through photoimmunotherapy induced tumor-specific immunity. Cancer Lett. 2015;359:169-77
37. Sartori S, Di Vece F, Ermili F, Tombesi P. Laser ablation of liver tumors: an ancillary technique, or an alternative to radiofrequency and microwave? World J Radiol. 2017;9:91-96
38. Hafez DM, Liekweg C, Leuthardt EC. Staged laser interstitial thermal therapy (LITT) treatments to left insular low-grade glioma. Neurosurgery. 2019;86:337-42
39. Liu S, Doughty A, West C, Tang Z, Zhou F, Chen WR. Determination of temperature distribution in tissue for interstitial cancer photothermal therapy. Int J Hyperther. 2018;34:756-63
40. Sweeney EE, Cano-Mejia J, Fernandes R. Photothermal therapy generates a thermal window of immunogenic cell death in neuroblastoma. Small. 2018;14:1800678
41. Dewhirst MW, Lee CT, Ashcraft KA. The future of biology in driving the field of hyperthermia. Int J Hyperther. 2016;32:4-13
42. Li X, Hode T, Guerra MC, Ferrel GL, Lunn JA, Adalsteinsson O. et al. Combined effects of selective photothermal therapy and immunoadjuvant against stage IV breast cancer. J Innov Opt Heal Sci. 2010;3:279-84
43. Chen WR, Adams RL, Carubelli R, Nordquist RE. Laser-photosensitizer assisted immunotherapy: a novel modality for cancer treatment. Cancer Lett. 1997;115:25-30
44. Chen WR, Liu H, Ritchey JW, Bartels KE, Lucroy MD, Nordquist RE. Effect of different components of laser immunotherapy in treatment of metastatic tumors in rats. Cancer Res. 2002;62:4295-9
45. Chen WR, Singhal AK, Liu H, Nordquist RE. Antitumor immunity induced by laser immunotherapy and its adoptive transfer. Cancer Res. 2001;61:459-61
46. Chen WR, Carubelli R, Liu H, Nordquist RE. Laser immunotherapy. Mol Biotechnol. 2003;25:37-43
47. Chen WR, Korbelik M, Battels KE, Liu H, Sun J, Nordquist RE. Enhancement of laser cancer treatment by a chitosan-derived immunoadjuvant. Photochem Photobiol. 2005;81:190-5
48. Zhou F, Wu S, Song S, Chen WR, Resasco DE, Xing D. Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials. 2012;33:3235-42
49. Zhou F, Yang J, Zhang Y, Liu M, Lang ML, Li M. et al. Local phototherapy synergizes with immunoadjuvant for treatment of pancreatic cancer through induced immunogenic tumor vaccine. Clin Cancer Res. 2018;24:5335-46
50. Qi S, Lu L, Zhou F, Chen Y, Xu M, Chen L. et al. Neutrophil infiltration and whole-cell vaccine elicited by N-dihydrogalactochitosan combined with NIR phototherapy to enhance antitumor immune response and T cell immune memory. Theranostics. 2020;10:1814-32
51. Guo L, Yan DD, Yang D, Li Y, Wang X, Zalewski O. et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 2014;8:5670-81
52. Xu X, Lu H, Lee R. Near infrared light triggered photo/immuno-therapy toward cancers. Front Bioeng Biotech. 2020;8:488
53. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350-5
54. Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P. Ipilimumab. Nat Rev Drug Discov. 2011;10:411-2
55. Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143-58
56. Li Y, Li X, Doughty A, West C, Wang L, Zhou F. et al. Phototherapy using immunologically modified carbon nanotubes to potentiate checkpoint blockade for metastatic breast cancer. Nanomed Nanotechnol. 2019;18:44-53
57. Yang Q, Peng J, Shi K, Xiao Y, Liu Q, Han R. et al. Rationally designed peptide-conjugated gold/platinum nanosystem with active tumor-targeting for enhancing tumor photothermal-immunotherapy. J Control Release. 2019;308:29-43
58. Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193
59. Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn S, Wang C. et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater. 2019;31:1900192
60. He Y, Cong C, Liu Z, Li X, Zhu R, Gao D. Stealth surface driven accumulation of “Trojan Horse” for tumor hypoxia relief in combination with targeted cancer therapy. Chem Eng J. 2019;378:122252
61. Waks AG, Winer EP. Breast cancer treatment: a review. Jama. 2019;321:288-300
62. Schadendorf D, Fisher DE, Garbe C, Gershenwald JE, Grob JJ, Halpern A. et al. Melanoma. Nat Rev Dis Primers. 2015;1:15003
63. Fan Q, Cohen S, John B, Riker AI. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-7
64. Murad A, William DA, Edward WC, St Pierre SA, Rommel J, Ciurea A. et al. In situ photoimmunotherapy: a surgery-and limb-sparing approach to the treatment of cutaneous metastases in advanced melanoma. Arch Dermatol. 2010;146:831-4
65. Xiaosong L, Nan D, Haijun L, Shan L, Dianjun C, Feifan Z. et al. Photothermal therapy combined with dinitrophenyl hapten for the treatment of late stage malignant melanoma. Proc SPIE. 2017 10065
66. Chen DJ, Li XS, Zhao H, Fu Y, Kang HR, Yao FF. et al. Dinitrophenyl hapten with laser immunotherapy for advanced malignant melanoma: a clinical study. Oncol Lett. 2017;13:1425-31
67. Shafirstein G, Bellnier D, Oakley E, Hamilton S, Potasek M, Beeson K. et al. Interstitial photodynamic therapy-a focused review. Cancers. 2017;9:12
68. Bolze F, Jenni S, Sour A, Heitz V. Molecular photosensitisers for two-photon photodynamic therapy. Chem Commun. 2017;53:12857-77
69. Zhen W, Liu Y, Jia X, Wu L, Wang C, Jiang X. Reductive surfactant-assisted one-step fabrication of a BiOI/BiOIO3 heterojunction biophotocatalyst for enhanced photodynamic theranostics overcoming tumor hypoxia. Nanoscale Horiz. 2019;4:720-6
70. Ni K, Lan G, Lin W. Nanoscale metal-organic frameworks generate reactive oxygen species for cancer therapy. ACS Central Sci. 2020;6:861-8
71. Sang W, Zhang Z, Dai Y, Chen X. Recent advances in nanomaterial-based synergistic combination cancer immunotherapy. Chem Soc Rev. 2019;48:3771-810
72. Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535-45
73. Nath S, Obaid G, Hasan T. The course of immune stimulation by photodynamic therapy: bridging fundamentals of photochemically induced immunogenic cell death to the enrichment of T-cell repertoire. Photochem Photobiol. 2019;95:1288-305
74. Nishinaga Y, Sato K, Yasui H, Taki S, Takahashi K, Shimizu M. et al. Targeted phototherapy for malignant pleural mesothelioma: near-infrared photoimmunotherapy targeting podoplanin. Cells. 2020;9:1019
75. Kobayashi H, Choyke PL. Near-infrared photoimmunotherapy of cancer. Accounts Chem Res. 2019;52:2332-9
76. Mew D, Wat CK, Towers G, Levy J. Photoimmunotherapy: treatment of animal tumors with tumor-specific monoclonal antibody-hematoporphyrin conjugates. J Immunol. 1983;130:1473-7
77. Vrouenraets MB, Visser GW, Stewart FA, Stigter M, Oppelaar H, Postmus PE. et al. Development of meta-tetrahydroxyphenylchlorin-monoclonal antibody conjugates for photoimmunotherapy. Cancer Res. 1999;59:1505-13
78. Del Governatore M, Hamblin MR, Shea CR, Rizvi I, Molpus KG, Tanabe KK. et al. Experimental photoimmunotherapy of hepatic metastases of colorectal cancer with a 17.1 A chlorine6 immunoconjugate. Cancer Res. 2000;60:4200-5
79. Savellano MD, Pogue BW, Hoopes PJ, Vitetta ES, Paulsen KD. Multiepitope HER2 targeting enhances photoimmunotherapy of HER2-overexpressing cancer cells with pyropheophorbide-a immunoconjugates. Cancer Res. 2005;65:6371-9
80. Kwitniewski M, Juzeniene A, Glosnicka R, Moan J. Immunotherapy: a way to improve the therapeutic outcome of photodynamic therapy? Photoch Photobio Sci. 2008;7:1011-7
81. Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med. 2011;17:1685-91
82. Spring BQ, AbuYousif AO, Palanisami A, Rizvi I, Zheng X, Mai Z. et al. Selective treatment and monitoring of disseminated cancer micrometastases in vivo using dual-function, activatable immunoconjugates. P Natl Acad Sci USA. 2014;111:933-42
83. Kobayashi H. Illuminating the cancer-targeting potential of near-infrared photoimmunotherapy. Biochem Soc. 2016;38:16-9
84. Harada T, Nakamura Y, Sato K, Nagaya T, Okuyama S, Ogata F. et al. Near-infrared photoimmunotherapy with galactosyl serum albumin in a model of diffuse peritoneal disseminated ovarian cancer. Oncotarget. 2016;7:79408-16
85. Sato K, Nagaya T, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy in the treatment of pleural disseminated NSCLC: preclinical experience. Theranostics. 2015;5:698-709
86. Sato K, Hanaoka H, Watanabe R, Nakajima T, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy in the treatment of disseminated peritoneal ovarian cancer. Mol Cancer Ther. 2015;14:141-50
87. Sato K, Nagaya T, Mitsunaga M, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy for lung metastases. Cancer Lett. 2015;365:112-21
88. Sato K, Choyke PL, Kobayashi H. Photoimmunotherapy of gastric cancer peritoneal carcinomatosis in a mouse model. PloS One. 2014;9:e113276
89. Sato K, Nagaya T, Nakamura Y, Harada T, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy prevents lung cancer metastases in a murine model. Oncotarget. 2015;6:19747-58
90. Isobe Y, Sato K, Nishinaga Y, Takahashi K, Taki S, Yasui H. et al. Near infrared photoimmunotherapy targeting DLL3 for small cell lung cancer. EBioMedicine. 2020;52:102632
91. Nagaya T, Nakamura Y, Okuyama S, Ogata F, Maruoka Y, Choyke PL. et al. Near-infrared photoimmunotherapy targeting prostate cancer with prostate-specific membrane antigen (PSMA) antibody. Mol Cancer Res. 2017;15:1153-62
92. Nagaya T, Friedman J, Maruoka Y, Ogata F, Okuyama S, Clavijo PE. et al. Host immunity following near-infrared photoimmunotherapy is enhanced with PD-1 checkpoint blockade to eradicate established antigenic tumors. Cancer Immunol Res. 2019;7:401-13
93. Sato K, Sato N, Xu B, Nakamura Y, Nagaya T, Choyke PL. et al. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci Transl Med. 2016;8:352ra110
94. Kochuparambil S, McDonald D, Fidler M, Stenson K, Vasan N, Razaq M. 1051PD-A phase 1, multicenter, open-label, dose-escalation, combination study of RM-1929 and photoimmunotherapy in patients with recurrent head and neck cancer. Ann Oncol. 2017;28:372-94
95. Cognetti DM, Johnson JM, Curry JM, Mott F, Kochuparambil ST, McDonald D. et al. Results of a phase 2a, multicenter, open-label, study of RM-1929 photoimmunotherapy (PIT) in patients with locoregional, recurrent head and neck squamous cell carcinoma (rHNSCC). J Clin Oncol. 2019;37:6014-6014
96. Denis TGS, Aziz K, Waheed AA, Huang YY, Sharma SK, Mroz P. et al. Combination approaches to potentiate immune response after photodynamic therapy for cancer. Photoch Photobio Sci. 2011;10:792-801
97. Kleinovink JW, van Driel PB, Snoeks TJ, Prokopi N, Fransen MF, Cruz LJ. et al. Combination of photodynamic therapy and specific immunotherapy efficiently eradicates established tumors. Clin Cancer Res. 2016;22:1459-68
98. Santos LL, Oliveira J, Monteiro E, Santos J, Sarmento C. Treatment of head and neck cancer with photodynamic therapy with redaporfin: a clinical case report. Case Rep Oncol. 2018;11:769-76
99. Lin B, Liu J, Wang Y, Yang F, Huang L, Lv R. Enhanced upconversion luminescence-guided synergistic antitumor therapy based on photodynamic therapy and immune checkpoint blockade. Chem Mater. 2020;32:4627-40
100. He Y, Cong C, He Y, Hao Z, Li C, Wang S. et al. Tumor hypoxia relief overcomes multidrug resistance and immune inhibition for self-enhanced photodynamic therapy. Chem Eng J. 2019;375:122079
101. Devirgiliis V, Panasiti V, Curzio M, Gobbi S, Rossi M, Roberti V. et al. Complete remission of nodular basal cell carcinoma after combined treatment with photodynamic therapy and imiquimod 5% cream. Dermatol Online J. 2008;14:25
102. Osiecka B, Jurczyszyn K, Ziółkowski P. The application of Levulan-based photodynamic therapy with imiquimod in the treatment of recurrent basal cell carcinoma. Med Sci Monit. 2012;18:15-9
103. Requena C, Messeguer F, Llombart B, Serra-Guillén C, Guillén C. Facial extensive recurrent basal cell carcinoma: successful treatment with photodynamic therapy and imiquimod 5% cream. Int J Dermatol. 2012;51:451-4
104. Sotiriou E, Apalla Z, Vrani F, Lallas A, Chovarda E, Ioannides D. Photodynamic therapy vs. imiquimod 5% cream as skin cancer preventive strategies in patients with field changes: a randomized intraindividual comparison study. J Eur Acad Dermatol. 2015;29:325-9
105. Zheng T, Wang W, Wu F, Zhang M, Shen J, Sun Y. Zwitterionic polymer-gated Au@TiO2 core-shell nanoparticles for imaging-guided combined cancer therapy. Theranostics. 2019;9:5035-48
106. Gao D, Guo X, Zhang X, Chen S, Wang Y, Chen T. et al. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Mater Today Bio. 2020;5:100035
Author contact
![]() Corresponding authors: Xiaosong Li: lixiaosongcom.cn; Wei R. Chen: Wei-R-Chenedu; Feifan Zhou: zhouffedu.cn
Corresponding authors: Xiaosong Li: lixiaosongcom.cn; Wei R. Chen: Wei-R-Chenedu; Feifan Zhou: zhouffedu.cn
 Global reach, higher impact
Global reach, higher impact