13.3
Impact Factor
Theranostics 2021; 11(5):2123-2136. doi:10.7150/thno.49368 This issue Cite
Research Paper
CYP1A2 suppresses hepatocellular carcinoma through antagonizing HGF/MET signaling
1. Department of Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China.
2. Department of Endoscopy, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou 510000, China.
3. Department of Orthopedics and Traumatology, Faculty of Medicine, The Chinese University of Hong Kong, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China.
4. Department of Otorhinolaryngology, Head and Neck Surgery, Faculty of Medicine, The Chinese University of Hong Kong, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong, China.
5. Shenzhen Research Institute, The Chinese University of Hong Kong, Shenzhen, Guangdong, China.
Abstract
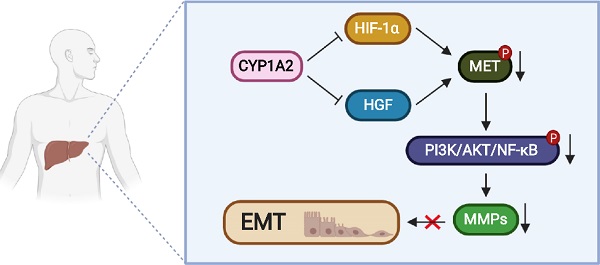
Rationale: Hyperactivation of HGF/MET signaling pathway is a critical driver in liver tumorigenesis. Cytochrome P450 1A2 (CYP1A2) was significantly down-regulated in hepatocellular carcinoma (HCC). However, little is explored about its tumor suppressive role in HCC. In this study, we examined the functional mechanisms and clinical implication of CYP1A2 in HCC.
Methods: The clinical impact of CYP1A2 was evaluated in HCC patients in Hong Kong cohort. The biological functions of CYP1A2 were investigated in vitro and in vivo. A series of biochemical experiments including Western blot assay, immunohistochemistry, quantitative reverse transcription-polymerase chain reaction, and Co-immunoprecipitation assay were conducted.
Results: CYP1A2 expression was prominently silenced in HCC tumor tissues and the high expression of CYP1A2 was significantly correlated with lower AFP level, less vascular invasion, and better tumor-free survival in local cohort of HCC patients. The overexpression of CYP1A2 inhibited HCC cell viability and clonogenicity, reduced cell migration and invasion abilities in vitro, and suppressed tumorigenicity in vivo, whereas CYP1A2 knockdown exhibited the opposite effects. CYP1A2 significantly hindered HGF/MET signaling and Matrix metalloproteinases (MMPs) expression in HCC cells. Mechanically, CYP1A2 decreased HGF level and diminished HIF-1α expression, both of which are recognized as key regulators of MET activation. As the transcriptional activator of MET, HIF-1α was identified as a binding partner of CYP1A2. Direct binding of CYP1A2 with HIF-1α induced ubiquitin-mediated degradation of HIF-1α, inhibiting HIF-1α-mediated transcriptions.
Conclusions: In conclusion, our results have identified CYP1A2 as a novel antagonist of HGF/MET signaling, and CYP1A2 may serve as an independent new biomarker for the prognosis of HCC patients.
Keywords: CYP1A2, HGF/MET signaling, hepatocellular carcinoma, HIF-1α, prognosis
 Global reach, higher impact
Global reach, higher impact