13.3
Impact Factor
Theranostics 2021; 11(5):2080-2097. doi:10.7150/thno.50701 This issue Cite
Review
PDE inhibition in distinct cell types to reclaim the balance of synaptic plasticity
1. Department of Neurosciences, European Graduate School of Neuroscience, Biomedical Research Institute, UHasselt, Hasselt University, Hasselt, Belgium.
2. Department Psychiatry and Neuropsychology, European Graduate School of Neuroscience, School for Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands.
3. Tomsk State University, Tomsk, Russia.
4. Department of Psychiatry, Psychosomatics, and Psychotherapy, University of Wuerzburg, Wuerzburg 97080, Germany.
Abstract
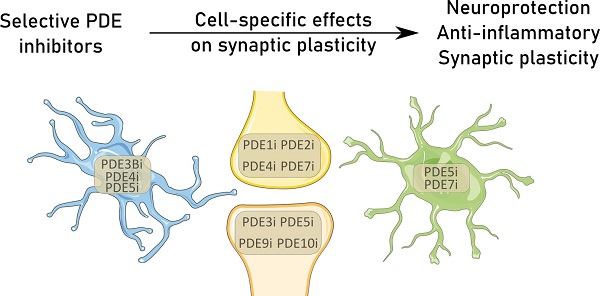
Synapses are the functional units of the brain. They form specific contact points that drive neuronal communication and are highly plastic in their strength, density, and shape. A carefully orchestrated balance between synaptogenesis and synaptic pruning, i.e., the elimination of weak or redundant synapses, ensures adequate synaptic density. An imbalance between these two processes lies at the basis of multiple neuropathologies. Recent evidence has highlighted the importance of glia-neuron interactions in the synaptic unit, emphasized by glial phagocytosis of synapses and local excretion of inflammatory mediators. These findings warrant a closer look into the molecular basis of cell-signaling pathways in the different brain cells that are related to synaptic plasticity. In neurons, intracellular second messengers, such as cyclic guanosine or adenosine monophosphate (cGMP and cAMP, respectively), are known mediators of synaptic homeostasis and plasticity. Increased levels of these second messengers in glial cells slow down inflammation and neurodegenerative processes. These multi-faceted effects provide the opportunity to counteract excessive synapse loss by targeting cGMP and cAMP pathways in multiple cell types. Phosphodiesterases (PDEs) are specialized degraders of these second messengers, rendering them attractive targets to combat the detrimental effects of neurological disorders. Cellular and subcellular compartmentalization of the specific isoforms of PDEs leads to divergent downstream effects for these enzymes in the various central nervous system resident cell types. This review provides a detailed overview on the role of PDEs and their inhibition in the context of glia-neuron interactions in different neuropathologies characterized by synapse loss. In doing so, it provides a framework to support future research towards finding combinational therapy for specific neuropathologies.
Keywords: neurodegeneration, synapses, phosphodiesterase, cell-signaling, glia-neuron
 Global reach, higher impact
Global reach, higher impact