13.3
Impact Factor
Theranostics 2021; 11(4):1753-1762. doi:10.7150/thno.53438 This issue Cite
Review
YAP in pancreatic cancer: oncogenic role and therapeutic strategy
1. Department of Clinical Laboratory, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China.
2. Department of Laboratory Medicine, West China Second Hospital, Sichuan University, Chengdu 610041, China.
#These authors contributed equally to this work.
Received 2020-9-18; Accepted 2020-11-12; Published 2021-1-1
Abstract
Pancreatic cancer, especially pancreatic ductal adenocarcinoma (PDAC), remains a fatal disease with few efficacious treatments. The Hippo signaling pathway, an evolutionarily conserved signaling module, plays critical roles in tissue homeostasis, organ size control and tumorigenesis. The transcriptional coactivator yes-associated protein (YAP), a major downstream effector of the Hippo pathway, is associated with various human cancers including PDAC. Considering its importance in cancer, YAP is emerging as a promising therapeutic target. In this review, we summarize the current understanding of the oncogenic role and regulatory mechanism of YAP in PDAC, and the potential therapeutic strategies targeting YAP.
Keywords: Pancreatic cancer, Hippo pathway, YAP, KRAS, therapeutic target
Introduction
Pancreatic cancer, of which 90% is attributable to pancreatic ductal adenocarcinoma (PDAC), remains the most lethal human malignancy, with an overall 5-year survival rate of 9% and a median survival of ~6 months [1, 2]. Because of the aggressive nature and difficulty in early diagnosis, greater than one-half of pancreatic cancer patients are typically diagnosed at an advanced stage, for which the 5-year survival rate is only 3% [1]. Despite new insights into the molecular mechanism of pancreatic cancer and advances in surgery, adjuvant therapy and chemotherapy, the overall prognosis of pancreatic cancer has not significantly improved. New approaches for the diagnosis and therapy of pancreatic cancer are urgently needed. Elucidating the signaling networks that regulate the development and progression of pancreatic cancer is critical to prevent or treat this lethal disease.
Yes-associated protein (YAP) and its paralog transcriptional co‑activator with PDZ-binding motif (TAZ) are the major downstream effectors of Hippo pathway [3]. Although TAZ and YAP share similar structures and display a degree of functional redundancy, YAP has been studied more extensively. YAP is increasingly identified as a potent oncogene [3] and its abundance and activity are frequently increased in many cancers [4-12]. In this review, we will comprehensively summarize the current studies of YAP in pancreatic cancer. Further understanding of the functions and regulatory mechanism of YAP is of great value for the prevention, early diagnosis and treatment of PDAC.
The core of the Hippo pathway
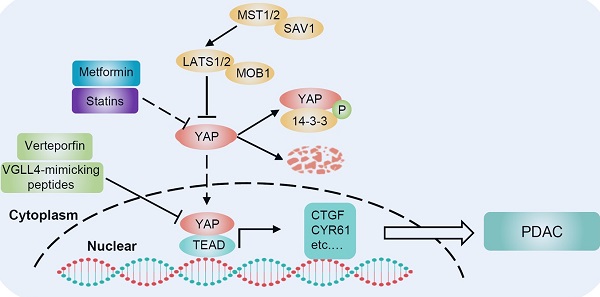
The Hippo pathway, originally identified in Drosophila, is highly conserved in mammals and is important for organ size control, tissue homeostasis and regeneration [13-21]. The core of the Hippo pathway in mammals consists of a kinase cascade, mammalian Ste20-like kinases 1/2 (MST1/2), large tumor suppressor 1/2 (LATS1/2), and transcriptional coactivators YAP and TAZ (Figure 1). MST1/2 form heterodimers with the adaptor protein SAV1, which functions as a partner of MST1/2 in promoting LATS1/2 phosphorylation [22]. RASSF1A interacts with MST1/2 and induces their autophosphorylation and activation [23]. MST1/2 then directly phosphorylates LATS1/2 and the cofactor MOB1. Phosphorylated LATS1/2 enhances autophosphorylation at their activation loop and phosphorylated MOB1 binds to the autoinhibitory domain of LATS1/2, leading to full activation [22, 24]. NF2 is another potent activator of LATS1/2, and NF2 can directly interact with LATS1/2 and recruit them to the plasma membrane for activation by MST1/2 [25, 26]. In parallel to MST1/2, members of the MAP4K family, including MAP4K1/2/3/5 and MAP4K4/6/7 can also directly phosphorylate LATS1/2 at their hydrophobic motifs, resulting in LATS1/2 activation [27-29]. Notably, TAO kinases have been found to act both upstream of and in parallel to MSTs to directly phosphorylate LATSs, raising the possibility that MST1/2 are not absolutely required for the regulation of LATS1/2 [30, 31]. Once activated, LATS1/2 directly phosphorylates YAP/TAZ and phosphorylated YAP/TAZ are sequestered in the cytoplasm by binding to 14-3-3 [32-34]. Moreover, subsequent phosphorylation of YAP/TAZ by CK1 leads to SCF β-TrCP-mediated ubiquitination and proteasomal degradation [35, 36]. When LATS1/2 are inactive, dephosphorylated YAP/TAZ translocate to the nucleus, where they compete with VGLL4 (vestigial-like protein 4) and initiate gene transcription by interacting with transcription factors, particularly TEA domain (TEAD) family members [37-39]. It is worth noting that the regulation of Hippo pathway is not static but rather dynamic, and this dynamic regulation leads to YAP rapidly shuttling between the cytoplasm and the nucleus [40].
Core components of the Hippo pathway in mammalian cells. The core components of Hippo pathway consist of MST1/2, LATS1/2 and YAP/TAZ. When the Hippo pathway is ON, MST1/2, in complex with SAV1, phosphorylate LATS1/2 and MOB1. MAP4Ks also phosphorylate and activate LATS1/2. NF2 is an additional and potent activator of LATS1/2 that is devoid of kinase activity. Activated LATS1/2 phosphorylate YAP and TAZ, resulting in 14-3-3-mediated YAP/TAZ cytoplasmic retention and ubiquitin-mediated proteasomal degradation. When the Hippo pathway is OFF, YAP/TAZ are dephosphorylated and translocate to the nucleus, where they compete with VGLL4 for TEADs binding and induce the transcription of downstream target genes, such as CTGF and CYR61, which are involved in growth, proliferation, and survival.
The posttranslational modifications of YAP
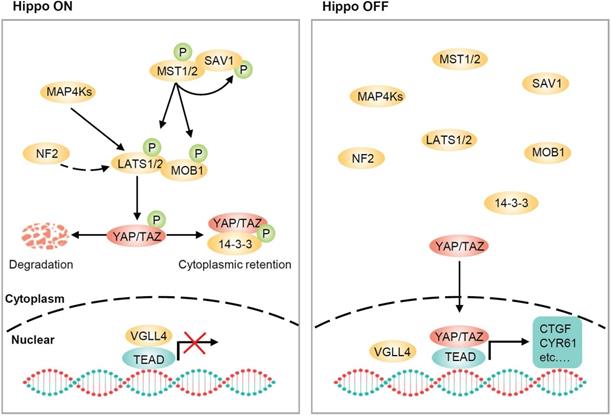
Phosphorylation is the most common posttranslational modification of YAP and LATS1/2 are the primary upstream kinases. Moreover, AMPK can also directly phosphorylate YAP on several residues including the S61 and S94. Phosphorylation of YAP S94 by AMPK disrupts its interaction with TEAD and inhibits YAP function [41]. However, exactly how AMPK-dependent S61 phosphorylation inhibits YAP transcriptional activity remains unknown [42]. In addition to phosphorylation, YAP activity can be regulated through several other posttranslational modifications, including O-GlcNAcylation, acetylation, and methylation (Figure 2). When glucose is sufficient, YAP is O-GlcNAcylated at serine 109 or threonine 241 by O-GlcNAc transferase (OGT). YAP O-GlcNAcylation activates its transcriptional activity by disrupting the LATS-mediated phosphorylation and blocking its ubiquitination-mediated degradation by β-TrCP [43, 44]. SIRT1-mediated deacetylation of YAP enhances the YAP/TEAD4 association and transcriptional activity, whereas acetyltransferases CBP and p300 are responsible for YAP acetylation [45, 46]. Furthermore, SETD7 methylates YAP on lysine 494, and this methylation specifically promotes the cytoplasmic retention of YAP [47]. Conversely, SET1A-mediated methylation of YAP at lysine 342 is essential for the nuclear retention of YAP [48]. Therefore, YAP can be regulated by diverse posttranslational mechanisms.
Schematic illustration of the posttranslational modifications of YAP. In addition to phosphorylation and ubiquitylation, YAP can also be regulated by several other posttranslational modifications including high glucose-stimulated YAP O-GlcNAcylation for increasing YAP stability, SIRT1-mediated YAP deacetylation for enhancing YAP/TEAD association, SETD7 methylated methylation for YAP cytoplasmic retention, and SET1A-mediated methylation for YAP nuclear retention.
YAP and cancer metabolism
One hallmark of cancer cells is metabolic reprogramming. Significant progress has been made in the crosstalk between cancer metabolism and YAP signaling. Studies have shown that YAP is regulated by cellular energy stress. Both LATS kinase and AMPK were activated during glucose starvation, resulting in phosphorylation of YAP and contributing to its inactivation [42, 49]. Besides, glycolysis is required to sustain YAP function, and the key enzyme of glycolysis, phosphofructokinase (PFK1), can bind to TEADs and promote their functional and biochemical cooperation with YAP [50]. On the other hand, YAP reprograms glucose metabolism. YAP promoted glucose metabolism through transcriptional upregulation of glucose-transporter 3 (GLUT3) expression [42]. In addition, YAP promoted glycolysis through upregulating the expression of lncRNA BCAR4, which subsequently coordinates the Hedgehog pathway to enhance the transcription of glycolysis activators HK2 and PFKFB3 [51]. Mitochondria are the powerhouses of the cell crucial for both anabolic and catabolic metabolism. The mitochondrial deoxyguanosine kinase (DGUOK) is a rate-limiting enzyme in the mitochondrial deoxynucleoside salvage pathway. DGUOK was found to inhibit mitochondrial respiration and self-renewal of lung cancer stem-like cells through preventing AMPK-mediated phosphorylation of YAP [52]. Activation of lipid biosynthesis, is a major event in the metabolic transformation of cancer [53]. Stearoyl-CoA-desaturase 1 (SCD1), the enzyme involved in fatty acids synthesis, is a key regulator of YAP in lung cancer stem cells through its involvement in wnt/β-catenin pathway [54].
In pancreatic cancer, activating mutation in Kras is the most prominent driver, present in over 90% of PDAC [55, 56]. Kras-induced metabolic reprogramming is essential for pancreatic cancer cells to survive the environmental and nutrient stresses. As the downstream target of KRAS, YAP cooperates with Myc to maintain the global transcription of metabolic genes and metabolic homeostasis in Kras-driven PDAC [57]. Notably, some drugs targeting metabolic regulation of YAP, such as metformin and statins, have been found to be potential treatment strategies for pancreatic cancer.
The functional role of YAP in pancreatic cancer
Studies have indicated that YAP is overexpressed in PDAC and active YAP promotes pancreatic cancer cell proliferation, survival and metastasis [58, 59]. Moreover, YAP has been identified as an independent prognostic marker of PDAC, and elevated YAP expression is associated with poor prognosis [60-62]. In mouse models of pancreatic duct cell-specific deletion of Lats1/2, YAP cooperates with AP-1 to initiate pancreatic cancer development from ductal cells [63]. Acinar cell-specific Lats1/2 deletions caused pancreatic inflammation and fibrosis are YAP dependent in adult mice [64]. Moreover, YAP is essential for PDAC progression in Kras-mutant mice [65, 66]. Zhang et al. found that, although YAP is dispensable for acinar to ductal metaplasia (ADM), an initial step in the progression to PDAC, YAP is essential for PanIN progression to PDAC [65]. Gruber et al. found that YAP is required for the induction of ADM and progression to PanIN by direct up-regulation of JAK-STAT3 signaling [66]. Mutant GNAS, as an onco-modulator of Kras-induced neoplasia, causes phosphorylated YAP to be sequestered in the cytoplasm and alters tumor progression [67]. In addition, oncogenic activation of YAP collaborates with heterozygous KrasMUT in driving PDAC tumorigenesis [68]. Besides, YAP is required for cancer recurrence in the absence of KRAS [69]. Recent studies have also demonstrated that YAP is a major driver of squamous subtype PDAC, independent of oncogenic KRAS [70]. These findings raise an important insight that YAP not only acts as a PDAC driver downstream of KRAS but also enables PDAC to escape KRAS dependence [65, 69, 71].
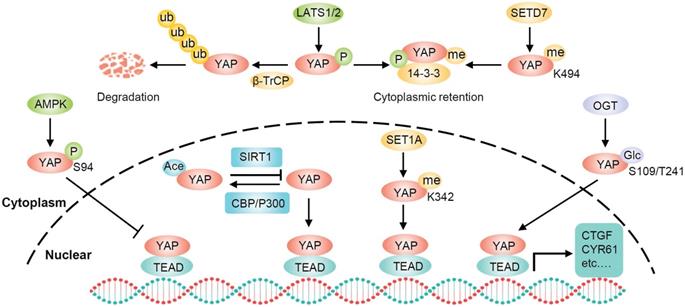
YAP exerts oncogenic functions in different aspects of PDAC (Figure 3). YAP is essential to maintain the metabolic homeostasis in Kras-driven PDAC [57]. Severe inflammation and profound immune suppression are common features of PDAC. Studies have demonstrated that YAP acts as a transcriptional driver of multiple cytokines, which in turn promote the differentiation and accumulation of myeloid-derived suppressor cells (MDSCs), and contributes to the strong immunosuppressive microenvironment in both mouse and human PDAC [72]. Cancer stem cells (CSCs), which express stem cell marker CD133, have the ability to self-renew. Recent studies have demonstrated that YAP is downstream of HMGB1-TLR2 signaling to induce CD133- cancer cell dedifferentiation and enhance pancreatic cancer stemness [73]. YAP overexpression in PDAC induces NMU expression and promotes cell motility and tumor metastasis via upregulation of epithelial-mesenchymal transition (EMT) [62]. Moreover, YAP also contributes to pancreatic cancer cell invasion and migration by disrupting tumor-stromal interactions [74].
Regulation of YAP in PDAC
A complex network of upstream inputs, including cell polarity, mechanical stress, cell junctions and G-protein-coupled receptor (GPCR) signaling, modulates Hippo pathway activity [75]. Hippo signaling can also crosstalk with other signaling pathways, such as transforming growth factor-β (TGFβ) [76, 77] and WNT/β-catenin pathways to modulate YAP activity [78-80]. GPCRs function as key transducers of extracellular signals into the cell [81]. Depending on the nature of downstream G proteins, GPCRs can either enhance or suppress YAP activity through LATS kinases [75]. TGF-β facilitates YAP/SMAD2 nuclear translocation by targeting RASSF1A, which is the scaffold of Hippo pathway [77]. WNT stimulation diverts YAP away from the β‑catenin destruction complex, causing YAP nuclear accumulation and β‑catenin stabilization [78]. In addition, the Hippo pathway also crosstalks with other pathways, such as PI3K/AKT, Hedgehog, Notch, and mTOR [82-87], to modulate YAP activity. In this section, we will focus on the regulation of YAP in PDAC (Figure 4).
Schematic overview of YAP functions in pancreatic cancer. YAP exerts oncogenic functions in different aspects of PDAC, including proliferation, survival, metastasis, metabolic reprogramming, and cancer stem cells, as well as inflammation and immunosuppression.
Regulation of YAP in PDAC. Multiple signals are integrated to regulate YAP activity. Soluble factors binding to GPCRs regulate LATS kinase through Rho. The insulin/IGF-1 receptor contributes to YAP activation through PI3K. WNT stimulation diverts YAP away from the β‑catenin destruction complex. TGF-β facilitates YAP nuclear translocation through RASSF1A. Other factors also affect YAP activity, in Hippo dependent or independent manners.
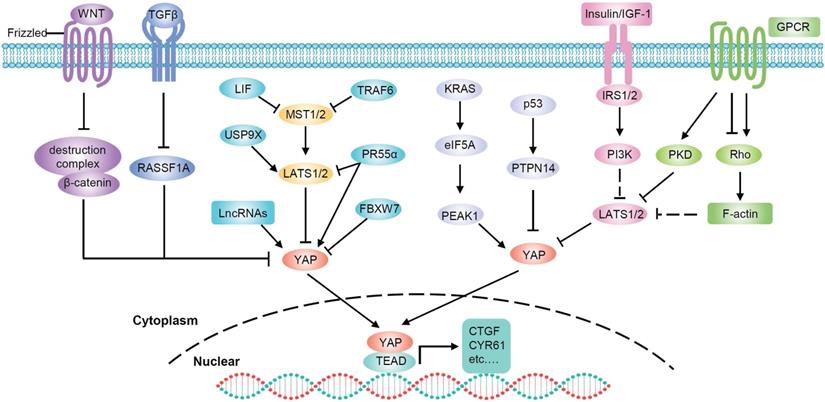
In human PDAC, the crosstalk between insulin/IGF-1 receptor and GPCR systems contributes to YAP activation through PI3K and PKD [88]. In addition, YAP is a major mediator of the pro-oncogenic mutant p53 [89] and p53 negatively regulates YAP through PTPN14 activation [90]. Therefore, the tumor suppressor activity of p53 in pancreatic cancer is at least partially mediated via the inactivation of YAP [90]. Moreover, in PDAC, mutant KRAS upregulates eukaryotic translation initiation factor 5A (eIF5A) and PEAK1, and eIF5A-PEAK1 signaling promotes YAP expression [91]. PR55α, a PP2A regulatory subunit, has been reported to be essential for the tumor-promoting functions of YAP in pancreatic cancer. There are two pathways by which PR55α regulates YAP: activating YAP by inhibiting the MOB1-triggered autoactivation of LATS1/2 or directly interacting with YAP [92]. Chen et al. identified an MST4-MOB4 complex, whose overall structure is similar to that of the MST1-MOB1 complex that can antagonize the MST1-MOB1 complex to promote YAP activity and play a pro-oncogenic role in pancreatic cancer [93]. Glucose-regulated protein 78kDa on the surface of cancer cells (CS-GRP78) activates YAP in a Rho-dependent manner, promoting motility and radiation-resistance of PDAC cells [94]. In addition, leukemia inhibitory factor (LIF) inhibits the activity of the Hippo-signaling pathway and subsequently increases YAP transcriptional activity in KRAS-driven pancreatic cancer. Therefore, blockade of LIF provides an attractive approach to improving therapeutic outcomes of pancreatic cancer [95].
Ubiquitylation is an important mechanism that regulates YAP activity through the proteasome degradation pathway [96-99]. It was reported that the E3 ubiquitin ligase SIAH2 activates YAP by destabilizing LATS2 in response to hypoxia [96]. Since hypoxia is a critical feature in PDAC, SIAH2 may also play an important role in pancreatic cancer through YAP. Moreover, deubiquitylase USP9X can suppress pancreatic cancer progression by inhibiting YAP through the deubiquitination and stabilization of LATS2 [100, 101]. FBXW7, which is a component of the Skp1-Cullin1-Fbox E3 ligase complex, has been identified as a potent suppressor of KrasG12D-induced pancreatic tumorigenesis. The anticancer effect of FBXW7 is, at least in part, due to its proteolytic regulation of YAP [102]. TRAF6 activates YAP by promoting the degradation of MST1 [12]. Recently, studies have found that TAK1 prevents YAP degradation through a complex with TRAF6 and inhibition of TAK1 can significantly impair the oncogenic functions of YAP in pancreatic cancer [103].
LncRNAs are also involved in the regulation of YAP in pancreatic cancer. LncRNA UCA1 increases YAP nuclear localization and stabilization by forming shielding composites, thus promoting the migration and invasion of pancreatic cancer cells [104]. LINC01559 accelerates pancreatic cancer cell proliferation and migration through a YAP-mediated pathway. On the one hand, LINC01559 serves as a ceRNA of YAP by sponging miR‐607; on the other hand, LINC01559 may inhibit YAP phosphorylation and enhance its activity by directly interacting with YAP [105]. LncRNA THAP9-AS1 promotes PDAC growth and leads to a poor clinical outcome via regulating YAP [61]. Mechanistically, THAP9-AS1 spongs miR-484, leading to YAP upregulation. In addition, THAP9-AS1 binds to YAP and inhibits the phosphorylation-mediated inactivation of YAP by LATS1. Reciprocally, YAP promotes THAP9-AS1 transcription to form a feed-forward circuit. Moreover, lncRNA MALAT1 and Linc-RoR have also been found to activate the Hippo/YAP pathway in PDAC cells [106, 107]. Taken together, YAP is modulated by a complex signaling network and is commonly overactivated in pancreatic cancer cells.
Therapeutic strategies targeting YAP
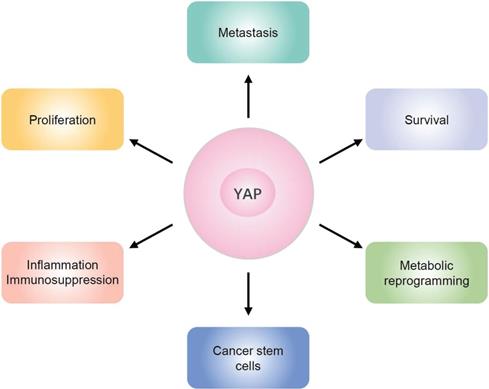
Kras mutation is the most prominent driver of pancreatic cancer, and YAP is a critical KRAS effector. To date, KRAS is not a viable therapeutic target for pancreatic cancer. Even if KRAS could be effectively inhibited by new therapies, YAP amplification could provide a potential pathway for cancer recurrence. Therapeutically targeting YAP is clearly a very challenging yet exciting goal for PDAC. Studies have found novel approaches to inhibit YAP activity, including drugs such as verteporfin, statins and metformin (Figure 5).
Verteporfin
Since YAP binds to TEAD transcription factors to induce target gene expression, designing compounds that directly dissociate YAP and TEAD interaction will be the most direct and effective approach to suppress YAP-induced oncogenic events [108]. Verteporfin, a photosensitizer clinically used in photodynamic therapy, abrogates the interaction between YAP and TEAD, thereby inhibiting YAP transcriptional activity [109]. Additionally, verteporfin has been clinically used for neovascular macular degeneration and exhibits limited toxicity, which provides an opportunity for its clinical application in cancer. Studies have demonstrated that verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of PDAC. The mechanism accounting for these activities of verteporfin is to suppress the expression of targeted genes, such as cyclinD1, cyclinE1, Ang2, MMP2, VE-cadherin and a-SMA, by disrupting the YAP-TEAD complex [110]. This study suggests that verteporfin may be a promising drug for pancreatic cancer by targeting YAP. Moreover, verteporfin can significantly enhance the antitumor efficacy of the pan-RAF inhibitor LY3009120 in pancreatic cancer by blocking the activation of alternative AKT signaling pathway after treatment with the RAF inhibitor [111]. Therefore, the combination of verteporfin and pan-RAF inhibitors may be a potential approach for treating KRAS-mutant pancreatic cancer. In addition, another study has shown that verteporfin moderately enhances the antitumor activity of gemcitabine in a PDAC model by inhibiting autophagy, but its inhibitory effect on YAP was not evaluated in that study [112].
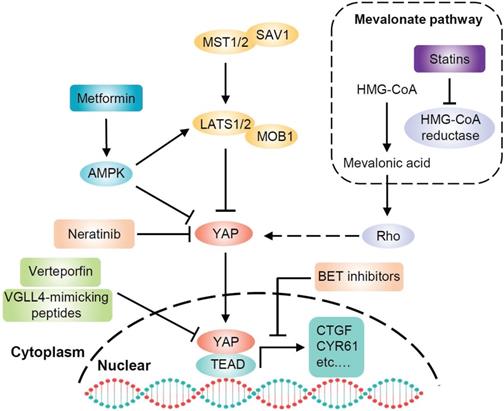
Targeting YAP for PDAC therapy. There are several strategies for targeting YAP in PDAC. Statin-mediated inhibition of HMG-CoA reductase in the mevalonate pathway reduces the geranylgeranylation and membrane localization of Rho GTPases, restricting YAP nuclear accumulation and thus its activity. Verteporfin and VGLL4-mimicking peptides disrupt the interaction between YAP and TEAD, inhibiting YAP-induced transcription. Metformin activates AMPK, which inhibits YAP both directly and by activating LATS kinases. BET inhibitors oppose the transcription of YAP-regulated genes. Neratinib increases the phosphorylation of LATS1 and YAP, causing YAP cytosolic accumulation and degradation.
Statins
Statins, inhibitors of the mevalonate pathway, are conventionally used to treat hypercholesterolemia and prevent cardiovascular disorders. Mechanistically, statins inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which is the rate limiting enzyme in the generation of mevalonate [113]. Mevalonate is a precursor of geranylgeranyl pyrophosphate, which is required for membrane localization and activation of Rho GTPases. Activated Rho plays a critical role in the nuclear accumulation of YAP through actin remodeling [34]. Thus, statins have been identified as potential YAP inhibitors that oppose YAP nuclear localization and transcriptional responses by inhibiting the mevalonate pathway [114, 115]. Preclinical studies have shown that statins delay the progression of pancreatic lesions to carcinoma or prevent the initial stages of PDAC in KrasG12D mice [116, 117]. It is also worth noting that substantial evidence indicates that statins have extensive tumor suppressor effects and accumulating clinical studies have shown a significant negative association between statins and cancer occurrence or survival [118].
Metformin
Metformin, a widely administered drug for type 2 diabetes worldwide [119], activates AMPK, inhibits cell growth and reduces cancer occurrence [120]. Moreover, the antidiabetic agent metformin has been found to inhibit YAP. Mechanistically, metformin activates AMPK, which in turn inhibits YAP via both LATS activation and direct YAP phosphorylation [41]. Inhibition of YAP may contribute to the antitumor effect of metformin. Indeed, multiple epidemiological studies have shown that metformin is significantly associated with a reduced risk of cancer, especially pancreatic cancer, in diabetic patients [121-123]. Another study found that oral administration of metformin strikingly decreases the incidence of PDAC in KrasG12D mice with diet-induced obesity [124]. This effect is associated with an increase in pancreatic AMPK activity and a decrease in pancreatic YAP expression. Furthermore, metformin can inhibit pancreatic cancer through other AMPK-mediated signaling pathways. For example, metformin inhibits pancreatic cancer growth by disrupting the crosstalk between GPCR and insulin receptor signaling systems [125]. These results raise the possibility that metformin may be a potential therapeutic strategy for human pancreatic cancer.
Other strategies
Bromodomain-containing protein 4 (BRD4) is a coactivator of the bromodomain and extraterminal domain (BET). A recent study has demonstrated that the transcriptional addiction in cancer cells is mediated by YAP through BRD4. Mechanistically, BRD4 interacts with YAP and is recruited to chromatin, enhancing the expression of a host of growth-regulating genes. The BET inhibitor JQ1 opposes the activity of BRD4 and downregulates the expression of YAP-regulated genes [126]. These findings suggest the potential of BET inhibitors to target YAP, but the effect of these inhibitors in pancreatic cancer remains to be investigated. Neratinib is an irreversible ERBB1/2/4 inhibitor, which can downregulate the expression of other RTKs and mutant RAS proteins. Prior studies have demonstrated that neratinib causes mutant KRAS to localize in intracellular vesicles, concomitant with its degradation. Moreover, neratinib increases the phosphorylation of LATS1 and YAP, causing the majority of YAP to be translocated into the cytosol and degraded. Therefore, neratinib can coordinately suppress the functions of both mutant KRAS and YAP to kill pancreatic cancer cells [127]. In addition, a peptide mimicking the function of VGLL4 acts as a YAP antagonist and potently suppresses tumor growth [128], suggesting that the VGLL4-mimicking peptide may be a promising therapeutic strategy against YAP-driven human cancers, however, its effect in pancreatic cancer has not been revealed.
Conclusions and further perspectives
Despite the major advances in revealing the molecular mechanism driving PDAC, this disease remains the most lethal human cancer. Almost all PDACs harbor Kras mutation, which is necessary but not completely sufficient for the development of invasive PDAC. YAP has been identified as a critical effector downstream of KRAS, but its activation enables bypass of KRAS addiction in pancreatic cancer. There is no doubt that YAP plays a critical role in cancer development and is emerging as an attractive therapeutic target for PDAC. However, some challenges remain unresolved. First, it is not fully elucidated what triggers the activation of YAP, since the core components of the Hippo pathway are typically not mutated in PDAC. Second, the downstream effectors and mechanisms that mediate the function of YAP remain incompletely elucidated. Importantly, more efforts are needed to develop effective inhibitors that directly target YAP or dissociate the YAP-TEAD interaction. These drugs can be used alone or in combination with other therapies, such as chemotherapy, radiotherapy and immunotherapy, for more effective treatment of advanced PDAC. Finally, it is also essential to develop biomarkers, especially noninvasive biomarkers, to accurately select patients for YAP-targeted therapy, and to facilitate the real-time evaluation of therapeutic effects.
Abbreviations
ADM: acinar to ductal metaplasia; BET: bromodomain and extraterminal domain; BRD4: Bromodomain-containing protein 4; CSC: cancer stem cell; DGUOK: deoxyguanosine kinase; eIF5A: eukaryotic translation initiation factor 5A; EMT: epithelial-mesenchymal transition; GLUT3: glucose-transporter 3; GPCR: G-protein-coupled receptor; LATS1/2: large tumor suppressor 1/2; LIF: leukemia inhibitory factor; MDSC: myeloid-derived suppressor cells; MST1/2: mammalian Ste20-like kinases 1/2; OGT: O-GlcNAc transferase; PDAC: pancreatic ductal adenocarcinoma; PFK1: phosphorfructokinase; SCD1: stearoyl-CoA-desaturase 1; TAZ: transcriptional co‑activator with PDZ-binding motif; TEAD: TEA domain; TGFβ: transforming growth factor-β; VGLL4: vestigial-like protein 4; YAP: Yes-associated protein.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81874188 and 31501123) and Key R&D and promotion projects of Henan province (202102310119).
Authors' contributions
Ting Sun conceived the structure of manuscript and revised the manuscript. Wenhao Mao and Jia Mai collected the related paper and drafted the manuscript. Hui Peng created the figures. Junhu Wan revised this manuscript. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34
2. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039-49
3. Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15:73-9
4. Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010;101:1279-85
5. Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T. et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70:8517-25
6. Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB. et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810-22
7. Kang W, Tong JH, Chan AW, Lee TL, Lung RW, Leung PP. et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17:2130-9
8. Orr BA, Bai H, Odia Y, Jain D, Anders RA, Eberhart CG. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J Neuropathol Exp Neurol. 2011;70:568-77
9. Yokoyama T, Osada H, Murakami H, Tatematsu Y, Taniguchi T, Kondo Y. et al. YAP1 is involved in mesothelioma development and negatively regulated by Merlin through phosphorylation. Carcinogenesis. 2008;29:2139-46
10. Muramatsu T, Imoto I, Matsui T, Kozaki K, Haruki S, Sudol M. et al. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32:389-98
11. Liu T, Liu Y, Gao H, Meng F, Yang S, Lou G. Clinical significance of yes-associated protein overexpression in cervical carcinoma: the differential effects based on histotypes. Int J Gynecol Cancer. 2013;23:735-42
12. Zhang J, Xu ZP, Yang YC, Zhu JS, Zhou Z, Chen WX. Expression of Yes-associated protein in gastric adenocarcinoma and inhibitory effects of its knockdown on gastric cancer cell proliferation and metastasis. Int J Immunopathol Pharmacol. 2012;25:583-90
13. Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534-46
14. Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053-63
15. Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457-67
16. Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514-9
17. Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921-7
18. Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914-20
19. Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445-56
20. Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D. et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467-78
21. Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N. et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675-85
22. Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. The FEBS journal. 2006;273:4264-76
23. Guo C, Zhang X, Pfeifer GP. The tumor suppressor RASSF1A prevents dephosphorylation of the mammalian STE20-like kinases MST1 and MST2. J Biol Chem. 2011;286:6253-61
24. Chan EH, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076-86
25. Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342-55
26. Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J. et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27-38
27. Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW. et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357
28. Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade. Dev Cell. 2015;34:642-55
29. Li S, Cho YS, Yue T, Ip YT, Jiang J. Overlapping functions of the MAP4K family kinases Hppy and Msn in Hippo signaling. Cell Discov. 2015;1:15038
30. Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell. 2011;21:888-95
31. Poon CL, Lin JI, Zhang X, Harvey KF. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev Cell. 2011;21:896-906
32. Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421-34
33. Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747-61
34. Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1-17
35. Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24:72-85
36. Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D. et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285:37159-69
37. Zhao B, Ye X, Yu J, Li L, Li W, Li S. et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962-71
38. Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S. et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009;284:13355-62
39. Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F. et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24:331-43
40. Ege N, Dowbaj AM, Jiang M, Howell M, Hooper S, Foster C. et al. Quantitative Analysis Reveals that Actin and Src-Family Kinases Regulate Nuclear YAP1 and Its Export. Cell systems. 2018;6:692-708.e13
41. Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S. et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500-10
42. Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL. et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490-9
43. Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, Liu X. et al. The essential role of YAP O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat Commun. 2017;8:15280
44. Peng C, Zhu Y, Zhang W, Liao Q, Chen Y, Zhao X. et al. Regulation of the Hippo-YAP Pathway by Glucose Sensor O-GlcNAcylation. Mol Cell. 2017;68:591-604.e5
45. Hata S, Hirayama J, Kajiho H, Nakagawa K, Hata Y, Katada T. et al. A novel acetylation cycle of transcription co-activator Yes-associated protein that is downstream of Hippo pathway is triggered in response to SN2 alkylating agents. J Biol Chem. 2012;287:22089-98
46. Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan F. et al. SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2014;33:1468-74
47. Oudhoff MJ, Freeman SA, Couzens AL, Antignano F, Kuznetsova E, Min PH. et al. Control of the hippo pathway by Set7-dependent methylation of Yap. Dev Cell. 2013;26:188-94
48. Fang L, Teng H, Wang Y, Liao G, Weng L, Li Y. et al. SET1A-Mediated Mono-Methylation at K342 Regulates YAP Activation by Blocking Its Nuclear Export and Promotes Tumorigenesis. Cancer Cell. 2018;34:103-18.e9
49. Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S. et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500-10
50. Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M. et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015;34:1349-70
51. Zheng X, Han H, Liu GP, Ma YX, Pan RL, Sang LJ. et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017;36:3325-35
52. Lin S, Huang C, Sun J, Bollt O, Wang X, Martine E. et al. The mitochondrial deoxyguanosine kinase is required for cancer cell stemness in lung adenocarcinoma. EMBO Mol Med. 2019;11:e10849
53. Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2:881-98
54. Noto A, De Vitis C, Pisanu ME, Roscilli G, Ricci G, Catizone A. et al. Stearoyl-CoA-desaturase 1 regulates lung cancer stemness via stabilization and nuclear localization of YAP/TAZ. Oncogene. 2017;36:4573-84
55. Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P. et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-6
56. Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL. et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399-405
57. Murakami S, Nemazanyy I, White SM, Chen H, Nguyen CDK, Graham GT. et al. A Yap-Myc-Sox2-p53 Regulatory Network Dictates Metabolic Homeostasis and Differentiation in Kras-Driven Pancreatic Ductal Adenocarcinomas. Dev Cell. 2019;51:113-28.e9
58. Yang S, Zhang L, Purohit V, Shukla SK, Chen X, Yu F. et al. Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation-dependent manner through LPAR3. Oncotarget. 2015;6:36019-31
59. Diep CH, Zucker KM, Hostetter G, Watanabe A, Hu C, Munoz RM. et al. Down-regulation of Yes Associated Protein 1 expression reduces cell proliferation and clonogenicity of pancreatic cancer cells. PLoS One. 2012;7:e32783
60. Salcedo Allende MT, Zeron-Medina J, Hernandez J, Macarulla T, Balsells J, Merino X. et al. Overexpression of Yes Associated Protein 1, an Independent Prognostic Marker in Patients With Pancreatic Ductal Adenocarcinoma, Correlated With Liver Metastasis and Poor Prognosis. Pancreas. 2017;46:913-20
61. Li N, Yang G, Luo L, Ling L, Wang X, Shi L. et al. lncRNA THAP9-AS1 Promotes Pancreatic Ductal Adenocarcinoma Growth and Leads to a Poor Clinical Outcome via Sponging miR-484 and Interacting with YAP. Clin Cancer Res. 2020;26:1736-48
62. Yoo W, Lee J, Jun E, Noh KH, Lee S, Jung D. et al. The YAP1-NMU Axis Is Associated with Pancreatic Cancer Progression and Poor Outcome: Identification of a Novel Diagnostic Biomarker and Therapeutic Target. Cancers (Basel). 2019 11
63. Park J, Eisenbarth D, Choi W, Kim H, Choi C, Lee D. et al. YAP and AP-1 Cooperate to Initiate Pancreatic Cancer Development from Ductal Cells in Mice. Cancer Res. 2020
64. Liu J, Gao M, Nipper M, Deng J, Sharkey FE, Johnson RL. et al. Activation of the intrinsic fibroinflammatory program in adult pancreatic acinar cells triggered by Hippo signaling disruption. PLoS Biol. 2019;17:e3000418
65. Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G. et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42
66. Gruber R, Panayiotou R, Nye E, Spencer-Dene B, Stamp G, Behrens A. YAP1 and TAZ Control Pancreatic Cancer Initiation in Mice by Direct Up-regulation of JAK-STAT3 Signaling. Gastroenterology. 2016;151:526-39
67. Ideno N, Yamaguchi H, Ghosh B, Gupta S, Okumura T, Steffen DJ. et al. GNAS(R201C) Induces Pancreatic Cystic Neoplasms in Mice That Express Activated KRAS by Inhibiting YAP1 Signaling. Gastroenterology. 2018;155:1593-607 e12
68. Mueller S, Engleitner T, Maresch R, Zukowska M, Lange S, Kaltenbacher T. et al. Evolutionary routes and KRAS dosage define pancreatic cancer phenotypes. Nature. 2018;554:62-8
69. Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q. et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185-97
70. Tu B, Yao J, Ferri-Borgogno S, Zhao J, Chen S, Wang Q. et al. YAP1 oncogene is a context-specific driver for pancreatic ductal adenocarcinoma. JCI insight. 2019 4
71. Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X. et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171-84
72. Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT. et al. Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene. 2017;36:1232-44
73. Zhang L, Shi H, Chen H, Gong A, Liu Y, Song L. et al. Dedifferentiation process driven by radiotherapy-induced HMGB1/TLR2/YAP/HIF-1α signaling enhances pancreatic cancer stemness. Cell Death Dis. 2019;10:724
74. Jiang Z, Zhou C, Cheng L, Yan B, Chen K, Chen X. et al. Inhibiting YAP expression suppresses pancreatic cancer progression by disrupting tumor-stromal interactions. J Exp Clin Cancer Res. 2018;37:69
75. Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780-91
76. Fujii M, Toyoda T, Nakanishi H, Yatabe Y, Sato A, Matsudaira Y. et al. TGF-β synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J Exp Med. 2012;209:479-94
77. Pefani DE, Pankova D, Abraham AG, Grawenda AM, Vlahov N, Scrace S. et al. TGF-β Targets the Hippo Pathway Scaffold RASSF1A to Facilitate YAP/SMAD2 Nuclear Translocation. Mol Cell. 2016;63:156-66
78. Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S. et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157-70
79. Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA. et al. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579-91
80. Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL. et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science (New York, NY). 2011;332:458-61
81. O'Hayre M, Vázquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S. et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412-24
82. Yuan Z, Kim D, Shu S, Wu J, Guo J, Xiao L. et al. Phosphoinositide 3-kinase/Akt inhibits MST1-mediated pro-apoptotic signaling through phosphorylation of threonine 120. J Biol Chem. 2010;285:3815-24
83. Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S. et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729-41
84. Si Y, Ji X, Cao X, Dai X, Xu L, Zhao H. et al. Src Inhibits the Hippo Tumor Suppressor Pathway through Tyrosine Phosphorylation of Lats1. Cancer Res. 2017;77:4868-80
85. Totaro A, Castellan M, Battilana G, Zanconato F, Azzolin L, Giulitti S. et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat Commun. 2017;8:15206
86. Artinian N, Cloninger C, Holmes B, Benavides-Serrato A, Bashir T, Gera J. Phosphorylation of the Hippo Pathway Component AMOTL2 by the mTORC2 Kinase Promotes YAP Signaling, Resulting in Enhanced Glioblastoma Growth and Invasiveness. J Biol Chem. 2015;290:19387-401
87. Liang N, Zhang C, Dill P, Panasyuk G, Pion D, Koka V. et al. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med. 2014;211:2249-63
88. Hao F, Xu Q, Zhao Y, Stevens JV, Young SH, Sinnett-Smith J. et al. Insulin Receptor and GPCR Crosstalk Stimulates YAP via PI3K and PKD in Pancreatic Cancer Cells. Mol Cancer Res. 2017;15:929-41
89. Di Agostino S, Sorrentino G, Ingallina E, Valenti F, Ferraiuolo M, Bicciato S. et al. YAP enhances the pro-proliferative transcriptional activity of mutant p53 proteins. EMBO reports. 2016;17:188-201
90. Mello SS, Valente LJ, Raj N, Seoane JA, Flowers BM, McClendon J. et al. A p53 Super-tumor Suppressor Reveals a Tumor Suppressive p53-Ptpn14-Yap Axis in Pancreatic Cancer. Cancer Cell. 2017;32:460-73.e6
91. Strnadel J, Choi S, Fujimura K, Wang H, Zhang W, Wyse M. et al. eIF5A-PEAK1 Signaling Regulates YAP1/TAZ Protein Expression and Pancreatic Cancer Cell Growth. Cancer Res. 2017;77:1997-2007
92. Hein AL, Brandquist ND, Ouellette CY, Seshacharyulu P, Enke CA, Ouellette MM. et al. PR55alpha regulatory subunit of PP2A inhibits the MOB1/LATS cascade and activates YAP in pancreatic cancer cells. Oncogenesis. 2019;8:63
93. Chen M, Zhang H, Shi Z, Li Y, Zhang X, Gao Z. et al. The MST4-MOB4 complex disrupts the MST1-MOB1 complex in the Hippo-YAP pathway and plays a pro-oncogenic role in pancreatic cancer. J Biol Chem. 2018;293:14455-69
94. Gopal U, Mowery Y, Young K, Pizzo SV. Targeting cell surface GRP78 enhances pancreatic cancer radiosensitivity through YAP/TAZ protein signaling. J Biol Chem. 2019;294:13939-52
95. Wang MT, Fer N, Galeas J, Collisson EA, Kim SE, Sharib J. et al. Blockade of leukemia inhibitory factor as a therapeutic approach to KRAS driven pancreatic cancer. Nat Commun. 2019;10:3055
96. Ma B, Chen Y, Chen L, Cheng H, Mu C, Li J. et al. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 2015;17:95-103
97. Bae SJ, Kim M, Kim SH, Kwon YE, Lee JH, Kim J. et al. NEDD4 controls intestinal stem cell homeostasis by regulating the Hippo signalling pathway. Nat Commun. 2015;6:6314
98. Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D. et al. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell. 2014;26:48-60
99. Zhou X, Li Y, Wang W, Wang S, Hou J, Zhang A. et al. Regulation of Hippo/YAP signaling and Esophageal Squamous Carcinoma progression by an E3 ubiquitin ligase PARK2. Theranostics. 2020;10:9443-57
100. Toloczko A, Guo F, Yuen HF, Wen Q, Wood SA, Ong YS. et al. Deubiquitinating Enzyme USP9X Suppresses Tumor Growth via LATS Kinase and Core Components of the Hippo Pathway. Cancer Res. 2017;77:4921-33
101. Zhu C, Ji X, Zhang H, Zhou Q, Cao X, Tang M. et al. Deubiquitylase USP9X suppresses tumorigenesis by stabilizing large tumor suppressor kinase 2 (LATS2) in the Hippo pathway. J Biol Chem. 2018;293:1178-91
102. Zhang Q, Zhang Y, Parsels JD, Lohse I, Lawrence TS, Pasca di Magliano M. et al. Fbxw7 Deletion Accelerates Kras-Driven Pancreatic Tumorigenesis via Yap Accumulation. Neoplasia (New York, NY). 2016;18:666-73
103. Santoro R, Zanotto M, Simionato F, Zecchetto C, Merz V, Cavallini C. et al. Modulating TAK1 Expression Inhibits YAP and TAZ Oncogenic Functions in Pancreatic Cancer. Mol Cancer Ther. 2020;19:247-57
104. Zhang M, Zhao Y, Zhang Y, Wang D, Gu S, Feng W. et al. LncRNA UCA1 promotes migration and invasion in pancreatic cancer cells via the Hippo pathway. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1770-82
105. Lou C, Zhao J, Gu Y, Li Q, Tang S, Wu Y. et al. LINC01559 accelerates pancreatic cancer cell proliferation and migration through YAP-mediated pathway. J Cell Physiol. 2020;235:3928-38
106. Zhou Y, Shan T, Ding W, Hua Z, Shen Y, Lu Z. et al. Study on mechanism about long noncoding RNA MALAT1 affecting pancreatic cancer by regulating Hippo-YAP signaling. J Cell Physiol. 2018;233:5805-14
107. Chen W, Wang H, Liu Y, Xu W, Ling C, Li Y. et al. Linc-RoR promotes proliferation, migration, and invasion via the Hippo/YAP pathway in pancreatic cancer cells. J Cell Biochem. 2020;121:632-41
108. Pobbati AV, Hong W. A combat with the YAP/TAZ-TEAD oncoproteins for cancer therapy. Theranostics. 2020;10:3622-35
109. Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA. et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300-5
110. Wei H, Wang F, Wang Y, Li T, Xiu P, Zhong J. et al. Verteporfin suppresses cell survival, angiogenesis and vasculogenic mimicry of pancreatic ductal adenocarcinoma via disrupting the YAP-TEAD complex. Cancer Sci. 2017;108:478-87
111. Zhao X, Wang X, Fang L, Lan C, Zheng X, Wang Y. et al. A combinatorial strategy using YAP and pan-RAF inhibitors for treating KRAS-mutant pancreatic cancer. Cancer Lett. 2017;402:61-70
112. Donohue E, Thomas A, Maurer N, Manisali I, Zeisser-Labouebe M, Zisman N. et al. The autophagy inhibitor verteporfin moderately enhances the antitumor activity of gemcitabine in a pancreatic ductal adenocarcinoma model. J Cancer. 2013;4:585-96
113. Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292:1160-4
114. Rozengurt E, Eibl G. Central role of Yes-associated protein and WW-domain-containing transcriptional co-activator with PDZ-binding motif in pancreatic cancer development. World J Gastroenterol. 2019;25:1797-816
115. Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S. et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357-66
116. Mohammed A, Qians L, Janakiram NB, Lightfoot S, Steele VE, Rao CV. Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48Cre/+ LSL-KrasG12D/+ mice. Int J Cancer. 2012;131:1951-62
117. Hao F, Xu Q, Wang J, Yu S, Chang HH, Sinnett-Smith J. et al. Lipophilic statins inhibit YAP nuclear localization, co-activator activity and colony formation in pancreatic cancer cells and prevent the initial stages of pancreatic ductal adenocarcinoma in KrasG12D mice. PLoS One. 2019;14:e0216603
118. Gronich N, Rennert G. Beyond aspirin-cancer prevention with statins, metformin and bisphosphonates. Nat Rev Clin Oncol. 2013;10:625-42
119. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403
120. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J. et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167-74
121. Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304-5
122. Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482-8
123. Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905-12
124. Chang HH, Moro A, Chou CEN, Dawson DW, French S, Schmidt AI. et al. Metformin Decreases the Incidence of Pancreatic Ductal Adenocarcinoma Promoted by Diet-induced Obesity in the Conditional KrasG12D Mouse Model. Sci Rep. 2018;8:5899
125. Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539-45
126. Zanconato F, Battilana G, Forcato M, Filippi L, Azzolin L, Manfrin A. et al. Transcriptional addiction in cancer cells is mediated by YAP/TAZ through BRD4. Nat Med. 2018;24:1599-610
127. Dent P, Booth L, Roberts JL, Liu J, Poklepovic A, Lalani AS. et al. Neratinib inhibits Hippo/YAP signaling, reduces mutant K-RAS expression, and kills pancreatic and blood cancer cells. Oncogene. 2019;38:5890-904
128. Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X. et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166-80
Author contact
![]() Corresponding author: Ting Sun, Department of Clinical Laboratory, The First Affiliated Hospital of Zhengzhou University, No.1 Jianshe Road East, Zhengzhou 450052, China. E-mail: suntingedu.cn, Tel: +86 371-66913230.
Corresponding author: Ting Sun, Department of Clinical Laboratory, The First Affiliated Hospital of Zhengzhou University, No.1 Jianshe Road East, Zhengzhou 450052, China. E-mail: suntingedu.cn, Tel: +86 371-66913230.
 Global reach, higher impact
Global reach, higher impact