13.3
Impact Factor
Theranostics 2021; 11(3):1513-1526. doi:10.7150/thno.53459 This issue Cite
Review
Recent advances in supramolecular antidotes
1. State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Taipa, Macau, China.
2. Green Catalysis Center, College of Chemistry, Zhengzhou University, 100 Kexue Road, Zhengzhou 450001, China.
3. Aix Marseille Univ, CNRS, ICR, Marseille, France.
*These authors contributed equally to this work.
Received 2020-9-19; Accepted 2020-10-27; Published 2021-1-1
Abstract
Poisons always have fascinated humankind. Initially considered as deleterious or hazardous substances, the modern era has witnessed the controlled utilization of dangerous poisons in medicine and cosmetics. Simultaneously, antidotes have become crucial as reversal agents to counteract the effects of a poison, and they are also used today to positively cancel the benefits of a poison after use. Currently, the majority of poisons are composed of small molecules. This review focuses on recent developments to reverse or prevent toxic effects of poisons by encapsulation in host molecules. Cyclodextrins, cucurbiturils, acyclic cucurbituril derivatives, calixarenes, and pillararenes, have been reported to largely impact the effects of toxic compounds, thus extending the current paradigm of small molecule antidotes by adding a new family of macrocyclic compounds to the current arsenal of antidotes. Along this line of research, endogenous "harmful" species are also sequestered by one or more of these supramolecular host molecules, expanding the potential of supramolecular antidotes to diverse therapeutic areas.
Keywords: Poisons, Macrocycles, Supramolecular Chemistry, Antidotes, Host-guest
Introduction
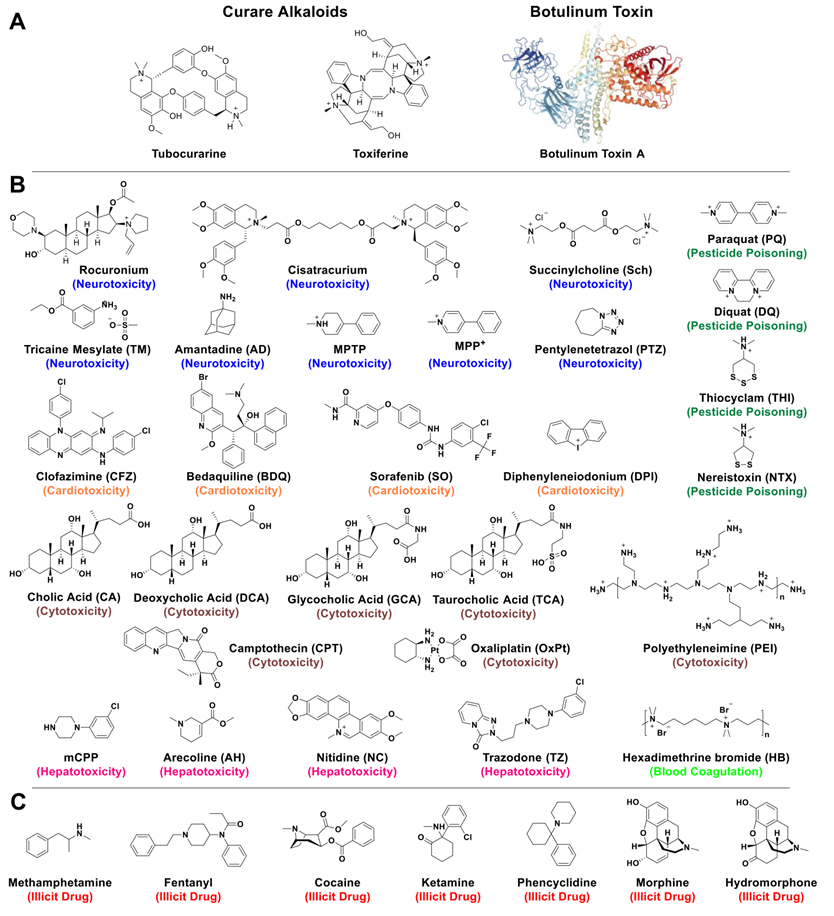
The utilization of poisons (compounds causing harm, injury or death) seems to have preceded the invention of writing (beginning of history) by at least 500 years [1]. With various applications including probable uses for hunting and social recognition/promotion within early human groups, poisons have then mostly been used for suicide, pest-control, and assassination since Antiquity and generally up to the Renaissance period. According to the World Health Organization, unintentional poisoning remains a problem for modern society, with several million people affected each year [2]. Yet, new forms of poisons have started to be widely applied for example as pesticides, key intermediates in the chemical industry, disinfectants, conservatives, or in medicine for instance in surgery (i. e. curare alkaloids [3], previously discovered and used by Amazonian Indians, Figure 1A) or in cosmetics (botulinum toxin [4], a neurotoxic protein that is also the most toxic substance known, Figure 1A). Excluding physical instances, poisons can be chemicals (ions or small molecules) or biologicals (peptides or proteins such as those found in animals' venoms).
In parallel, many antidotes (substances able to counter the effects of a poison or a disease) have been discovered with time (such as mithridate, an ancient antidote with complex formulation) and most lethal poisons today have antidotes, such as cyanide (antidote sodium nitrite or thiosulfate), opioids (antidote naloxone), lead (antidote the succimer chelator), heparin (antidote protamine sulfate) or the three medications methotrexate, trimethoprim and pyrimethamine sharing the same antidote: leucovorin. Modern approaches include the production of specific antibodies to counter the effects of venoms [5]. However, several poisons still do not have antidotes, such as the alkaloids aconitine (from acotinum plant sometimes used in medicine), or coniine, N-methylconiine and conhydrine (from hemlock plant), famous as the basis for the beverage forced to swallow and having caused the death of the Greek philosopher Socrates (399 BC). The glycoprotein ricin, besides its mention in several fictions, is one of the most toxic substances [6] for which there is no known antidote and that has seriously been considered as a warfare agent. As mentioned above, besides antibodies used as antidotes against venoms, most antidotes are small molecules.
Structures of toxic compounds discussed in this review. Examples of natural toxins (A) present in curares extracted from Amazonian plants or synthesized by bacteria like botulinum toxin. The initial clinical use of curare toxins as muscle relaxants has progressively been replaced by synthetic analogues like Rocuronium or Cisatracurium. Structures of toxic (B) and illicit (C) molecules discussed in this review.
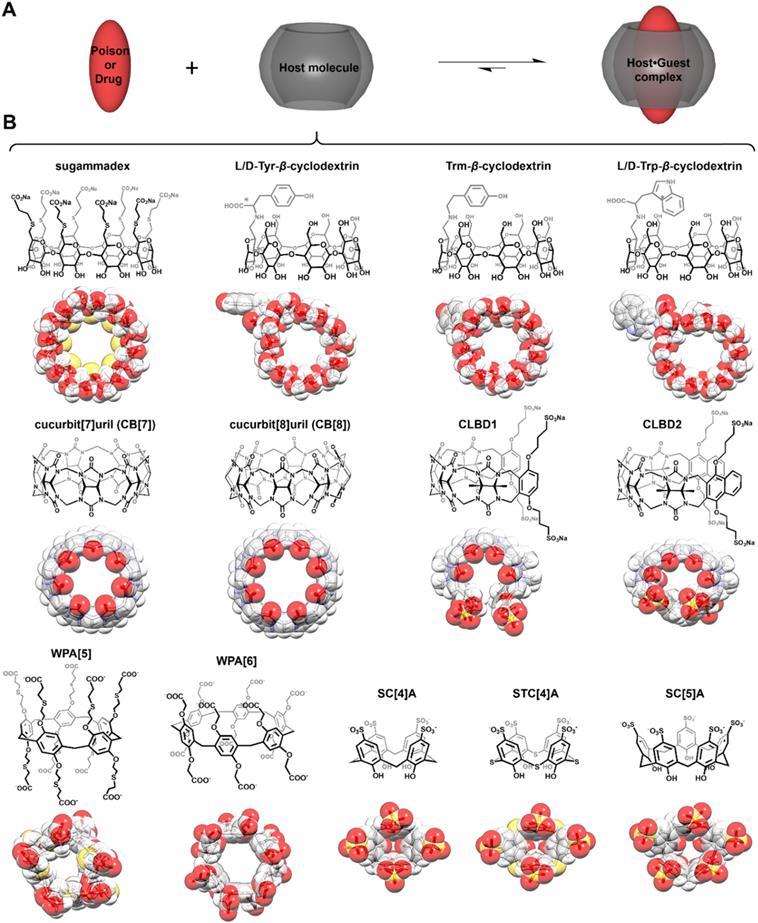
In the past 30 years, the Nobel Prize in Chemistry has been awarded twice to researchers focused on supramolecular systems, more specifically for their contributions toward “the development and use of molecules with structure-specific interactions of high selectivity” (1987) and for “the design and synthesis of molecular machines” (2016) [7]. For both research fields, macrocyclic compounds were central in the findings that have led to major breakthroughs. Yet, despite these significant advances, practical applications stemming from supramolecular chemistry remain relatively limited [8-11]. As perhaps one of the most famous families of macrocycles, cyclodextrins (artificial host molecules of natural origin) have started to be used in the pharmaceutical and cosmetic industries (Figure 2) [12-13]. In the meantime, supramolecular chemists have developed numerous other host molecules with various topologies, including some resembling crowns (crown ethers), cups (calix[n]arenes), pumpkins (cucurbit[n]urils), clips (acyclic cucurbit[n]uril congeners), or tubes (pillar[n]arenes, see Figure 2), n denoting the number of monomer repeat units, among others [14].
Structures of host molecules used as antidotes. Principle of poison (or drug) capture (A) by synthetic hosts (B). In panel B, cyclodextrins are on the top line, cucurbiturils and acyclic cucurbiturils in the middle one, and pillararenes and calixarenes are at the bottom.
Indeed, supramolecular systems have achieved preliminary success for biomedical applications [15-16]. For instance, some supramolecular polymers even reached the clinic [17-18]. Among a variety of supramolecular systems, macrocycles and related host molecules have been widely studied in biomedical applications due to their stable physical/chemical properties, batch-to-batch consistency, and relatively high biocompatibility [19]. Scientists have discovered that simple host-guest complexes can make good drug delivery systems, with marked advantages for some of them to alleviate or reverse side-effects or toxicities of drugs both in vitro and in vivo when these drugs are encapsulated by host molecules, thanks to their relatively strong host-guest interactions mediated via hydrogen-bonding, electrostatic interaction, and/or hydrophobic interactions [19-21]. Indeed, the toxicity or unforeseen side-effects of biologically active molecules can limit their clinical applicability or cause the interruption of a drug development program. With the rapid growth of the number of reported host molecules [22-24], many macrocycles have shown interesting binding properties toward bioactive compounds, and some of them, good inhibition/reversal effects against toxic compounds and drugs exhibiting adverse side-effects, although there has been only one commercial success (Sugammadex) in the development of host molecules as clinically approved antidotes [20-21, 25]. However, the potential of host molecules as promising antidotes that have been extensively investigated in vitro and in vivo has never been summarized. In this context, host molecules having shown inhibition or reversal of biological properties for a given compound are the objects of the present review with highlights on the most promising host molecules used as antidotes, and on products that are now on the market.
Cyclodextrins
Cyclodextrins (CDs, Figure 2B) are considered as one of the oldest known families of host molecules, and their discovery over a century ago predates the establishment of supramolecular chemistry as a scientific discipline. CDs are sugar-based cyclic oligomers made from the bacterial degradation of starch and have a versatile hydrophobic cavity amenable for binding a plethora of guest molecules [26]. The excellent biocompatibility and the ability to enhance solubility of insoluble drugs via host-guest interactions have made CDs as one of the most widely applied excipients in pharmaceutics [27]. However, chemically functionalized CDs may bind strongly with toxic species, thereby exhibiting reversal effects.
Reversal of neuromuscular blocking agents
Even if CDs are usually considered as non-toxic, they did not attract much attention as an antidote until Zhang and co-workers used a γ-CD derivative (sugammadex, Figure 2B) to reverse the effects of neuromuscular blocking agents (NMBAs) in 2002 (Figure 3) [28-29].
Proposed mechanism for the recovery of natural partners of nAChR membrane proteins by sugammadex. Schematic representation of the mechanism of sugammadex as a supramolecular antidote to reverse NMBAs-induced neuromuscular blocking effects by competitive binding.
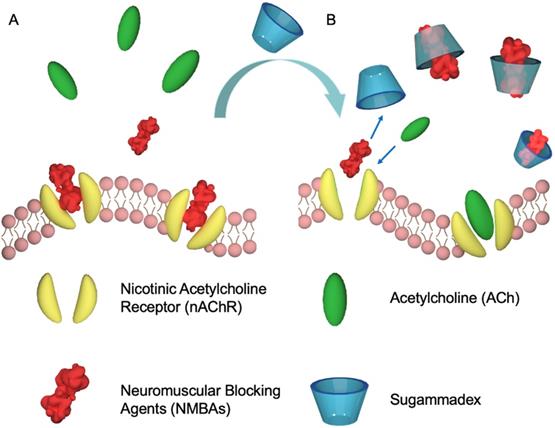
NMBAs are mono- and most often di-quaternary ammonium compounds derived from curare or malouetine (like rocuronium or cisatracurium, Figure 1), binding post-synaptic acetylcholine receptors and causing paralysis of skeletal muscles [30]. They are often used to complement anesthesia. With a high binding affinity Ka of (1.05±0.16)×107 M-1 between sugammadex and rocuronium, the host compound could efficiently reverse the rocuronium-induced neuromuscular blocking effect in vivo, that has paved the way to the development of supramolecular antidotes [28]. The success of this approach was soon followed by subsequent studies, further establishing sugammadex as an effective antidote [31]. Finally, sugammadex, with the trade name of Bridion®, has been developed and commercialized by Organon, which was acquired by Schering-Plough and subsequently merged with Merck. Despite this success, sugammadex is not without shortcomings. Its approval by the U.S. Food and Drug Administration (FDA) has been delayed due to its association with anaphylaxis [32]. In addition, sugammadex-induced bradycardia and asystole cannot be ignored [33]. These side-effects could be due to unspecific binding and hence, new host molecules with better affinities would be interesting to test.
Clearance of bile acids
Liu and co-workers studied the binding of bile acids (BAs) such as cholic acid (CA), deoxycholic acid (DCA), glycocholic acid (GCA), and taurocholic acid (TCA, Figure 1B), with L/D-tyrosine-modified β-CDs (L/D-Tyr-β-CDs) or L/D-tryptophan-modified β-CDs (L/D-Trp-β-CDs, Figure 2B) and strong affinities were observed in most cases [34]. However, Liu et al. postulated that the -COOH groups on both host and guest molecules could have hindered an efficient recognition between these β-CD derivatives and BAs due to electrostatic repulsions between carboxylate groups. They thus synthesized a tyramine-modified β-CD (Trm-β-CD) to verify their assumption [35]. In general, this new host has shown stronger affinities than L/D-Tyr-β-CDs and L/D-Trp-β-CDs toward BAs. Both in vitro (HT-29 and HCT-116 cell lines) and in vivo (mice) studies showed that Trm-β-CD can reverse the cytotoxicity of DCA and accelerate the clearance of blood DCA, suggesting a promising future for this host with respect to intrahepatic cholestasis and other BA-related diseases.
Calixarenes
Calix[n]arenes (C[n]As) were named like this on account of their shape, resembling that of a chalice or grail (Figure 2B) [36]. These host molecules have been studied for decades and many accomplishments have been achieved in various research areas [14]. However, the poor water solubility and relatively high toxicity of these compounds have limited their application in biomedical sciences [37]. Despite these drawbacks, scientists have developed several methods to facilitate the use of C[n]As in the biomedical field. The most promising strategy turned to be the synthesis of water soluble and less toxic C[n]A derivatives: p-sulfonatocalix[n]arenes (SC[n]As, n = 4-8, Figure 2B) [37].
Antidote for pesticide poisoning
Liu and co-authors have studied the recognition of the pesticide paraquat (PQ, Figure 1B) by SC[n]A (n = 4, 5) in several phosphate buffers (pH = 2.0, 7.2, and 12.0) [38]. PQ forms a stable complex with SC[4]A (binding constant Ka ≈ 104 M-1 at all pH), while the corresponding value for the PQ•SC[5]A complex (Ka ≈ 103 M-1) raised to 105 M-1 when the pH was increased from 2.0 to 12.0 [38]. Later, the potential of SC[4]A, SC[5]A, and a newly introduced p-sulfonatothiacalix[4]arene (STC[4]A) was studied as host compounds for the treatment of viologens poisoning using paraquat and diquat as poisons [20]. Upon encapsulation, the transformation of oxygen to superoxide (O2●―) was reduced, thereby inhibiting the generation of hydroxyl radicals (HO●), and decreasing the toxicity of viologens. Subsequently, the PQ•SC[4]A complex was selected for an in vivo pharmacokinetic study involving oral administration [39]. Compared to the group of rats treated with PQ alone, the group treated with PQ•SC[4]A showed lower PQ concentrations in plasma (Figure 4).
Plasma concentration of PQ against time after oral administrations of PQ, inclusion complex, and treatment with C[4]AS 30 min after PQ poisoning. Reprinted from ref. [39]. Copyright 2011 American Chemical Society.
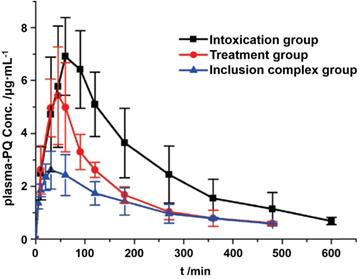
These results were supported and tentatively explained by an in vitro intestinal absorption study, that showed an inhibition of PQ absorption by the intestines, when SC[4]A was present [39]. SC[n]A macrocycles are further chemically tunable and could thus be another family of supramolecular antidotes in the near future.
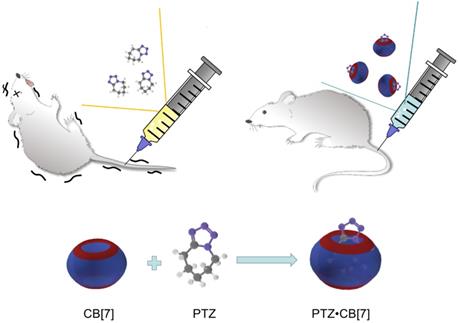
Scheme showing the reduction of neurotoxicity of PTZ administered with CB[7] in mice.
Antidote of heparin
Unfractionated heparin (UFH) is widely used in clinical practice as an anticoagulant agent. However, when overdosed or administered in sensitive populations, UFH may cause several side effects including excessive bleeding [40]. The only clinically available UFH neutralization agent, protamine, has several risks as well associated with its use, including allergic reactions in a large sub-population of patients [40]. Hence, it is essential to develop novel UFH neutralizers to reverse heparinization in an effective and safe manner, as a replacement or supplementary of protamine. Very recently, a biocompatible oligoethylene glycol functionalized guanidinecalixarene (GC[4]AOEG), which binds with UFH with a high binding affinity (107 M-1), was designed as a supramolecular antidote against UFH. In vivo tests in three mouse bleeding models showed that GC[4]AOEG can effectively alleviate excessive bleeding induced by UFH with an excellent safety profile, indicating a great potential of GC[4]AOEG for clinical translation as a UFH neutralizer [40].
Cucurbiturils
Cucurbit[6]uril had received only modest attention until the groups of Kim and Day successfully isolated several homologues (cucurbit[n]urils: CB[n]s, n = 5-8, Figure 2B) forming in 2000 a new family of host molecules and opening new avenues for macrocyclic chemistry [41-51]. Like CDs, these synthetic macrocycles are usually considered as relatively non-toxic compounds [52-55]. In 2004 [56] and 2005 [57], two seminal papers have reported the encapsulation of platinum drugs in CB[7] as an approach to reduce their toxicity. In 2011, Gilson and co-authors found that CB[7] could tightly bind amantadine (AD, Figure 1B, an antiviral medication also used against Parkinson's disease; Ka = 1.7±0.8×1014 M-1) [58]. This discovery demonstrated that CB[7] could be employed as a latent competitor against natural receptors for the binding of small molecules. In parallel, the Wang's group has systemically studied a series of cases for which CB[7] was used as a potential antidote or toxicity-inhibitory agent. In particular, CB[7] was found to have a significant potential as a neural toxicity reversal agent [59-61].
Alleviation of neurotoxicity
CB[7] was found to accelerate the recovery of zebrafish that had been anaesthetized by tricaine mesylate (TM, Figure 1B), an FDA-approved general anesthetic [59]. In addition, CB[7] was found to inhibit the neurotoxicities of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, Figure 1B), a neurotoxin that is used to construct Parkinson's disease models, and its active metabolite N-methyl-4-phenylpyridine (MPP+) in a study relying on zebrafish [60]. CB[7] was then shown to reduce the toxicity of pentylenetetrazol (PTZ, Figure 1B), a neurotoxin inducing seizure, on zebrafish and mice (Figure 5) [61].
Results obtained with these models are presumably due to the relatively strong binding affinities between CB[7] and the neurotoxic compounds. Indeed, CB[7] forms 1:1 host-guest complexes with TM, MPTP, MPP+, and PTZ, with relatively high binding constants of the order of ~105 M-1, starting to make CB[7] a good competitor impeding the binding between neurotoxins and their natural receptors [59-61]. Following the reversal of neurotoxicities by CB[7], the ability of this macrocycle to reverse or inhibit other toxicities has been evaluated.
Alleviation of cardiotoxicity
Cardiotoxicity is one of the high-risk side effects of bioactive compounds, often inducing cardiac dysfunctions. In this context, CB[n]s pleasingly showed good efficacy in alleviating cardiotoxicities of several bioactive molecules [62-65]. For instance, clofazimine (CFZ, Figure 1B) is a drug that was developed for the treatment of tuberculosis but suffers from cardiotoxicity and poor solubility in water [62]. After encapsulation in CB[7] (Ka ≈ 104~105 M-1), the water solubility of CFZ increased in both acidic and neutral media, and in vitro and in vivo tests have shown drops of CFZ cardiotoxicity without affecting its antimycobacterial activity [62]. Bedaquiline (BDQ, Figure 1B), another anti-tuberculosis drug, is also prone to the same issues. The solubility of BDQ increased with CB[7] by a factor of 0.27-fold of the concentration of CB[7]. Meanwhile, in vivo tests including a set of physiological parameters for cardiac functions showed that the cardiotoxicity of BDQ was dramatically decreased in the presence of CB[7] [63]. Sorafenib (SO, Figure 1B) is a small-molecule kinase inhibitor (SMKI) that is widely used in the treatment of various cancers [64], but is also characterized by a significant cardiotoxicity. Relatively high affinity between SO and CB[7] (Ka = (2.87 ± 0.13)×105 M-1) enabled this host to decrease SO cardiotoxicity without affecting the desired activity, as confirmed in vitro (with SMMC7721 cell lines) and in vivo (with zebrafish models) [64]. Diphenyleneiodonium (DPI, Figure 1B) is an uncompetitive inhibitor of flavoenzymes able to reduce the activity of NADPH oxidase, xanthine oxidase, and nitric oxide synthase [65]. However, a latent cardiotoxicity of DPI presents risks when used in vivo. Both CB[7] and CB[8] form stable complexes with DPI in aqueous solution, CB[7] being singly complexed and CB[8] doubly bound (Ka DPI•CB[7] = (3.13±0.16)×104 M-1, and Ka DPI•CB[8] = 2.26×1012 M-2), but CB[7] showed better performance in reducing the cardiotoxicity of DPI, likely due to its better solubility compared to that of CB[8] [65].
Alleviation of hepatotoxicity
Natural products are an important source of new medicines, but their hepatotoxicities can present serious concerns. For instance, arecoline (AH, Figure 1B) is an active compound extracted from Areca nut that has been studied for the treatment of several neurological disorders, but also relatively to the cardiovascular and digestive systems. However, AH can show severe hepatotoxic effects [66] so CB[7] was tested as a supramolecular antidote to minimize its hepatotoxicity. The association between AH and CB[7] was first studied and has shown formation of a 1:1 complex with a binding affinity Ka of (1.21±0.12)×103 M-1. Subsequent work demonstrated a reduction of the hepatic toxicity of AH in vitro based on tests performed on L02 cell lines [66]. Nitidine chloride (NC, Figure 1B) is a natural alkaloid extracted from the root of Zanthoxylum nitidum [67] and a promising drug candidate against cancer, but further studies were impeded by its hepatic toxicity. A novel formulation of NC with CB[7] was tested, enabling to boost its anti-cancer activity (IC50 = 2.94±0.15 μM compared to 7.28±0.36 μM for free NC) [67] while reducing its hepatotoxicity (IC50 = 3.48±0.49 μM), by a factor of ~2 (IC50 = 6.87±0.80 μM for free NC) in vitro. Trazodone (TZ, Figure 1B) is an FDA-approved drug that has been developed for the treatment of depression. However, its active metabolite, m-chlorophenylpiperazine (mCPP), has been associated with hepatotoxicity. An in vitro study showed that CB[7] could complex these two compounds and significantly decrease the hepatotoxicities caused by TZ and mCPP [68]. These results were confirmed in vivo highlighting the potential of this formulation for clinical applications [68].
Alleviation of general cytotoxicity
The administration of anti-cancer drugs is often accompanied by unexpected side-effects induced by their cytotoxicity. Camptothecin (CPT, Figure 1B) is a potent anti-cancer agent used to treat various cancers [69] but it can be converted into an active lactone form and a toxic carboxylate analogue, thereby limiting its applicability. Studies have shown that the structural changes of CPT can be limited by formation of supramolecular complexes with CB[7]. Both in vitro and in vivo investigations have shown that the presence of CB[7] could alleviate the non-specific toxicity of CPT [69]. Zhang and co-authors have studied a supramolecular chemotherapy based on the formation of complexes between the anti-cancer drug oxaliplatin (OxPt, Figure 1B) and CB[7, 57, 70]. Their study has demonstrated that the presence of CB[7] could significantly reduce the cytotoxicity of OxPt in vitro (on healthy colorectal NCM460 cells), and improve its anti-cancer activity by competitive binding with spermine which is overexpressed in some cancer cells such as HCT116 or HT-29 cells.
Besides common drugs, the toxicity of polymers can also be a serious source of concern. Polyethylenimine (PEI, Figure 1B) has been studied as a gene delivery vehicle [71]. However, the applicability of high molecular weight PEI (branched, 25 kDa) that have shown better gene delivery, is limited by their non-specific cytotoxicity. In this context, CB[7] was shown to significantly decrease the cytotoxicity of PEI most likely by multiple host binding [71]. Likewise, the supramolecular shielding strategy works also well with hexadimethrine bromide (HB, or polybrene Figure 1), a polycation that can neutralize heparin and control internal bleeding. However, its use can induce severe cases of blood coagulation raising major concerns for the lives of patients [72]. CB[7] can complex each repeating unit of HB with a binding affinity Ka of (1.04±0.19)×107 M-1. Both in vitro and in vivo tests showed that coagulation effects induced by HB can be significantly decreased by this supramolecular encapsulation strategy [72].
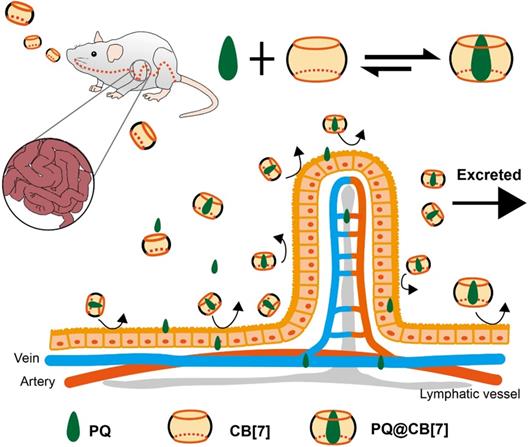
Proposed function of CB[7] as treatment in PQ detoxification. CB[7] is orally administered after PQ ingestion. In the stomach or intestine, PQ is trapped by CB[7], preventing further damages to the intestines and reducing the absorption and tissue distribution of PQ. Most of the pesticide will be excreted as PQ•CB[7] complexes. Reprinted from ref. [74]. Copyright 2019 Ivyspring.
Antidote for pesticide poisoning
The extensive utilization of pesticides in agriculture is a significant source of pollution and can cause severe harm to ecosystems or humans. For instance, the pesticide nereistoxin (NTX, Figure 1B) and its derivative thiocyclam (THI, Figure 1B) have shown non-selective teratogenic toxicities, tested for alleviation by formation of supramolecular complexes with CB[7] [73]. Relatively high binding constants of (1.4±0.15)×105 M-1 and (7.46±0.10)×105 M-1 for NTX and THI respectively, enabled assessing antidotal effects. In vivo tests performed on zebrafish embryos and larvae showed that CB[7] significantly constrained the teratogenicity of NTX and THI [73]. Another example is that of the toxic and famous pesticide paraquat (PQ), still responsible for serious troubles in public health [74]. Recently, the Wang's group has studied the antidotal effect of CB[7] on PQ toxicity both in vitro and in vivo (Figure 6) [74]. While a reduction of PQ's toxicity was noted previously in studies focused on PQ•CB[7] complexes as anti-cancer agents (Ka ~ 105 M-1) [75], the Wang's group developed a new method to reverse PQ poisoning [74]. This work showed that CB[7] could significantly decrease PQ levels in plasma and major organs, and alleviate their adverse effects via oral administration. In addition, oral administration of CB[7] within 2 hours post-PQ ingestion could improve the survival rates of mice and extend their survival time, outperforming activated charcoal that is usually used for the treatment of PQ poisoning [74]. Finally, with the antidotal effect of CB[7], the PQ•CB[7] complex can also potentially be used as a safer herbicide [76].
Acyclic cucurbiturils
One of the key advantages of CB[n]s is their rigid structures, but this can also limit their use in certain cases. Isaacs and co-authors have developed a series of flexible, clip-like CB[n]s known as acyclic cucurbit[n]uril-type congeners (acyclic CB[n]) [77]. Calabadion 1 and calabadion 2 (abbreviated CLBD1 and CLBD2, respectively, see Figure 2B) have a flexible C-shape structure that can grasp many guest molecules in water. These acyclic CB[n]s have shown good biocompatibility and strong binding affinities toward a wide range of molecules [25, 77-78], including NMBAs such as rocuronium and cisatracurium [25, 79-80].
Reversal of neuromuscular blocking agents
In vivo studies have revealed that CLBD1 could accelerate the recovery of spontaneous breathing, and reduce the time required to reach a train-to-four ratio of 0.9 for rats (given rocuronium and cisatracurium), from minutes to seconds, outperforming the recommended neostigmine/glycopyrrolate treatment [79]. On the other hand, rocuronium was shown to be 89-fold better complexed by CLBD2 (Ka = 3.4×109 M-1) than by sugammadex (Ka = 3.8×107 M-1). Accordingly, CLBD2 provided a better reversal effect than sugammadex in vivo [80].
Reversal of toxicity of illicit drugs
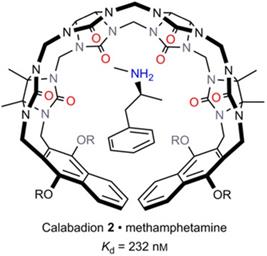
More recently, Isaacs and co-authors further explored the potential of acyclic CB[n] derivatives as supramolecular antidotes. The complexation of various illicit drugs (methamphetamine, fentanyl, cocaine, ketamine, phencyclidine, morphine and hydromorphone, Figure 1B) by CLBD1, CLBD2, and other host molecules (CB[7], C[4]AS and HP-β-CD) was investigated. Among all hosts, CLBD2 showed a good binding affinity for methamphetamine (Ka = (4.3±1.0)×106 M-1, Figure 7), enabling to reverse the hyperlocomotive activity of rats treated with methamphetamine [81].
Capture of methamphetamine by CLBD2. Reprinted from ref. [81]. Copyright 2017 John Wiley & Sons, Inc.
Pillararenes
As one of the latest series of host molecules discovered, pillar[n]arenes (PA[n]s) have experienced a rapid development during the past decade [82-85]. Many applications of PA[n]s have been reported. However, as is the case for most host molecules, water solubility was a key barrier impeding their biological applications. In 2012, Huang and co-workers prepared a water soluble pillar[6]arene (WPA[6], Figure 2B) with an excellent potential for biological applications [86]. The strong binding affinity of WPA[6] for PQ (Ka = (1.02±0.10)×108 M-1) suggested that WPA[6] could be a potent antidote [87].
Alleviation of cytotoxicity
This macrocycle was so used in a supramolecular chemotherapy approach to decrease the cytotoxicity of OxPt while improving its anti-cancer activity. The good binding affinity between WPA[6] and OxPt in phosphate buffer at pH 7.4 and 37.0 °C enabled to decrease the cytotoxicity of OxPt [88-89].
Reversal of neuromuscular blocking agents
Succinylcholine (Sch, Figure 1) is the only depolarizing NMBA that is widely used for rapid sequence induction in emergency rooms [90]. However, its use can be accompanied by severe side-effects, such as hyperkalemia and cardiac arrest. Sch can be complexed by SC[4]A, CB[7] and WPA[6] in vitro with respective association constants Ka Sch•C[4]AS ~ 104 M-1, Ka Sch•CB[7] ~ 106 M-1 and Ka Sch•WPA[6] ≈ 3.4×106 M-1. Among these hosts, WPA[6] exhibited excellent antidotal effects. In vivo studies showed that the encapsulation of Sch by WPA[6] reduced the guest side-effects', such as the incidence of cardiac arrhythmia, high serum potassium levels, and muscular damage [90]. Inspired by the success of sugammadex, Stoikov and co-workers synthesized a water soluble PA[5], namely WPA[5] (Figure 2B), containing similar side-chains as those of sugammadex, and aimed at achieving a better reversal effect for the use of rocuronium bromide. Although WPA[5] required a longer time than enabled by sugammadex for the recovery of muscle contraction after restriction by rocuronium, it still showed a great potential as a new kind of antidote [91]. The main properties of macrocycles as antidotes are summarized in Table 1.
Supramolecular therapeutics via sequestration of endogenous species
All of these above-discussed examples are about sequestration of exogenous toxic/harmful species and counteracting their toxicities via supramolecular encapsulation. Indeed, very often some diseases are induced by, or are featured with, a high concentration of harmful endogenous species or biomarkers, and elimination of these species may inhibit the disease progression. Thus, along the line of “supramolecular antidote” research, efficient sequestration or “trap” of disease-related endogenous species may provide a promising therapeutic strategy for various diseases.
Supramolecular “trap” of spermine for cancer treatment
Polyamines, including spermine (SPM), spermidine (SPD), and putrescine (PUT), exist in a wide range of living organisms and are essential for cell proliferation and differentiation. Therefore, elimination of free polyamines in cancer cells has become a potential approach to induce cancer cell apoptosis to improve cancer treatment. For instance, Zhang et al. have designed a series of macrocycles-based drug delivery systems, where chemotherapeutic agents get released via competitive binding of SPM to the host molecules [70, 75, 88, 92]. The first two CB[7]-based supramolecular drug delivery systems, PQ-CB[7] [75] and OxPt-CB[7] [70], showed enhanced anti-cancer activity due to the synergetic effect of controlled drug release and capture of SPM. Subsequently, a CB[7] based polyethylene glycol (PEG) was designed as a platform for drug delivery aiming at long systemic circulation while preserving all the benefits of the previously reported supramolecular drug delivery systems [92]. Additionally, WPA[6] was investigated as a supramolecular drug carrier, similar to CB[7] [88]. The OxPt-WPA[6] drug delivery system, with a large difference between the binding affinities of SPM-WAP[6] and OxPt-WPA[6], showed much better anti-cancer activity than those based on CB[7]. Very recently, Li et al. synthesized a peptide-PA[5] conjugate (denoted as P1PA[5], where P1 refers to RGDSK(N3)EEEE) as a supramolecular trap for efficient elimination of free polyamines in cancer cells [93]. P1PA[5] exhibited high binding affinities toward a wide range of polyamines (Ka of (3.98±0.68)×105 M-1, (1.45±0.14)×105 M-1, and (2.32±0.68)×106 M-1 for SPM, SPD, and PUT, respectively). The efficient, specific cell penetration of the host molecule and the subsequent “trap” of free polyamines in cells by P1PA[5] yielded good anti-cancer activities both in vitro and in vivo.
Effects of supramolecular antidotes toward toxic compounds
| Molecule | Toxicity | Antidote | Effect of Antidote | Binding Affinity [Solvent/pH/Temperature (°C)] | Ref. |
|---|---|---|---|---|---|
| Rocuronium | Neurotoxicity | Sugammadex | Accelerate recovery from anaesthesia | (1.05±0.16)×107 M-1 [50 mM PBS/7.0/25] | [28] |
| CLBD1 | Accelerate recovery from anaesthesia | (8.4±0.9)×106 M-1 [N.A./N.A./N.A.] | [79] | ||
| CLBD2 | Accelerate recovery from anaesthesia | 3.4×109 M-1 [N.A./7.4/25] | [80] | ||
| WPA[5] | Accelerate recovery from anaesthesia | 4.5×103 M-1 [H2O/N.A./19.85] | [91] | ||
| DCA | Cytotoxicity | Trm-β-CD | Decrease the cytotoxicity and accelerate the clearance of blood DCA | (1.57±0.07) ×104 M-1 [3% DMSO-PBS/N.A./25] | [35] |
| PQ | Pesticide Poisoning | C[4]AS | Alleviate poisoning | ~104 M-1 [PBS/N.A./25] | [39] |
| C[5]AS | Alleviate poisoning | ~103 M-1 to 105 M-1 [PBS/2.0-12.0/25] | [20] | ||
| CB[7] | Alleviate poisoning | ~105 M-1 [HCl-PBS/1.2-7.4/25] | [74] | ||
| WPA[6] | Alleviate cytotoxicity | (1.02±0.10)×108 M-1 [H2O/N.A./R.T.] | [87] | ||
| UFH | Complex side effects | GC[4]AOEG | Reverse heparinization | (1.25±0.13) ×107 M-1 [HEPES/7.4/25] | [40] |
| TM | Neurotoxicity | CB[7] | Accelerate recovery from anaesthesia | (8.0±0.5)×104 M-1 [E3/7.2/25] | [59] |
| MPTP | Neurotoxicity | CB[7] | Inhibit neurodegeneration | (4.8±0.2)×104 M-1 [10 mM PBS/7.4/25] | [60] |
| MPP+ | Neurotoxicity | CB[7] | Inhibit neurodegeneration | (1.05±0.05)×105 M-1 [10 mM PBS/7.4/25] | [60] |
| PTZ | Neurotoxicity | CB[7] | Inhibit PTZ induced seizure | (1.94±0.11)×105 M-1 [H2O/N.A./25] | [61] |
| CFZ | Cardiotoxicity | CB[7] | Decrease cardiotoxicity | 104~105 M-1 [HCl-H2O/2.0/25] | [62] |
| BDQ | Cardiotoxicity | CB[7] | Decrease cardiotoxicity | 3.98×103 M-1 [CH3CN/Neutral/25] | [63] |
| SO | Cardiotoxicity | CB[7] | Decrease cardiotoxicity | (2.87±0.13)×105 M-1 [D2O/7/25] | [64] |
| DPI | Cardiotoxicity | CB[7] | Decrease cardiotoxicity | (3.13±0.16)×104 M-1 [H2O/Neutral/25] | [65] |
| CB[8] | Decrease cardiotoxicity | 2.26×1012 M-2 [H2O/Neutral/25] | [65] | ||
| AH | Hepatotoxicity | CB[7] | Alleviate hepatotoxicity | (1.21±0.12)×103 M-1 [H2O/Neutral/25] | [66] |
| NC | Hepatotoxicity | CB[7] | Alleviate hepatotoxicity | N.A. | [67] |
| TZ | Hepatotoxicity | CB[7] | Alleviate hepatotoxicity | (1.50±0.13)×106 M-1 [H2O/Neutral/25] | [68] |
| mCPP | Hepatotoxicity | CB[7] | Alleviate hepatotoxicity | (6.90±0.49)×105 M-1 [H2O/2.4/25] | [68] |
| CPT | Cytotoxicity | CB[7] | Alleviate non-specific toxicity | 9.5×107 M-2 [DMSO-H2O/Neutral/N.A.] | [69] |
| OxPt | Cytotoxicity | CB[7] | Reduce cytotoxicity | 2.89×106 M-1 [20 mM PBS/6.0/37] | [70] |
| WPA[6] | Reduce cytotoxicity | 1.66×104 M-1 [20 mM PBS/7.4/37] | [88] | ||
| PEI | Cytotoxicity | CB[7] | Decrease cytotoxicity | (1.03±0.19)×105 M-1 per repeating unit [H2O/Neutral/25] | [71] |
| HB | Blood coagulation | CB[7] | Decrease coagulation effects | (1.04±0.19)×107 M-1 per repeating unit [H2O/7.4/25] | [72] |
| NTX | Non-selective teratogenic toxicity | CB[7] | Alleviate teratogenicity | (1.4±0.15)×105 M-1 [H2O/Neutral/25] | [73] |
| THI | Non-selective teratogenic toxicity | CB[7] | Alleviate teratogenicity | (7.46±0.10)×105 M-1 [H2O/Neutral/25] | [73] |
| Cisatracurium | Neurotoxicity | CLBD1 | Accelerate recovery from anaesthesia | (9.7±0.8)×105 M-1 [N.A./N.A./N.A.] | [79] |
| Methamphetamine | Illicit drug | CLBD2 | Reverse the hyperlocomotive activity | (4.3±1.0)×106 M-1 [20 mM PBS/7.4/25] | [81] |
| Sch | Neurotoxicity | WPA[6] | Reduce side-effects | 3.42×106 M-1 [PBS/7.4/25] | [90] |
Note: N.A. “not available”. PBS: phosphate buffer saline. R.T.: room temperature. HEPES: 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid buffer.
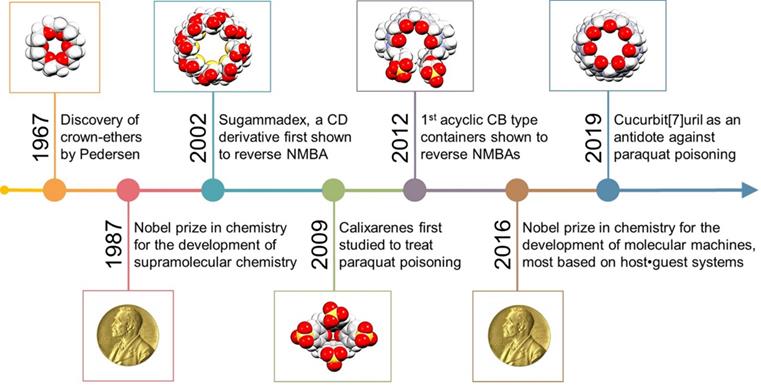
Timeline highlighting selected key developments in supramolecular chemistry and prime macrocyclic antidotes in the fight against poisons.
Supramolecular sequestration of cholesterol for treatment of atherosclerosis and NPC disease
Cholesterol plays a vital role in the progression of atherosclerosis (among several other diseases), since cholesterol is a major component of atherosclerotic plagues and its accumulation and deposition triggers a complex inflammatory response to further promote atherosclerosis. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD), an FDA-approved excipient to enhance the solubility of numerous lipophilic agents, was found to enhance the solubility of cholesterol [94]. In fact, HP-β-CD not only increases the solubility of cholesterol to reduce the formation of cholesterol crystals, but also enhances the production of water-soluble oxysterol to finally realize the anti-inflammatory effects after a complex intracellular metabolism process. As a result, HP-β-CD showed good therapeutic effects toward atherosclerosis in vivo [94]. However, the poor pharmacokinetics and ototoxicity of HP-β-CD still limit its clinical translation for atherosclerosis treatment. Recently, a β-CD based polymer (β-CDP) was designed to overcome these disadvantages of HP-β-CD, by showing a safer profile and better atherosclerosis treatment effects than HP-β-CD, ascribed to the enhanced pharmacokinetics in vivo [95].
In general, there are two metabolism pathways of cholesterol after cellular uptake: 1) transportation from lysosome to endoplasmic reticulum with the assistance of Niemann-Pick type C1/C2 (NPC1/NPC2) transporter, and transformed into cholesteryl esters; 2) metabolized into water-soluble oxysterols [94]. The mutations of NPC1 or NPC2 may induce the accumulation of cholesterol in lysosomes and further influence subsequent biological processes, which is known as Niemann-Pick disease type C (often known as NPC disease). NPC causes hepatosplenomegaly, neonatal cholestatic jaundice, splenomegaly, and even death [96]. With the same action mechanism to elimination of cholesterol for atherosclerosis treatment [94], HP-β-CD has emerged as a potential agent for the treatment of NPC. Both in vitro [97], and in vivo studies (including clinical trials) [96] have demonstrated the efficacy of HP-β-CD for NPC treatment with a decent safety profile, providing the only therapeutic hope for NPC patients, and thus far only supramolecular medicine shows the charm.
Conclusion, Challenges and Perspectives
In summary, we believe that the macrocyclic chemistry has entered a gold era. The number of host compounds reported has never been so high and researchers are now strongly interested in testing rapidly new macrocycles for an also ever-increasing number of applications. Starting with sugammadex, which is the initial host that has been developed to reverse the effects of NMBAs (Figure 8), other host molecules have started to be studied for their inhibition/reversal effects toward bioactive compounds.
As the sole clinically and commercially successful case and as a supramolecular antidote, sugammadex still presents significant risks that have limited its extensive applications. But many host molecules such as cyclodextrins, pillararenes, cucurbiturils, and acyclic cucurbituril derivatives have shown a real potential for the next generation supramolecular antidotes, they can additionally be functionalized to improve affinity and selectivity, and new host molecules are periodically described [14, 24]. In the definition given for “Antidotes” by the Encyclopedia Britannica, it is mentioned that such a substance can be able to: “keep … (a poison) … from fitting a receptor at its site of action; or binding to a receptor to prevent the poison's binding there, blocking its action”. The definition implies that binding affinity and selectivity are key factors that influence the antidotal effects of any given host molecules. A high binding affinity and selectivity would likely afford an effective antidote toward a specific guest species; however, the general applicability would be compromised by the high selectivity, making such an antidote of less "commercial" interest. A low-selectivity host may offer broad-spectrum antidote against a variety of toxic guests, but this often comes with a relatively low efficacy and likely more side effects. These factors have to be considered and balanced in future development of supramolecular antidotes.
Besides the toxicities induced by exogenous species, harmful endogenous species (broadly defined as internal “toxins”/”poisons” once the local concentration is high) may also be sequestered via supramolecular encapsulation for various therapeutic purposes. Thus far, supramolecular medicine has exhibited significant potential for the treatment of cancer, atherosclerosis and NPC disease in vivo and even in humans. The unique role of supramolecular host molecules in this case provides a new strategy to treat various diseases that are otherwise difficult to tackle. Under the same principles, diseases caused by other endogenous, harmful substances, such as uric acid, bile acid, high blood sugar, etc. may find practical solutions with delicately designed host molecules that can specifically “trap” these molecules in vivo. The field of supramolecular medicine will certainly become burgeoning by then.
Furthermore, with the emergence of protein binding by macrocycles such as calixarenes or cucurbiturils [98-102] there is only one step to imagine macrocycles able to target proteins expressed on the surface of viruses, thereby reducing virus binding and impeding early stages of viral infection, something highly topical after the emergence of the pandemic SARS-CoV-2 virus. There still may be a long way to reach that purpose, but macrocycles and analogous host compounds have already opened fascinating perspectives as supramolecular antidotes in a broader context.
Abbreviations
CD: cyclodextrin; NMBA: neuromuscular blocking agent; nAChR: nicotinic acetylcholine receptor; ACh: acetylcholine; FDA: Food and Drug Administration; BA: bile acid; CA: cholic acid; DCA: deoxycholic acid; GCA: glycocholic acid; TCA: taurocholic acid; L/D-Tyr-β-CD: L/D-tyrosine-modified β-CD; L/D-Trp-β-CD: L/D-tryptophan-modified β-CD; Trm-β-CD: tyramine-modified β-CD; C[n]A: calix[n]arene; SC[n]A: p-sulfonatocalix[n]arene; PQ: paraquat; DQ: diquat; STC[4]A: p-sulfonatothiacalix[4]arene; UFH: unfractionated heparin; GC[4]AOEG: oligoethylene glycol functionalized guanidinocalixarene; CB[n]: cucurbit[n]uril; AD: amantadine; TM: tricaine mesylate; MPTP: N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MPP+: N-methyl-4-phenylpyridine; PTZ: pentylenetetrazol; CFZ: clofazimine; BDQ: Bedaquiline; SO: sorafenib; SMKI: small-molecule kinase inhibitor; DPI: Diphenyleneiodonium; AH: arecoline; NC: nitidine chloride; TZ: Trazodone; mCPP: m-chlorophenylpiperazine; CPT: camptothecin; OxPt: oxaliplatin; PEI: polyethylenimine; HB: hexadimethrine bromide; NTX: nereistoxin; THI: thiocyclam; Acyclic CB[n]: acyclic cucurbit[n]uril-type; CLBD1: calabadion 1; CLBD2: calabadion 2; PA[n]: pillar[n]arene; WPA[n]: water soluble pillar[n]arene; Sch: succinylcholine; SPM: spermine; SPD: spermidine; PUT: putrescine; PEG: polyethylene glycol; P1PA[5]: peptide-PA[5] conjugate; HP-β-CD: 2-Hydroxypropyl-β-cyclodextrin; β-CDP: β-CD based polymer; NPC1: Niemann-Pick type C1; NPC2: Niemann-Pick type C2; NPC: Niemann-Pick disease type C.
Acknowledgements
The Science and Technology Development Fund (FDCT), Macau SAR (Grant No.: 020/2015/A1, 0007/2020/A and SKL-QRCM(UM)-2020-2022), National Natural Science Foundation of China (Grant No.: 21871301), and University of Macau (Grant No.: MYRG2019-00059-ICMS) are gratefully acknowledged for providing financial support to this work. DB acknowledges CNRS and Aix-Marseille University for continuous support.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Nepovimova E, Kuca K. The history of poisoning: from ancient times until modern ERA. Arch Toxicol. 2019;93:11-24
2. World Health Organization, The public health impact of chemicals: knowns and unknowns, World Health Organization, Geneva; 2016
3. Raghavendra T. Neuromuscular Blocking Drugs: Discovery and Development. J R Soc Med. 2002;95:363-7
4. Cohen JL, Ozog DM, Porto DA. Botulinum toxins: cosmetic and clinical applications. John Wiley & Sons. 2017
5. Julve Parreño JM, Huet E, Fernández-del-Carmen A, Segura A, Venturi M, Gandía A. et al. A synthetic biology approach for consistent production of plant-made recombinant polyclonal antibodies against snake venom toxins. Plant Biotechnol J. 2018;16:727-36
6. Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin PoisoningA Comprehensive Review. JAMA. 2005;294:2342-51
7. Amabilino DB, Gale PA. Supramolecular chemistry anniversary. Chem Soc Rev. 2017;46:2376-7
8. Peters DH, Clissold SP. Clarithromycin. Drugs. 1992;44:117-64
9. Rios P, Carter TS, Mooibroek TJ, Crump MP, Lisbjerg M, Pittelkow M. et al. Synthetic Receptors for the High-Affinity Recognition of O-GlcNAc Derivatives. Angew Chem Int Ed. 2016;55:3387-92
10. Tromans RA, Carter TS, Chabanne L, Crump MP, Li H, Matlock JV. et al. A biomimetic receptor for glucose. Nat Chem. 2019;11:52-6
11. Mateus P, Chandramouli N, Mackereth CD, Kauffmann B, Ferrand Y, Huc I. Allosteric Recognition of Homomeric and Heteromeric Pairs of Monosaccharides by a Foldamer Capsule. Angew Chem Int Ed. 2020;59:5797-805
12. Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023-35
13. Crini G, Fourmentin S, Fenyvesi É, Torri G, Fourmentin M, Morin-Crini N. Fundamentals and Applications of Cyclodextrins. In: Fourmentin S, Crini G, Lichtfouse E, Eds. Cyclodextrin Fundamentals, Reactivity and Analysis. Cham: Springer International Publishing. 2018 p: 1-55
14. Liu Z, Nalluri SKM, Stoddart JF. Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes. Chem Soc Rev. 2017;46:2459-78
15. Yu G, Zhao X, Zhou J, Mao Z, Huang X, Wang Z. et al. Supramolecular Polymer-Based Nanomedicine: High Therapeutic Performance and Negligible Long-Term Immunotoxicity. J Am Chem Soc. 2018;140:8005-19
16. Sun C, Wang Z, Yue L, Huang Q, Cheng Q, Wang R. Supramolecular Induction of Mitochondrial Aggregation and Fusion. J Am Chem Soc. 2020;142:16523-7
17. Goor OJGM, Hendrikse SIS, Dankers PYW, Meijer EW. From supramolecular polymers to multi-component biomaterials. Chem Soc Rev. 2017;46:6621-37
18. Yan X, Wang F, Zheng B, Huang F. Stimuli-responsive supramolecular polymeric materials. Chem Soc Rev. 2012;41:6042-65
19. Zhou J, Yu G, Huang F. Supramolecular chemotherapy based on host-guest molecular recognition: a novel strategy in the battle against cancer with a bright future. Chem Soc Rev. 2017;46:7021-53
20. Wang K, Guo D-S, Zhang H-Q, Li D, Zheng X-L, Liu Y. Highly Effective Binding of Viologens by p-Sulfonatocalixarenes for the Treatment of Viologen Poisoning. J Med Chem. 2009;52:6402-12
21. Yin H, Wang R. Applications of Cucurbit[n]urils (n=7 or 8) in Pharmaceutical Sciences and Complexation of Biomolecules. Isr J Chem. 2018;58:188-98
22. Yu Y, Rebek J. Reactions of Folded Molecules in Water. Acc Chem Res. 2018;51:3031-40
23. Sullivan MR, Yao W, Gibb BC. The thermodynamics of guest complexation to octa-acid and tetra-endo-methyl octa-acid: reference data for the sixth statistical assessment of modeling of proteins and ligands (SAMPL6). Supramol Chem. 2019;31:184-9
24. Yang L-P, Wang X, Yao H, Jiang W. Naphthotubes: Macrocyclic Hosts with a Biomimetic Cavity Feature. Acc Chem Res. 2020;53:198-208
25. Ma D, Zhang B, Hoffmann U, Sundrup MG, Eikermann M, Isaacs L. Acyclic Cucurbit[n]uril-Type Molecular Containers Bind Neuromuscular Blocking Agents In vitro and Reverse Neuromuscular Block In vivo. Angew Chem Int Ed. 2012;51:11358-62
26. Szejtli J. Introduction and General Overview of Cyclodextrin Chemistry. Chem Rev. 1998;98:1743-54
27. Strickley RG. Solubilizing Excipients in Oral and Injectable Formulations. Pharm Res. 2004;21:201-30
28. Bom A, Bradley M, Cameron K, Clark JK, van Egmond J, Feilden H. et al. A Novel Concept of Reversing Neuromuscular Block: Chemical Encapsulation of Rocuronium Bromide by a Cyclodextrin-Based Synthetic Host. Angew Chem Int Ed. 2002;41:265-70
29. Adam JM, Bennett DJ, Bom A, Clark JK, Feilden H, Hutchinson EJ. et al. Cyclodextrin-Derived Host Molecules as Reversal Agents for the Neuromuscular Blocker Rocuronium Bromide: Synthesis and Structure-Activity Relationships. J Med Chem. 2002;45:1806-16
30. Hunter JM. New Neuromuscular Blocking Drugs. N Engl J Med. 1995;332:1691-9
31. Naguib M. Sugammadex: Another Milestone in Clinical Neuromuscular Pharmacology. Anesth Analg. 2007;104:575-81
32. Tsur A, Kalansky A. Hypersensitivity associated with sugammadex administration: a systematic review. Anaesthesia. 2014;69:1251-7
33. Hunter JM, Naguib M. Sugammadex-induced bradycardia and asystole: how great is the risk? Br J Anaesth. 2018;121:8-12
34. Chen Y, Li F, Liu B-W, Jiang B-P, Zhang H-Y, Wang L-H. et al. Thermodynamic Origin of Selective Binding of β-Cyclodextrin Derivatives with Chiral Chromophoric Substituents toward Steroids. J Phys Chem B. 2010;114:16147-55
35. Zhang Y-M, Xu X, Yu Q, Liu Y-H, Zhang Y-H, Chen L-X. et al. Reversing the Cytotoxicity of Bile Acids by Supramolecular Encapsulation. J Med Chem. 2017;60:3266-74
36. Gutsche CD. Calixarenes. Acc Chem Res. 1983;16:161-70
37. Guo D-S, Liu Y. Supramolecular Chemistry of p-Sulfonatocalix[n]arenes and Its Biological Applications. Acc Chem Res. 2014;47:1925-34
38. Guo D-S, Wang L-H, Liu Y. Highly Effective Binding of Methyl Viologen Dication and Its Radical Cation by p-Sulfonatocalix[4,5]arenes. J Org Chem. 2007;72:7775-8
39. Wang G-F, Ren X-L, Zhao M, Qiu X-L, Qi A-D. Paraquat Detoxification with p-Sulfonatocalix-[4]arene by a Pharmacokinetic Study. J Agric Food Chem. 2011;59:4294-9
40. Huang Q, Zhao H, Shui M, Guo D-S, Wang R. Heparin reversal by an oligoethylene glycol functionalized guanidinocalixarene. Chem Sci. 2020;11:9623-9
41. Kim J, Jung I-S, Kim S-Y, Lee E, Kang J-K, Sakamoto S. et al. New Cucurbituril Homologues: Syntheses, Isolation, Characterization, and X-ray Crystal Structures of Cucurbit[n]uril (n = 5, 7, and 8). J Am Chem Soc. 2000;122:540-1
42. Day A, Arnold A, Blanch R. Method for synthesis cucurbiturils. Patent WO. 2000 68232
43. Lee JW, Samal S, Selvapalam N, Kim H-J, Kim K. Cucurbituril Homologues and Derivatives: New Opportunities in Supramolecular Chemistry. Acc Chem Res. 2003;36:621-30
44. Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L. The Cucurbit[n]uril Family. Angew Chem Int Ed. 2005;44:4844-70
45. Masson E, Ling X, Joseph R, Kyeremeh-Mensah L, Lu X. Cucurbituril chemistry: a tale of supramolecular success. RSC Adv. 2012;2:1213-47
46. Barrow SJ, Kasera S, Rowland MJ, del Barrio J, Scherman OA. Cucurbituril-Based Molecular Recognition. Chem Rev. 2015;115:12320-406
47. Assaf KI, Nau WM. Cucurbiturils: from synthesis to high-affinity binding and catalysis. Chem Soc Rev. 2015;44:394-418
48. Cheng Q, Yin H, Rosas R, Gigmes D, Ouari O, Wang R. et al. A pH-driven ring translocation switch against cancer cells. Chem Commun. 2018;54:13825-8
49. Gao C, Huang Q, Lan Q, Feng Y, Tang F, Hoi MPM. et al. A user-friendly herbicide derived from photo-responsive supramolecular vesicles. Nat Commun. 2018;9:2967
50. Combes S, Tran KT, Ayhan MM, Karoui H, Rockenbauer A, Tonetto A. et al. Triangular Regulation of Cucurbit[8]uril 1:1 Complexes. J Am Chem Soc. 2019;141:5897-907
51. Yin H, Cheng Q, Rosas R, Viel S, Monnier V, Charles L. et al. A Cucurbit[8]uril 2:2 Complex with a Negative pKa Shift. Chem Eur J. 2019;25:12552-9
52. Chen H, Chan JYW, Yang X, Wyman IW, Bardelang D, Macartney DH. et al. Developmental and organ-specific toxicity of cucurbit[7]uril: in vivo study on zebrafish models. RSC Adv. 2015;5:30067-74
53. Zhang X, Xu X, Li S, Wang L-H, Zhang J, Wang R. A systematic evaluation of the biocompatibility of cucurbit[7]uril in mice. Sci Rep. 2018;8:8819
54. Uzunova VD, Cullinane C, Brix K, Nau WM, Day AI. Toxicity of cucurbit[7]uril and cucurbit[8]uril: an exploratory in vitro and in vivo study. Org Biomol Chem. 2010;8:2037-42
55. Hettiarachchi G, Nguyen D, Wu J, Lucas D, Ma D, Isaacs L. et al. Toxicology and Drug Delivery by Cucurbit[n]uril Type Molecular Containers. PLOS ONE. 2010;5:e10514
56. Wheate NJ, Day AI, Blanch RJ, Arnold AP, Cullinane C, Grant Collins J. Multi-nuclear platinum complexes encapsulated in cucurbit[n]uril as an approach to reduce toxicity in cancer treatment. Chem Commun. 2004;12:1424-5 10.1039/B404358H
57. Jin Jeon Y, Kim S-Y, Ho Ko Y, Sakamoto S, Yamaguchi K, Kim K. Novel molecular drug carrier: encapsulation of oxaliplatin in cucurbit[7]uril and its effects on stability and reactivity of the drug. Org Biomol Chem. 2005;3:2122-5
58. Moghaddam S, Yang C, Rekharsky M, Ko YH, Kim K, Inoue Y. et al. New Ultrahigh Affinity Host-Guest Complexes of Cucurbit[7]uril with Bicyclo[2.2.2]octane and Adamantane Guests: Thermodynamic Analysis and Evaluation of M2 Affinity Calculations. J Am Chem Soc. 2011;133:3570-81
59. Chen H, Chan JYW, Li S, Liu JJ, Wyman IW, Lee SMY. et al. In vivo reversal of general anesthesia by cucurbit[7]uril with zebrafish models. RSC Adv. 2015;5:63745-52
60. Li S, Chen H, Yang X, Bardelang D, Wyman IW, Wan J. et al. Supramolecular Inhibition of Neurodegeneration by a Synthetic Receptor. ACS Med Chem Lett. 2015;6:1174-8
61. Huang Q, Kuok KI, Zhang X, Yue L, Lee SMY, Zhang J. et al. Inhibition of drug-induced seizure development in both zebrafish and mouse models by a synthetic nanoreceptor. Nanoscale. 2018;10:10333-6
62. Li S, Chan JY-W, Li Y, Bardelang D, Zheng J, Yew WW. et al. Complexation of clofazimine by macrocyclic cucurbit[7]uril reduced its cardiotoxicity without affecting the antimycobacterial efficacy. Org Biomol Chem. 2016;14:7563-9
63. Kuok KI, In Ng PC, Ji X, Wang C, Yew WW, Chan DPC. et al. Supramolecular strategy for reducing the cardiotoxicity of bedaquiline without compromising its antimycobacterial efficacy. Food Chem Toxicol. 2018;119:425-9
64. Yang X, Huang Q, Bardelang D, Wang C, Lee SMY, Wang R. Supramolecular alleviation of cardiotoxicity of a small-molecule kinase inhibitor. Org Biomol Chem. 2017;15:8046-53
65. Yin H, Huang Q, Zhao W, Bardelang D, Siri D, Chen X. et al. Supramolecular Encapsulation and Bioactivity Modulation of a Halonium Ion by Cucurbit[n]uril (n = 7, 8). J Org Chem. 2018;83:4882-7
66. Li S, Yang X, Niu Y, Andrew GL, Bardelang D, Chen X. et al. Alleviation of Hepatotoxicity of Arecoline (Areca Alkaloid) by a Synthetic Receptor. ChemistrySelect. 2017;2:2219-23
67. Li W, Yin H, Bardelang D, Xiao J, Zheng Y, Wang R. Supramolecular formulation of nitidine chloride can alleviate its hepatotoxicity and improve its anticancer activity. Food Chem Toxicol. 2017;109:923-9
68. Huang Q, Li S, Yin H, Wang C, Lee SMY, Wang R. Alleviating the hepatotoxicity of trazodone via supramolecular encapsulation. Food Chem Toxicol. 2018;112:421-6
69. Yang X, Wang Z, Niu Y, Chen X, Lee SMY, Wang R. Influence of supramolecular encapsulation of camptothecin by cucurbit[7]uril: reduced toxicity and preserved anti-cancer activity. MedChemComm. 2016;7:1392-7
70. Chen Y, Huang Z, Zhao H, Xu J-F, Sun Z, Zhang X. Supramolecular Chemotherapy: Cooperative Enhancement of Antitumor Activity by Combining Controlled Release of Oxaliplatin and Consuming of Spermine by Cucurbit[7]uril. ACS Appl Mater Interfaces. 2017;9:8602-8
71. Huang Q, Li S, Ding Y-F, Yin H, Wang L-H, Wang R. Macrocycle-wrapped polyethylenimine for gene delivery with reduced cytotoxicity. Biomater Sci. 2018;6:1031-9
72. Huang Q, Cheng Q, Zhang X, Yin H, Wang L-H, Wang R. Alleviation of Polycation-Induced Blood Coagulation by the Formation of Polypseudorotaxanes with Macrocyclic Cucurbit[7]uril. ACS Appl Bio Mater. 2018;1:544-8
73. Yang X, Li S, Wang Z, Lee SMY, Wang L-H, Wang R. Constraining the Teratogenicity of Pesticide Pollution by a Synthetic Nanoreceptor. Chem Asian J. 2018;13:41-5
74. Zhang X, Xu X, Li S, Li L, Zhang J, Wang R. A Synthetic Receptor as a Specific Antidote for Paraquat Poisoning. Theranostics. 2019;9:633-45
75. Chen Y, Huang Z, Xu J-F, Sun Z, Zhang X. Cytotoxicity Regulated by Host-Guest Interactions: A Supramolecular Strategy to Realize Controlled Disguise and Exposure. ACS Appl Mater Interfaces. 2016;8:22780-4
76. Zhang X, Huang Q, Zhao Z-Z, Xu X, Li S, Yin H. et al. An Eco- and User-Friendly Herbicide. J Agric Food Chem. 2019;67:7783-92
77. Ma D, Hettiarachchi G, Nguyen D, Zhang B, Wittenberg JB, Zavalij PY. et al. Acyclic cucurbit[n]uril molecular containers enhance the solubility and bioactivity of poorly soluble pharmaceuticals. Nat Chem. 2012;4:503-10
78. Ganapati S, Zavalij PY, Eikermann M, Isaacs L. In vitro selectivity of an acyclic cucurbit[n]uril molecular container towards neuromuscular blocking agents relative to commonly used drugs. Org Biomol Chem. 2016;14:1277-87
79. Hoffmann U, Grosse-Sundrup M, Eikermann-Haerter K, Zaremba S, Ayata C, Zhang B. et al. Calabadion: A New Agent to Reverse the Effects of Benzylisoquinoline and Steroidal Neuromuscular-blocking Agents. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2013;119:317-25
80. Haerter F, Simons JCP, Foerster U, Moreno Duarte I, Diaz-Gil D, Ganapati S. et al. Comparative Effectiveness of Calabadion and Sugammadex to Reverse Non-depolarizing Neuromuscular-blocking Agents. Anesthesiology: The Journal of the American Society of Anesthesiologists. 2015;123:1337-49
81. Ganapati S, Grabitz SD, Murkli S, Scheffenbichler F, Rudolph MI, Zavalij PY. et al. Molecular Containers Bind Drugs of Abuse in vitro and Reverse the Hyperlocomotive Effect of Methamphetamine in Rats. ChemBioChem. 2017;18:1583-8
82. Ogoshi T, Yamagishi T-a, Nakamoto Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem Rev. 2016;116:7937-8002
83. Chi X, Yu G, Shao L, Chen J, Huang F. A Dual-Thermoresponsive Gemini-Type Supra-amphiphilic Macromolecular [3]Pseudorotaxane Based on Pillar[10]arene/Paraquat Cooperative Complexation. J Am Chem Soc. 2016;138:3168-74
84. Murray J, Kim K, Ogoshi T, Yao W, Gibb BC. The aqueous supramolecular chemistry of cucurbit[n]urils, pillar[n]arenes and deep-cavity cavitands. Chem Soc Rev. 2017;46:2479-96
85. Xiao T, Xu L, Zhong W, Zhou L, Sun X-Q, Hu X-Y. et al. Advanced Functional Materials Constructed from Pillar[n]arenes. Isr J Chem. 2018;58:1219-29
86. Yu G, Xue M, Zhang Z, Li J, Han C, Huang F. A Water-Soluble Pillar[6]arene: Synthesis, Host-Guest Chemistry, and Its Application in Dispersion of Multiwalled Carbon Nanotubes in Water. J Am Chem Soc. 2012;134:13248-51
87. Yu G, Zhou X, Zhang Z, Han C, Mao Z, Gao C. et al. Pillar[6]arene/Paraquat Molecular Recognition in Water: High Binding Strength, pH-Responsiveness, and Application in Controllable Self-Assembly, Controlled Release, and Treatment of Paraquat Poisoning. J Am Chem Soc. 2012;134:19489-97
88. Hao Q, Chen Y, Huang Z, Xu J-F, Sun Z, Zhang X. Supramolecular Chemotherapy: Carboxylated Pillar[6]arene for Decreasing Cytotoxicity of Oxaliplatin to Normal Cells and Improving Its Anticancer Bioactivity Against Colorectal Cancer. ACS Appl Mater Interfaces. 2018;10:5365-72
89. Hao Q, Chen Y, Huang Z, Xu J-F, Sun Z, Zhang X. Correction to “Supramolecular Chemotherapy: Carboxylated Pillar[6]arene for Decreasing Cytotoxicity of Oxaliplatin to Normal Cells and Improving Its Anticancer Bioactivity Against Colorectal Cancer”. ACS Appl Mater Interfaces. 2018;10:28250
90. Zhang X, Cheng Q, Li L, Shangguan L, Li C, Li S. et al. Supramolecular therapeutics to treat the side effects induced by a depolarizing neuromuscular blocking agent. Theranostics. 2019;9:3107-21
91. Shurpik DN, Mostovaya OA, Sevastyanov DA, Lenina OA, Sapunova AS, Voloshina AD. et al. Supramolecular neuromuscular blocker inhibition by a pillar[5]arene through aqueous inclusion of rocuronium bromide. Org Biomol Chem. 2019;17:9951-9
92. Chen H, Chen Y, Wu H, Xu J-F, Sun Z, Zhang X. Supramolecular polymeric chemotherapy based on cucurbit[7]uril-PEG copolymer. Biomaterials. 2018;178:697-705
93. Chen J, Ni H, Meng Z, Wang J, Huang X, Dong Y. et al. Supramolecular trap for catching polyamines in cells as an anti-tumor strategy. Nat Commun. 2019;10:3546
94. Zimmer S, Grebe A, Bakke SS, Bode N, Halvorsen B, Ulas T. et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci Transl Med. 2016;8:333ra50
95. Kim H, Han J, Park J-H. Cyclodextrin polymer improves atherosclerosis therapy and reduces ototoxicity. J Control Release. 2020;319:77-86
96. Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, Weissfeld L. et al. Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1-2 trial. Lancet. 2017;390:1758-68
97. Abi-Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. P Natl Acad Sci USA. 2009;106:19316
98. Nguyen HD, Dang DT, van Dongen JLJ, Brunsveld L. Protein Dimerization Induced by Supramolecular Interactions with Cucurbit[8]uril. Angew Chem Int Ed. 2010;49:895-8
99. Chinai JM, Taylor AB, Ryno LM, Hargreaves ND, Morris CA, Hart PJ. et al. Molecular Recognition of Insulin by a Synthetic Receptor. J Am Chem Soc. 2011;133:8810-3
100. Urbach AR, Ramalingam V. Molecular Recognition of Amino Acids, Peptides, and Proteins by Cucurbit[n]uril Receptors. Isr J Chem. 2011;51:664-78
101. Dang DT, Schill J, Brunsveld L. Cucurbit[8]uril-mediated protein homotetramerization. Chem Sci. 2012;3:2679-84
102. Rennie ML, Fox GC, Pérez J, Crowley PB. Auto-regulated Protein Assembly on a Supramolecular Scaffold. Angew Chem Int Ed. 2018;57:13764-9
Author contact
![]() Corresponding authors: Ruibing Wang, E-mail: rwangedu.mo; and David Bardelang, E-mail: david.bardelangfr.
Corresponding authors: Ruibing Wang, E-mail: rwangedu.mo; and David Bardelang, E-mail: david.bardelangfr.
 Global reach, higher impact
Global reach, higher impact