13.3
Impact Factor
Theranostics 2021; 11(2):506-521. doi:10.7150/thno.49812 This issue Cite
Research Paper
Highly sensitive magnetic particle imaging of vulnerable atherosclerotic plaque with active myeloperoxidase-targeted nanoparticles
1. Medical School of Chinese PLA, Chinese PLA General Hospital, Beijing, 100853, China.
2. Department of Cardiology, the Sixth Medical Centre, Chinese PLA General Hospital, Beijing, 100853, China.
3. CAS Key Laboratory of Molecular Imaging, Institute of Automation, Chinese Academy of Sciences, Beijing, 100190, China.
4. University of Chinese Academy of Sciences, Beijing, China.
5. Beijing Advanced Innovation Center for Big Data-Based Precision Medicine, School of Medicine, Beihang University, Beijing, 100083, China.
#These authors contributed equally to this work.
Abstract
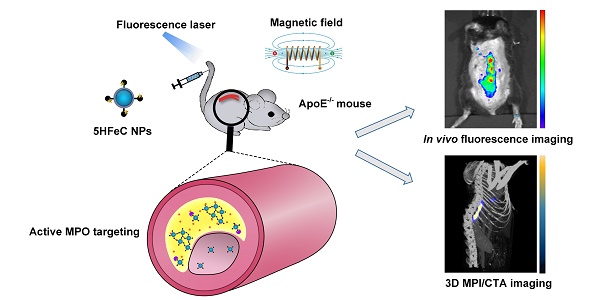
Inflammation is a pivotal driver of atherosclerotic plaque progression and rupture and is a target for identifying vulnerable plaques. However, challenges arise with the current in vivo imaging modalities for differentiating vulnerable atherosclerotic plaques from stable plaques due to their low specificity and sensitivity. Herein, we aimed to develop a novel multimodal imaging platform that specifically targets and identifies high-risk plaques in vivo by detecting active myeloperoxidase (MPO), a potential inflammatory marker of vulnerable atherosclerotic plaque.
Methods: A novel multimodal imaging agent, 5-HT-Fe3O4-Cy7 nanoparticles (5HFeC NPs), used for active MPO targeting, was designed by conjugating superparamagnetic iron oxide nanoparticles (SPIONs) with 5-hydroxytryptamine and cyanine 7 N-hydroxysuccinimide ester. The specificity and sensitivity of 5HFeC NPs were evaluated using magnetic particle imaging (MPI), fluorescence imaging (FLI), and computed tomographic angiography (CTA) in an ApoE-/- atherosclerosis mouse model. Treatment with 4-ABAH, an MPO inhibitor, was used to assess the monitoring ability of 5HFeC NPs.
Results: 5HFeC NPs can sensitively differentiate and accurately localize vulnerable atherosclerotic plaques in ApoE-/- mice via MPI/FLI/CTA. High MPI and FLI signals were observed in atherosclerotic plaques within the abdominal aorta, which were histologically confirmed by multiple high-risk features of macrophage infiltration, neovascularization, and microcalcification. Inhibition of active MPO reduced accumulation of 5HFeC NPs in the abdominal aorta. Accumulation of 5HFeC NPs in plaques enabled quantitative evaluation of the severity of inflammation and monitoring of MPO activity.
Conclusions: This multimodal MPI approach revealed that active MPO-targeted nanoparticles might serve as a method for detecting vulnerable atherosclerotic plaques and monitoring MPO activity.
Keywords: myeloperoxidase, vulnerable plaque, magnetic particle imaging, fluorescence imaging, computed tomographic angiography
 Global reach, higher impact
Global reach, higher impact