13.3
Impact Factor
Theranostics 2021; 11(1):27-47. doi:10.7150/thno.48987 This issue Cite
Review
Small molecule-based treatment approaches for intervertebral disc degeneration: Current options and future directions
1. AO Research Institute Davos, Davos, Switzerland.
2. Department of Orthopaedic and Trauma Surgery, University Medical Center Freiburg, Albert-Ludwigs University of Freiburg, Freiburg, Germany.
3. Department of Biomedical Engineering, Medical Faculty of the University of Basel, Basel, CH.
4. The first affiliated hospital of Sun Yat-sen University, Guangzhou, China.
Received 2020-6-2; Accepted 2020-9-1; Published 2021-1-1
Abstract
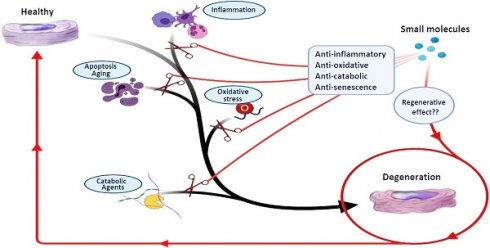
Low back pain (LBP) is a major reason for disability, and symptomatic intervertebral disc (IVD) degeneration (IDD) contributes to roughly 40% of all LBP cases. Current treatment modalities for IDD include conservative and surgical strategies. Unfortunately, there is a significant number of patients in which conventional therapies fail with the result that these patients remain suffering from chronic pain and disability. Furthermore, none of the current therapies successfully address the underlying biological problem - the symptomatic degenerated disc. Both spinal fusion as well as total disc replacement devices reduce spinal motion and are associated with adjacent segment disease. Thus, there is an unmet need for novel and stage-adjusted therapies to combat IDD. Several new treatment options aiming to regenerate the IVD are currently under investigation. The most common approaches include tissue engineering, growth factor therapy, gene therapy, and cell-based treatments according to the stage of degeneration. Recently, the regenerative activity of small molecules (low molecular weight organic compounds with less than 900 daltons) on IDD was demonstrated. However, small molecule-based therapy in IDD is still in its infancy due to limited knowledge about the mechanisms that control different cell signaling pathways of IVD homeostasis. Small molecules can act as anti-inflammatory, anti-apoptotic, anti-oxidative, and anabolic agents, which can prevent further degeneration of disc cells and enhance their regeneration. This review pursues to give a comprehensive overview of small molecules, focusing on low molecular weight organic compounds, and their potential utilization in patients with IDD based on recent in vitro, in vivo, and pre-clinical studies.
Keywords: small molecules, discogenic pain, intervertebral disc, degeneration, inflammation
Introduction
Discogenic back pain
Globally, chronic low back pain (CLBP) symptoms occur in ~60-80% of people during their lifetime, which has a significant socioeconomic impact via reduced quality of life and work efficacy [1-4]. CLBP is a multifactorial and complex clinical presentation, and symptomatic intervertebral disc (IVD) degeneration (IDD) is considered as the major cause of CLBP [5, 6]. The IVD is a fibrocartilaginous tissue that lies between two vertebrae and functions as a shock-absorber. It includes the jelly-like nucleus pulposus (NP), the surrounding fibrocartilaginous annulus fibrosus (AF), and the cartilaginous endplate (CEP) anchoring the IVD to the corpus vertebrae. IVDs are crucial structural components that form a fibrocartilage joint allowing for slight intervertebral motion [7].
IDD features extracellular matrix (ECM) degradation, accelerated cartilaginous and bone remodeling, the release of proinflammatory cytokines, altered spine biomechanics, angiogenesis, and neoinnervation, altogether causing CLBP and disability [8-10]. IDD can be induced or accelerated by mechanical stress, trauma, infection, genetic predisposition, or inflammation [10, 11]. Due to the limited healing potential and harsh nutritional conditions of adult IVDs, IVD ECM degradation is irreversible and requires restoration if disc regeneration is pursued. Previous in vitro and in vivo animal and human studies showed cellular senescence as a critical mechanism in the progression of IVD aging, increased inflammation, elevated catabolism, and subsequently IDD [12-14]. There is an unmet need for causative therapies especially for young patients affected by IDD that do not benefit from conservative treatments but, at the same time, do not qualify for spinal surgery. Therefore, the diagnosis and treatment of IDD in young patients would be a priority as in these disease stages the IVD still contains viable cells [15].
Most therapeutic options for IDD like analgesics, anti-inflammatory medications, and physical therapy are currently limited to symptomatic treatments, which only delay or mask the degradation process of the IVD. Surgical intervention is used as a last resort, with procedures such as total disc replacement or spinal fusion, which are associated with a substantial risk of intraoperative and postoperative complications [16]. Recently, new strategies like stem cell, gene, and molecular therapy have been used for the regeneration of the IVD. Even though these methods opened new possibilities, they also have their limitations [17, 18].
Therefore, there is a strong demand to find new therapeutic agents (or utilize well-known drugs which were proven effective in treating other diseases) aiming to relieve discogenic pain and regenerate damaged IVDs through restoration of tissue homeostasis. In this regard, several small molecules have shown promising results as alternative therapeutic agents in in vitro, in vivo, and clinical studies [19, 20]. These therapeutic agents demonstrate various phenomena to induce regeneration and prevent degeneration of the IVD, which include anti-oxidative, anti-inflammatory, anti-senescence, anti-apoptotic, anti-catabolic, and anabolic effects. This review is focused on low molecular weight organic compounds that have been investigated for their regenerative effects on IVDs. Furthermore, we will discuss how these small molecules may facilitate new treatment approaches for IVD regeneration.
Anatomy of the intervertebral disc
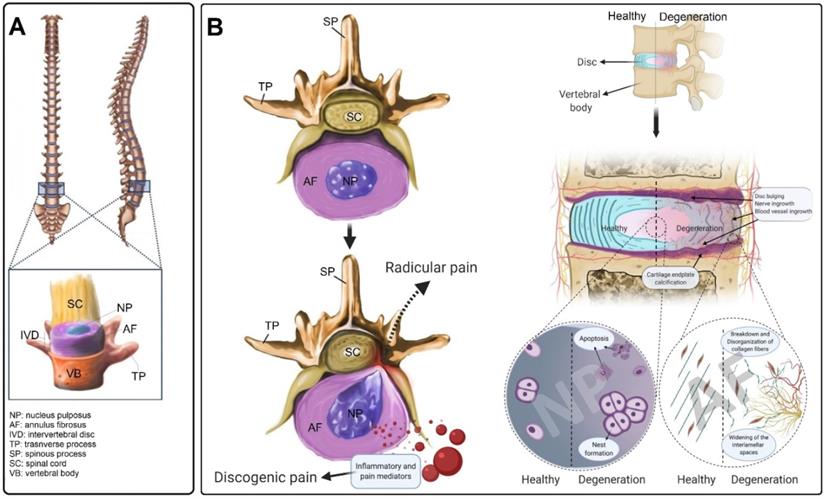
The IVD is the largest avascular structure in the human body that contains three main components; the soft mucoid NP core, the lamellar AF tissue that encloses the NP, and the CEPs which cover both top and bottom of the IVD (Figure 1A) [21]. Different cell populations produce a unique composition of ECM, forming a special microenvironment for the IVD, which plays an important role in its functionality and mechanical properties [22]. The high density of negatively charged proteoglycan (PG) molecules provides a capacity to absorb approximately three times their weight in water, giving the NP its mechanical resilience during compressive loading [23]. The NP is circumferentially surrounded by the AF, a fibrocartilaginous tissue consisting of highly organized collagen fibers that are arranged in concentric layers [24]. The AF is predominantly composed of both type I and II collagen and small quantities of PGs [25]. The outer layer of the AF is mainly made of collagen type I (95%); however, the amount of this collagen type is significantly decreasing in an almost a linear negative gradient when approaching the NP, where it constitutes less than 5% of collagen type I [26]. In contrast, an opposing pattern exists for collagen type II, decreasing in content towards the outer layers of the AF [26]. The endplate is an osteochondral structure that consists of two parts, including CEP and bony endplate (BEP) that physically limit the NP and AF to their anatomical partitions. Along with its mechanically supporting role, the CEP controls the fluid exchange, as well as an exchange of nutrients or metabolic waste, and acts as a semipermeable barrier between discs and vertebrae [27].
Damage, inflammation and IDD
The etiology of IDD is multifactorial and usually associated with genetic and environmental factors. [28]. IDD often occurs when the balance between catabolism and anabolism of the ECM is disturbed by decreased ECM production and enhanced ECM degradation [29]. The IVD (NP and AF) cells are responsible for keeping the balance between anabolic and catabolic processes including the synthesis, breakdown, and accumulation of ECM components [30]. The quality of ECM composition and the IVD mechanical properties are determined by these cellular processes that are high energy demanding and require glucose and oxygen consumption. During the IDD process, the expression of inflammatory cytokines (i.e., IL-1 and TNF) in disc cells is increased, which subsequently up-regulates matrix remodelling. Through inflammatory matrix remodelling processes, the concentration of PGs and collagen type II is dramatically decreased, which is mainly mediated by two extracellular enzyme types: matrix metalloproteinases (MMP) and a disintegrin and metalloproteinase with thrombospondin motifs proteins (ADAMTS). Simultaneously, the amount of collagen type I is increased which altogether can change the ECM shear stresses [30]. Moreover, an in vitro study showed that aggrecan, the major PG of the IVD, can inhibit neural ingrowth, which is associated with the development of CLBP [30, 31]. Therefore, it is suggested that detrimental changes in the ECM are linked with discogenic pain.
Damage to the CEP can be another reason for IVD degeneration through both mechanical and nutritional factors. Damage to the CEP changes mechanical loading of the NP, stimulating metabolic disturbances in the disc [32]. With increasing age, calcification of the endplate occurs, which may disturb its permeability and transportation of nutrients and other metabolites, leading to hypoxia and an acidic pH. This impairs the normal activity of IVD cells in synthesizing and supporting the ECM [33].
Inflammation is another factor that is thought to play an important role in the development of IDD [34]. It is not known whether inflammation is the cause or consequence of disc degeneration and herniation. However, pro-inflammatory cytokines and chemokines, which are produced during both systemic and local inflammation, have been associated with IDD and lower back pain. Overproduction of chemokines and cytokines including interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and interleukins (IL-1, 2, 4, 6, 8, and 17) by inflammatory cells present in the IVD can trigger the cascade of tissue degeneration. Moreover, several angiogenic and neurogenic factors (i.e., vascular endothelial growth factor, nerve growth factor) are also released during the IDD process, leading to blood vessel and nerve in-growth [35]. It is hypothesized that endogenous factors, such as ECM breakdown products, can induce IVD inflammatory responses [36]. Fibronectin, collagen, elastin, laminins, and low molecular weight hyaluronan are produced in response to an imbalance of homeostasis in ECM proteins. These products, in turn, induce an inflammatory response in the IVD [37-40]. Finally, all these processes can lead to discogenic and/or radicular pain (Figure 1B).
The intervertebral disc structure and the hallmarks of IVD degeneration (IDD). (A) The structure of the IVD and its anatomical location in the vertebral column. (B) Compared to healthy IVD, inflammation, blood vessel and neuronal ingrowth escalated in the degenerated disc. Moreover, the disorganization of collagen fibers and increasing of the interlamellar distances between collagen bundles in the annulus fibrosus were usually observed during the degeneration process that often results in disc bulging. During IDD, the number of apoptotic disc cells dramatically increased as well as the cell-cluster formation of nucleus pulposus (NP) cells. The calcification of the cartilage endplate and osteophyte formation occur in advanced degeneration. IVD degeneration is diagnosed when the degenerated IVD in the spine becomes symptomatic and causes discogenic pain. Additionally, the degenerated IVD (protrusion, bulging etc.) presses on spinal nerves, often producing radicular pain.
Mechanism of action of small molecules used for IVD regeneration
| Small molecule | Anti-apoptotic | Anti-inflammatory | Anti-oxidative | Anti-catabolic | Anabolic | Miscellaneous |
|---|---|---|---|---|---|---|
| Natural origin | ||||||
| Cannabidiol | [58] | [58, 127] | [58] | |||
| Epigallocatechin 3-gallate | [50] | [50, 78] | [50, 78] | [50] | ||
| Naringin | [63, 128] | [87, 128] | [63, 128] | [63, 87, 129] | [63, 87, 129] | |
| Urolithin A | [54] | [130] | ||||
| Rhein | [67] | [55, 67] | [67] | [67] | ||
| Estradiol | [89, 131] | [65, 132, 133] | [132, 134] | |||
| Curcumin | [135] | [135] | [104] Senolytic; [102] mTOR inhibitor. | |||
| o-Vanillin | [104] | [104] Senolytic | ||||
| Icariin | [80, 136-136] | [136] | [80, 136, 137] | [138] | [80] | |
| Resveratrol | [82, 139] | [82, 91, 140] | [82, 91, 139] | [82, 140] | [81, 82, 91] | |
| Celecoxib | [61, 141] | [61] | ||||
| Kaempferol | [52] | [52] | [52] | [105] BMP2 activator | ||
| Berberine | [70, 71, 86] | [70] | [86] | [70, 71, 86] | ||
| Luteoloside | [57] | [57] | [57] | [57] | [57] | |
| Chemical/ Synthetic | ||||||
| Statins | [142] | [107, 142] | [106] BMP2 activator | |||
| Metformin | [92] | [59] | [92] | [92] | [92] Autophagy | |
| APO866 | [93] | [93] | [93] Autophagy | |||
| Dexmedetomidine | [74] Inhibit pyroptosis | |||||
| SM04690 | [109] | 113 | [109]Wnt pathway Inhibitor | |||
| Gefitinib | [20] | [20] | [20] Autophagy | |||
| Tofacitinib | [56] | [56] | ||||
| INK-128 | [102] mTOR inhibitor | |||||
| NVP-BEZ235 | [102] mTOR inhibitor | |||||
| MK-2206 | [102] mTOR inhibitor |
Small molecules and IVD regeneration
In the context of this review, we focus on small molecules as low molecular weight (<900 daltons) compounds, including synthetic or natural products [41]. In the area of pharmaceuticals, small molecules are defined as compounds that bind to certain biological macromolecules and help regulating a particular biological process. The upper molecular weight limit for a small molecule, which requires rapid diffusion across the cell membrane and digestive system absorption, is 900 daltons. Basically, the molecules larger than 550 daltons face more challenges for absorption, while there are some up to 900 daltons that successfully cross barriers [42, 43]. Historically, they were provided as drugs (such as celecoxib) to modulate different cell processes. In the last few years, several small molecules that can selectively regulate cell fate and signaling pathways have been developed [44]. Indeed, small molecules have several advantages and only few limitations compared to large molecular compounds, as outlined in Table S1. Here we focus on recent advances in the use of small molecules that are effective in the regeneration of IVD cells through attenuation of inflammation, cell damage, and stimulation of anabolic processes. These molecules are listed in Table S2, and their mechanism of natural action is summarized in Table 1. In addition, we will discuss new strategies and approaches which were used in recent studies and the future direction for using these molecules to regenerate IVD cells. In vitro, in vivo, and clinical studies related to the discussed small molecules are listed in Table 2. The effective in vitro concentrations of different small molecules for regeneration of disc cells are listed in Table S3.
Study setup of different investigations using small molecules for IVD regeneration
| Small molecule | In vitro (Cell culture) | Ex vivo (Organ culture) | In vivo | Clinical study |
|---|---|---|---|---|
| Natural origin | ||||
| Cannabidiol | [58] | Rat [127] | ||
| Epigallocatechin 3-gallate | [50, 78] | Rat [50] | ||
| Naringin | [63, 87, 128, 129] | Rat [63] | ||
| Urolithin A | [143] | Rat [54] | ||
| Estradiol | [89, 90] | Rat [121, 132] | ||
| Curcumin | [144] | Rat [145] | ||
| o-Vanillin | [104] | |||
| Icariin | [80, 136-138] | Rat [146] | ||
| Resveratrol | [82, 139, 140] | [91] | Rabbit, Rat [91, 117] | |
| Celecoxib | [61, 141] | Dog [61, 115, 141] | ||
| Kaempferol | [52] | |||
| Berberine | [70, 71] | Rat [71] | ||
| Luteoloside | [57] | Rat [57] | ||
| Chemical/Synthetic | ||||
| Statins | [107] | Rat [142] | [118, 119] | |
| Metformin | [92] | Rat [92] | ||
| APO866 | [93] | |||
| Dexmedetomidine | [74] | |||
| SM04690 | [109] | Rat [109] | ||
| Gefitinib | [20] | Rat [20] | [20] | |
| Tofacitinib | [56, 60] | [56] | ||
| INK-128 | [102] | |||
| NVP-BEZ235 | [102] | |||
| MK-2206 | [102] | |||
Anti-inflammatory effects of small molecules
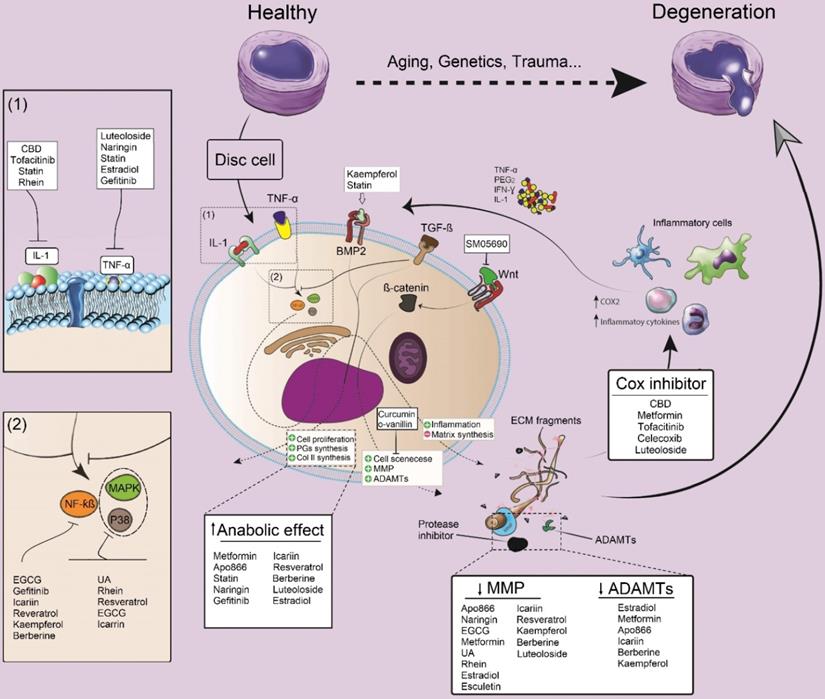
Although detailed pathways and molecular interactions between discogenic pain, disc degeneration, and inflammation remain to be elucidated, some inflammatory cytokines and related pathways are known as potential targets for therapies in IDD [19]. Pro-inflammatory cytokines such as IL-1 and TNF-α are key cytokines, triggering ECM degeneration through activation of NF-κB and p38/MAPK pathways. One of the most important cell signaling pathways that seem to play a crucial role in IDD is MAPK signaling. Through this pathway, both matrix synthesis and degradation are modulated in the IVD by influencing PG degradation as well as by changing anabolic and catabolic gene expression levels [45]. Particularly, the PG metabolism is regulated by p38/MAPK/extracellular signal-regulated kinase (ERK) signaling pathways, as treatment with inhibitors of p38 or ERK considerably attenuated the cytokine-induced decrease in synthesis and release of PG. Moreover, ERK can activate the Wnt/β-catenin signaling pathway, which may contribute to the pathogenesis of IDD. Activation of p38 and ERK, which was shown to be higher in degenerated IVD cells can enhance apoptosis induced by experimental loading stress [45]. During IDD development, several growth promoting factors such as insulin-like growth factor 1 (IGF-I), platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF) exert their beneficial effects (i.e., mitogenic action) via activating ERK by phosphorylation and subsequent DNA synthesis in degenerated disc cells, indicating that MAPKs may be involved in metabolic processes in the IVD [46]. TNF-α is a cytokine that has been closely related to IDD. This cytokine has two receptors (TNFR1 and TNFR2) that bind the ligand with high affinity. Upon TNF binding to TNFR1, two distinct signaling complexes can be activated, 1) the anti-apoptotic complex I, and 2) the death inducing signaling complex (complex II). Signaling downstream of anti-apoptotic complex I is mediated by NFκB/MAPK signaling pathways. IL-1 is another cytokine that has strongly been linked to IDD. Among 11 cytokines of the IL-1 family, IL-1α and IL-1β are the most studied cytokines regarding IDD. Like TNF, IL-1α and IL-1β can activate NFκB and MAPK signaling pathways [47]. As downstream effects, these cytokines activate MMPs and ADAMTS, which finally elevate ECM degradation.
In various in vitro studies, small molecules, including naringin, cannabidiol (CBD), epigallocatechin gallate (EGCG), curcumin, icariin, resveratrol, berberine, and tofacitinib showed an impact on the downregulation of IL-1 and TNF-α levels in IVD cells (Figure 2). According to previous literature, icariin, resveratrol, and EGCG can inhibit NF-kB and p38/MAPK signaling pathways, thereby modulating inflammatory responses and preventing the development of a degenerative cascade [48-51]. Gefitinib, kaempferol, and berberine are other small molecules that exclusively block the NF-kB signaling pathway [20, 52, 53]. On the other hand, intracellular p38/MAPK signals could be blocked by rhein and urolithin A in vitro [54, 55].
Lang et al. investigated the effects of tofacitinib in an inflammatory and degenerative bovine IVD organ culture model. Tofacitinib citrate (2.5 mg/mL) was added daily to the culture medium to simulate a systemic application of the drug. The results showed that tofacitinib could slow down the degenerative response and reduce inflammation in the organ culture model by selectively inhibiting the Janus kinase 3 (JAK3) pathway [56]. A recent in vitro study showed that luteoloside, a flavonoid glycoside, could suppress inflammatory factors, such as TNF-α and IL-6, in IL-1β-primed NP cells through inhibition of the NF-κB signaling cascade. They demonstrated that luteoloside promoted the nuclear factor erythroid 2-related factor 2 (Nrf2) translocation to the nuclei; Nrf2 can act through activation of the Nrf2/HO-1 (heme oxygenase-1) signaling in NP cells and mitigate inflammation by suppressing the NF-κB signaling cascade and by anti-apoptotic function [57].
Cyclooxygenase-2 (COX-2), which regulates prostaglandin E2 (PGE2) synthesis, is another candidate for modulation of inflammatory responses in IVDs. Chen et al. showed that pre-treatment with CBD suppressed the production of COX‑2 and inflammatory cytokines (i.e. IL-6 and IL‑1β) in NP cells [58]. Moreover, metformin, luteoloside, and icariin exhibited their anti-inflammatory impact on IL-1 primed NP cells via inhibition of COX‑2 and inducible nitric oxide synthase (iNOS) expression, leading to a decreased synthesis of PGE2 [51, 57, 59]. Suzuki et al. used the JAK antagonist tofacitinib for pre-treatment of rat AF cells and then cells were incubated with inflammatory cytokines such as IL-6. Tofacitinib significantly decreased the expression level of COX-2 and, subsequently, the production of PGE2 [60]. Celecoxib is one of the most popular anti-inflammatory small molecules which has recently been used for regeneration of the IVD. Of the selective COX-2-inhibitors, celecoxib was the first on the market and has been in clinical use since 1999. In a preclinical canine study, local delivery and sustained release of this small molecule (as celecoxib-loaded microspheres) in the IVD showed promising results in the control of inflammation, attenuation of discogenic pain and inhibition of IDD development [61] (Figure 2).
Anti-apoptotic effect of small molecules
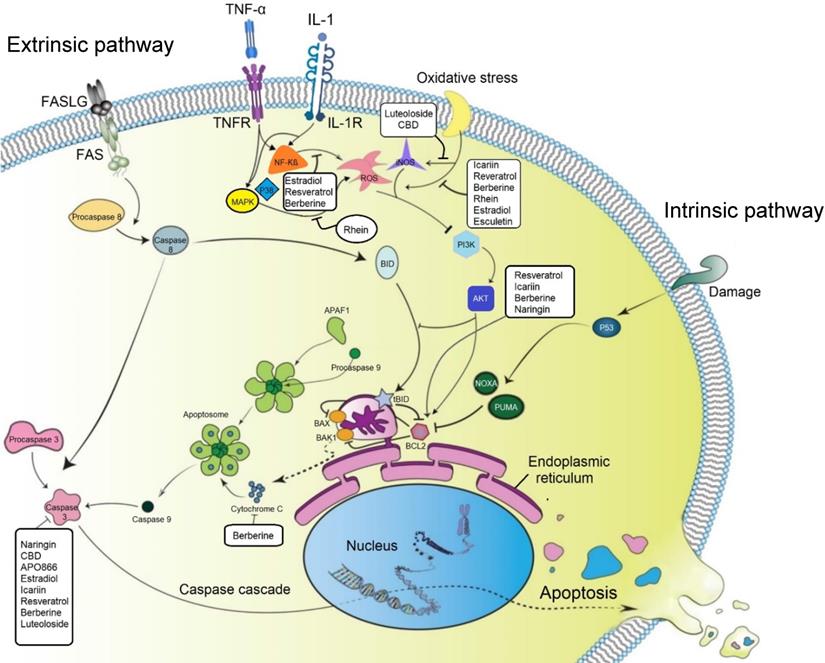
Therapeutic regulation of transduction pathways can modulate the programmed cell death (PCD) process, which is considered to play a significant role in IVD cell degeneration [62]. Several studies investigated the anti-apoptotic effect of small molecules in IVD cells (Figure 3). Recent studies showed that naringin and icariin could upregulate the cellular concentrations of anti-apoptotic protein B-cell lymphoma 2 (Bcl-2) and downregulate the apoptotic effect of promoter proteins, including cleaved caspase 3 and BCL-associated X (Bax) to modulate the apoptotic rate of human NP derived cells [63, 64]. Furthermore, after 4 hours of treatment with icariin, the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway was significantly activated. Based on previous studies, activation of this signaling pathway is correlated to anti-apoptosis and anti-oxidative stress; therefore, the anti-apoptotic effect of icariin is linked to the PI3K/AKT signaling pathway [63, 64]. Chen et al. confirmed the protective effect of CBD on hydrogen peroxide-induced apoptosis. They identified that CBD increased cell viability and reduced apoptosis in NP cells after exposure to hydrogen peroxide by reducing the expression level of caspase 3 and promoting the Bcl-2 protein expression [58].
The modulatory effect of small molecules on inflammation, anabolic and catabolic processes and their impacts on IDD. Pro-inflammatory cytokines including IL-1 and TNF-a, are the key cytokines triggering IVD matrix degeneration through activation of NF-κB and P38/MAPK pathways. Moreover, these cytokines activate MMPs and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTs) which finally will elevate ECM degradation. Small molecules can prevent IDD progression by inhibiting the activity of pro-inflammatory cytokines and the subsequent factors (NF-κB and P38/MAPK), or modulating the anabolic, catabolic and even some alternative pathways such as BMP-2 or Wnt pathways. IL: interleukin; ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs; MMPs: matrix metalloproteinase; CBD: cannabidiol; ECGC: Epigallocatechin gallate; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK: MAP Kinase; UA: urolithin A, TGF-β: transforming growth factor-beta; Cox: cyclooxygenase; PGE2: prostaglandin E2; IFN-γ: interferon-gamma; TNF-α: tumor necrosis factor-alpha.
The effect of small molecules on programmed cell death (apoptosis) and oxidative stress in degenerated IVD cells. Both intrinsic and extrinsic pathways of apoptosis are playing critical roles in IVD cell degeneration. Small molecules can increase the expression level of Bcl-2, leading to inhibition of the intrinsic pathway by its inhibitory effect on BAX and BAK1. BAX and BAK1 control the release of cytochrome C from mitochondria to cytosol or bind to Apaf-1, leading to inhibition of caspase 9 activity. Several small molecules can also decrease the expression level of caspase 3 in disc cells and subsequently inhibit apoptosis. Inflammatory cytokines and oxidative stresses can also increase the number of apoptotic cells in degenerated IVD cells by activating the ROS-mediated PI3K/Akt pathway. Production of ROS and the expression level of related products such as iNOS can be suppressed by different small molecules. TNF-α: tumor necrosis factor-alpha; IL: interleukin; NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK: MAP Kinase; Bcl2: B-cell lymphoma 2; Bax: BCL-Associated X; BAK1: BRI1-associated receptor kinase 1; PI3K: phosphatidylinositol 3-kinase; Akt: Protein Kinase B; ROS: reactive oxygen species; iNOS: inducible nitric oxide synthase; CBD: cannabidiol.
The effect of 17 beta-estradiol (E2) was also assessed on isolated NP cells from healthy rats and their intact IVDs, which were cultured with or without TNF-α. It was shown that in NP cells, E2 significantly increased matrix macromolecules expression, telomerase activity, and cell proliferation potential but attenuated senescence markers (p53 and p16). P53 plays a critical role in intrinsic pathways of apoptosis; therefore, the reduction of this marker could lead to a decrease in apoptosis [65]. Rhein acts through several closely interacting pathways affecting apoptosis. Rhein blocks the p38/MAPK pathway which in turn activates the PI3K/AKT parallel signaling pathways. Consequentially several downstream pathways are activated which regulate the cell cycle and apoptosis [66]. Therefore, the therapeutic potential of rhein as a multitarget molecule is due to its synergistic modulation of multiple pathways [67].
Resveratrol is another small molecule which could induce anti-apoptotic genes (e.g., Bcl-2) and simultaneously reduce the expression level of pro-apoptotic genes such as Bax or caspase 3. Moreover, when LY294002 was used as a strong inhibitor of PI3K/AKT, the anti-apoptotic effects of resveratrol in IL-1β-primed NP cells were attenuated [68]. Therefore, it can be concluded that resveratrol activated the PI3K/AKT signaling pathway, which in turn downregulated NP cell apoptosis. Furthermore, another study showed that resveratrol could activate sirtuin 1 (NAD (+)-dependent deacetylase), which reduces apoptosis in degenerated human NP cells [69]; while treatment with LY294002 again increased the rate of apoptosis. This study also suggested that resveratrol could increase the survival rate of degenerative human NP cells by activation of sirtuin 1 through the PI3K/AKT anti-apoptotic signaling pathway [69].
The protective effect of berberine by inhibiting NF-κB activation has been shown on IL-1β stimulated human NP cells undergoing apoptosis [70]. The modulatory effects of berberine on the expression levels of anti-apoptotic protein (Bcl-2), activation of caspase 3, pro-apoptotic Bax, Bak, and release of cytochrome c have also been reported [71].
Pyroptosis is another form of programmed cell death which can lead to production of proinflammatory mediators [72]. This process is mediated by nod-like receptor protein 3 (NLRP3) inflammasome, and it has been shown that suppressing the activation of NLRP3 inflammasome could diminish the IDD process [73]. New evidence indicates that microorganisms such as Cutibacterium acnes can induce inflammatory response (IL-1β) and initiate IDD. The number of NLRP-positive cells significantly increased in C. acnes infected disc tissue, which suggested that pyroptosis activation may be induced by C. acnes [73]. A recent study showed that dexmedetomidine, a sedative small molecule drug, inactivated NLRP3 through the suppression of NF-κB and JNK signals, subsequently alleviating pyroptosis during inflammation and IDD [74].
Anti-oxidative effect of small molecules
During IDD, usually excessive reactive oxygen species (ROS) are produced and released locally, suggesting a contribution of oxidative stress to the degeneration process and opening a new horizon regarding the pathogenesis of IDD. ROS, as active mediators, are involved in various cell signaling pathways and cell metabolisms, including matrix degradation, inflammation, apoptosis, autophagy, and senescence of IVD cells. Moreover, ROS can change the structure of matrix proteins in NP, AF, and CEP leading to the impairment of the IVD's mechanical function and acceleration of IVD degeneration processes [75]. Therefore, a therapeutic option for regulation of oxidative stress in disc cells could be a novel strategy for IVD regeneration. Apoptosis can be triggered by oxidative stress during IDD which has been elaborated in several studies. IL-1β treated NP cells produce more ROS in comparison to untreated cells, which consequently decreases the PG levels and triggers apoptosis [76]. Moreover, the ratio of apoptosis is increased in NP cells exposed to hydrogen peroxide and the expression levels of ECM proteins such as aggrecan and type II collagen are decreased [77]. Notably, the detrimental effects of oxidative stress on the cells may be efficiently prevented using anti-oxidative agents, such as different small molecules that protect IVD cells from apoptosis (Figure 3). For instance, the effect of naringin on oxidative stress-induced apoptosis was investigated in rat NP-derived mesenchymal stem cells (MSCs). The findings showed that naringin had protective effects against hydrogen peroxide induced NP cell apoptosis. The potential mechanism of naringin to alleviate apoptosis may be due to the activation of the ROS-mediated PI3K/AKT pathway [63]. In the same way, the protective effect of CBD on NP cells against oxidative stress was also reported. The results demonstrated that the pre-treatment with CBD suppressed the expression level of iNOS, which activated PI3K/AKT signaling pathway [58]. Another study by Krupkova et al. evaluated the anti-oxidative effect of EGCG on human IVD cells exposed to hydrogen peroxide. Their results demonstrated that survival of the treated disc cells by EGCG under severe oxidative stress was considerably enhanced in comparison to the control cells, which happened through activation of PI3K/AKT pathway and inhibition of cytochrome c release from mitochondria [78].
The inhibitory effect of E2 on ROS generation was studied by several investigators. ROS/NF-κB pathway of rat NP cells is affected by the interaction of estrogen receptor and E2, which inhibits TNF-α-induced premature senescence [65].
Cryopreservation can be used to allow the storage of cells over prolonged periods of time. While cryopreservation at -196°C would render IVD cells metabolically inactive, cells usually suffer insults during freeze-thawing such as the generation of ROS [79]. For this reason, the effect of icariin (25 µM) as an addition to cryopreservation media was investigated by Chan et al. They found that icariin improved the viability and function of human NP derived stem cells by preserving the phenotype after thawing the cells [80]. The increased activity of glutathione peroxidase (GPx) and superoxide dismutase (SOD) can explain the oxidation resistance, which provides oxidative stress protection to the cryopreserved cells. Several studies investigated the anti-oxidative effect of resveratrol on human, rat, and bovine NP cells in vitro. The protective effect of resveratrol is due to the stimulation of sirtuin 1 and the PI3K/AKT pathway which was activated in different settings [81-85]. Also, icariin could inhibit induced oxidative stress in NP cells in vitro [64]. Luo et al. observed that the production of ROS under hydrogen peroxide exposure was down-regulated with berberine, which protected human NP cells against oxidative stress-induced apoptosis [86]. A recent in vitro study showed that luteoloside could successfully suppress iNOS and modulate ROS production in IL-1β treated NP cells [57].
Although most of the studies showed an anti-oxidative effect of these small molecules on IVD cells, there is not enough in vivo and preclinical evidence to support the efficiency of these molecules to retard the process of IDD. Further in vivo and clinical studies are required to develop effective anti-oxidative therapies for IVD regeneration.
Anabolic and anti-catabolic effect of small molecules
Several studies assessed the anabolic and anti-catabolic effect of small molecules on IVD cells and reported their beneficial impacts on IDD (Figure 2). Li et al. evaluated the influence of naringin on the growth of degenerative NP cells and its regenerative effects on protein and gene expression. Naringin treatment elevated the protein expression of collagen type II, aggrecan, and SOX6, and decreased the gene expression of MMP3 [87]. Another study showed that naringin could promote the expression of anabolic genes such as collagen II, aggrecan, and reduce catabolic gene expression such as MMP13 to sustain the ECM [63].
Through an in vitro study, the TNF-α-induced production of MMPs and degradation of collagen II have been investigated, and the anti-senescence and anti-catabolic effects of urolithin A have been confirmed [88]. Estradiol can increase the anabolic activity of NP cells and induce the downregulation of MMPs, indicating protective capabilities of estradiol, as shown in vitro [89]. The down-regulation of the protein level of caspase-3, MMP3, and MMP13 and up-regulation of the protein level of type II collagen were closely related to the anti-degenerative mechanism [89]. With the therapeutic application of E2 to degenerated CEP cells, increased expression of collagen II and aggrecan was noted. In addition, an increase in the TGF-β secretion was reported [90]. Hua et al. demonstrated the anti-catabolic effect of icariin by decreased MMP and ADAMTS gene expression in human NP cells stimulated by IL-1β [51]. An anabolic effect of 200 μM resveratrol and decrease of the catabolic effects of pro-inflammatory stimuli (IL-1β) added to bovine NP cells was also reported by Li et al [82]. The anabolic effect of resveratrol in cell culture could be reproduced in an in vivo study by Kwon et al., who induced degeneration by annulotomy in rabbit discs followed by two intradiscal injections of resveratrol or carrier (DMSO) percutaneously. The regeneration of discs was assessed by magnetic resonance imaging (MRI), real-time polymerase chain reaction (RT-PCR), and histological analysis. An increased aggrecan and decreased MMP13 gene expression in the treatment group compared to the carrier was observed, and increased matrix PG production was confirmed by histology [91]. The effect of statins on the homeostasis of disc cells was also investigated in previous studies. It has been shown that hydrophilic statins had more regenerative potential on NP cells than lipophilic statins. They also showed that hydrophilic statins increased the expression of type II collagen and SRY-box transcription factor 9 (SOX9) in a lower dosage than lipophilic statins.
It has been shown that the expression level of anabolic genes in NP cells increased by metformin, while the expression of catabolic genes considerably decreased [92]. Gefitinib, a small molecule which inhibits epidermal growth factor receptor (EGFR), was also investigated for its potential effects on IDD regeneration. In an in vitro study, gefitinib at 10 µM was administered 30 minutes before the treatment of rat NP cells with 10 ng/ml TGF-α. After 48h of treatment with TGF-α, RNA and protein analyses were performed. It was concluded that gefitinib caused therapeutic inhibition of EGFR signaling thereby inhibiting IVD degeneration and enhancing IVD matrix synthesis in TGF-α treated NP cells [20]. The modulating effect of tofacitinib on anabolic and catabolic processes was also investigated in rat and human degenerated IVD cells. Tofacitinib was used to pre-treat disc cells for 30 minutes, followed by incubation with soluble IL-6 receptor (Sil-6R) and IL-6. Tofacitinib decreased the expression of catabolic factors such as MMP13. The study showed the therapeutic potential of tofacitinib in lessening the development of IDD through suppressing the catabolic effects of IL-6 [60]. Two similar in vitro studies showed that luteoloside and APO 866 (daporinad) increased the content of ECM-related proteins such as type II collagen and aggrecan and reduced the expression level of MMP13 and ADAMTS5 in IL-1β primed disc cells [57, 93].
Additional targets
Autophagy
Several investigations have demonstrated that autophagy occurs in IVD cells [94-96]. Autophagy is a conserved cellular process that continues to occur in all types of cells throughout life. Through this well-coordinated and multi-step process, cells remove unnecessary or dysfunctional components. Usually, a low basal level of autophagy occurs in IVD cells, which was confirmed in cells isolated from non-degenerative adult rat discs [94]. However, autophagy can considerably increase in degenerative IVD cells [97]. This process can successfully reduce apoptosis in both NP and AF cells, leading to the attenuation of IVD degeneration [94]. It has been demonstrated that nutrition deprivation markedly induced IVD cell apoptosis through the intrinsic pathway, whereas this process can be blocked by sirtuin 1 via acceleration of autophagy [98]. Moreover, the suppression of autophagy by exposure of the cells to 3-methyladenine (an autophagy inhibitor) increased the apoptosis of the cells [99].
The impact of small molecules on autophagy was assessed in different in vitro studies. Shi et al. used autophagy markers (LC3 and Beclin-1) to assess the impact of APO866 on autophagy of NP cells as well as their apoptosis. The results showed that APO866 inhibited IL-1β-induced NP cell apoptosis by induction of autophagy. These findings showed the therapeutic potential of APO866 for IDD [93]. Metformin activates the upstream regulator AMPK, which directly induces autophagy in NP cells in a dose- and time-dependent manner to block apoptosis [92]. In a rat IDD model, the controlled-release of gefitinib protected IVDs from degeneration possibly through the modulation of the EGFR-autophagy axis which was shown to not only suppress cartilage matrix degradation but also boost type II collagen synthesis [20].
Mammalian target of rapamycin (mTOR)
In molecular signaling, the mammalian target of rapamycin (mTOR) acts as a negative regulator of autophagy. The mTOR is a serine/threonine kinase which regulates cellular activation such as cell growth and division, cell motility and cell survival. The mTOR exists in two distinct protein complexes including mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). Protein kinase B (known as AKT), an essential pro-survival mediator by suppressing apoptosis, regulates mTORC1 and mTORC2 [100]. It has been shown that the IVD cells would utilize the mTOR signaling and autophagy to cope with stressful conditions such as low oxygen, pH and nutrient concentration [101]. Rapamycin is the primarily isolated mTORC1 inhibitor which can extend mammalian lifespan via inhibiting the cell cycle progression and lethal neoplastic diseases. Today, serious adverse effects of rapamycin including immunosuppression limited its extensive clinical use. A very recent study assessed the effects of mTOR inhibitors on human IVD cells. In this study, four different small molecules and mTORC inhibitors including INK-128, NVP-BEZ235, MK-2206 and curcumin were examined; the results showed the pharmacological modulation of mTOR signaling and autophagy increased the survival rate of IVD cells by suppression of apoptosis [102].
Anti-senescence effect
Senescent cells are non-dividing cells, which are still metabolically active. These cells have been shown to contribute to the catabolic shift in IVD tissue during degeneration by secreting the senescence-associated secretory phenotype (SASP) which contributes to a pro-inflammatory milieu [103]. Curcumin and o-vanillin have been shown to both exert anti-inflammatory and anti-oxidative effects and act as senomorphic drugs. Cherif et al. observed a reduced number of senescent disc (NP and AF) cells and decreased SASP factors as well as an increase in cell proliferation after treatment with curcumin or o-vanillin. This effect was due to the selective induction of apoptosis in senescent cells without negative effects on the proliferating cells [103, 104].
Bone morphogenetic protein activators
Bone morphogenetic proteins (BMPs such as BMP-2 and BMP-7) have shown promise in IVD regeneration. Li et al. evaluated the effect of BMPs for regeneration of IVD both in vitro and ex vivo. Their results showed that BMP heterodimers could successfully upregulate the aggrecan and type II collagen gene expression, as well as glycosaminoglycan (GAG) synthesis of NP cells [93]. The investigations of kaempferol and statins in the treatment of IDD have shown a potential regenerative effect via the BMP-2 signaling pathway [105, 106]. Lovastatin (at concentrations ≥1 µM) could significantly enhance the expression of anabolic genes encoding BMP-2 and BMP-7 [107]. Although these small molecules show great potential in slowing down the progression of IVD degeneration via activation of the BMP-2 signaling pathway, more studies are needed to further elucidate their effects as BMP activators.
Wnt pathway Inhibitor
In IDD, increased Wnt signaling suppresses progenitor cell proliferation and induces apoptosis of NP cells [108]. Moreover, it has been suggested that the Wnt signaling pathway is involved in fibrosis of the AF. SM04690, a small molecule inhibitor of the Wnt pathway, has been utilized in drug development for the treatment of IDD and osteoarthritis. SM04690 demonstrated regenerative properties in preclinical studies, including reduction of inflammation, inhibition of fibrosis, activation of NP cell proliferation, and production of ECM in a rat IVD model. These findings suggest SM04690 as a potential treatment for IDD [109].
The in vivo and clinical studies focused on the role of small molecules on IVD regeneration. ''n'' denotes the number of in vivo or clinical studies, Abbreviation; IP: intraperitoneal injection; ID: intradiscal injection/delivery; CBD: cannabidiol; EGCG: epigallocatechin 3-gallate.
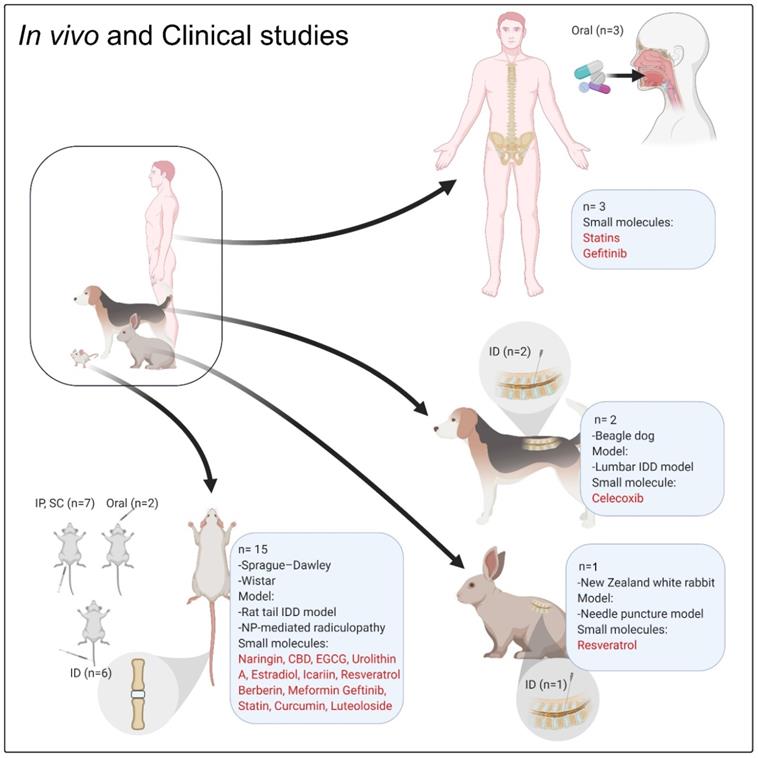
In vivo and clinical studies
To date, several in vivo studies using small molecules and targeting IDD and related degenerative processes have been published (Table 3, Figure 4). All these in vivo studies showed promising results of different small molecules on IDD; however, there is still limited evidence for a regenerative impact of small molecules on degenerated disc tissue. Most of the cited studies showed the preventive effects of the drugs on IDD progression. Only one study showed that EGCG could reach the MRI and histological scoring levels measured in the sham group [50]. Other studies showed that the symptoms or progression of IDD were alleviated in comparison with the vehicle-treated animals (negative control). Approximately 90% of in vivo investigations (Table 3) followed the animal cases up to 12 weeks, which is a very short-term follow-up and could explain such insignificant results [110].
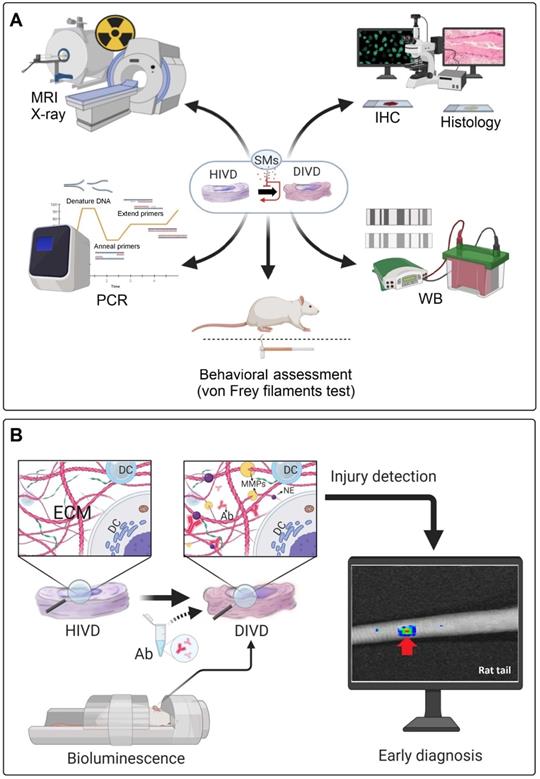
Most of the in vivo investigations used standard methods to analyze the regenerative process or the inhibition of IDD progression, including X-ray, MRI, histopathology and immunohistochemistry (IHC) (Figure 5A). However, these methods have some limitations to assess the beneficial effects of small molecules on IDD. For instance, MRI was used as a technique to analyze the disc height index (DHI) or the Pfirrmann grade in different treatment groups, whereby 3.0 Tesla (T) or lower (1.5T) clinical MRIs were frequently used (7 out of 10 in vivo studies on rodents). The 1.5T or 3.0T MRI and related image quality are not suitable for small animal imaging (mouse, rat, and rabbit), which may lead to an irreproducible scoring by the radiologists [111].
An overview of the in vivo studies for individual small molecules
| Molecule | |
|---|---|
| Naringin | |
| Study | Zhang et al. 2018 [63] |
| Aim | To assess the potential therapeutic effect of Naringin on IVD regeneration |
| Animal/Patient-model | Sprague-Dawley Rat (G: NI, n=36), puncture-induced rat IDD model |
| Intervention | Intraperitoneal injection of Naringin (80mg/kg/day) |
| Analysis | Histopathology and MRI at 4 and 12 weeks after surgery |
| Results/Conclusion | Histology: protection of CEP, no significant difference between saline (vehicle) and Naringin treated groups regarding the damage to NP cells after 12 weeks; MRI: There is a significant difference between (vehicle) and Naringin treated groups in terms of Pfirrmann MRI grade scores. Conclusion: Naringin may exert a protective effect on IVD after an initial injury. |
| Cannabidiol (CBD) | |
| Study | Silveria et al. 2014 [127] |
| Aim | To assess the protective effect of CBD on lesion-induced IDD |
| Animal/Patient-model | Wistar Rat (G: male, n=19), puncture-induced rat IDD model |
| Intervention | Intradiscal injection of CBD (60-80-120nm) |
| Analysis | Histopathology (15 days after surgery), MRI (2 and 15 days after surgery) |
| Results/Conclusion | Histology: CBD (120 nmol) prevented the typical histological changes in the AF, no significant protective effect seen on NP. MRI: Injection of CBD (120 nmol) immediately after lesion significantly improved MRI pixel intensity. Conclusion: Considering that CBD presents an extremely safe profile, only high dose of CBD (120 nmol) could halt the IDD progression. |
| Epigallocatechin 3-gallate (EGCG) | |
| Study | Krupkova et al. 2014 [50] |
| Aim | To analyze the effect of EGCG on discogenic pain |
| Animal/Patient-model | Sprague-Dawley Rat (G: female, n=60), Autologous NP was harvested from the tail and applied to the dorsal root ganglion (DRG, L5-L6) |
| Intervention | Local injection of 0.1ml EGCG (10 and 100μM) into the underlayer of epineurium |
| Analysis | Hind paw withdrawal response to von Frey Filament test (2, 7, 14, 21 and 28 d post-surgery) |
| Results/Conclusion | von Frey Filament test: During 28 days, NP+EGCG treatment significantly increased mechanical withdrawal thresholds in comparison to the NP+vehicle group, and reached levels measured in the sham group. Conclusion: EGCG (10 and 100μM) inhibits pain behaviour in vivo. |
| Urolithin A (UA) | |
| Study | Liu et al. 2018 [54] |
| Aim | To assess the beneficial effect of UA on IDD |
| Animal/Patient-model | Sprague-Dawley Rat (G: male, n=30), puncture-induced rat IDD model |
| Intervention | Oral delivery of UA (0.25 g per kg of diet or 25 mg/kg/day) |
| Analysis | X-ray, MRI and histopathology (4 weeks post-surgery) |
| Results/Conclusion | X-ray: UA treatment group showed no significant disc space. MRI: Pfirrmann grade scores were lower in the UA treatment group than the IDD control Histopathology: UA treatment group considerably alleviated IVD destruction in comparison to the IDD control Conclusion: UA may be a useful small molecule for the treatment of IDD. |
| Estradiol (E2) | |
| Study | Jin et al. 2018 [121] |
| Aim | To analyze the effect of E2 on IDD in the model of menopause rats |
| Animal/Patient-model | Sprague-Dawley Rat (G: female, n=30), oophorectomy (OVX) to induce menopausal in rats |
| Intervention | 10 µg/kg/day E2 supplementation for 12 weeks |
| Analysis | MRI, histopathology, IHC (LC3 for autophagy) (12 weeks post-surgery) |
| Results/Conclusion | MRI: T2 mapping showed a marked increase in results in OVX + E2 and sham when compared to OVX + vehicle Histopathology: The OVX + E2 treatment group showed the NP tissues were similar to those observed in the sham group IHC: There are no significant differences between OVX + E2 treatment and sham group in terms of autophagy Conclusion: E2 via regulating the redox balance (autophagy) of IVD could be a potential therapeutic agent for IDD in the postmenopausal women. |
| Study | Liu et al. 2018 [132] |
| Aim | To further explore whether estradiol (E2) had protective effects on IDD in OVX rats |
| Animal/Patient-model | Sprague-Dawley Rat (G: male, n=40), puncture-induced OVX-rat IDD model |
| Intervention | Subcutaneous injection of 20 µg/kg/day E2 for 28 d |
| Analysis | X-ray (disc height index-DHI), histopathology, IHC, western blot (WB) (30 d post-surgery) |
| Results/Conclusion | X-ray: In OVX + E2 treated animals, X-ray showed a markedly higher DHI in comparison to the OVX+ vehicle group. Histopathology: Mean histological scores in Sham and OVX + E2 group were significantly lower than OVX+ vehicle group IHC: E2 downregulated caspase-3, MMP-3 and MMP-13 proteins level but upregulated collagen Type II WB: Confirmed IHC results Conclusion: E2 shows protective effects against IDD by down-regulating catabolic proteins and up-regulating anabolic ones in OVX- animal models. |
| Icariin | |
| Study | Hua et al. 2020 [146] |
| Aim | To explore the effect of icariin on IDD |
| Animal/Patient-model | Sprague-Dawley Rat (G: male, n=24), needle puncture model |
| Intervention | Intraperitoneal administration of icariin (30 mg/kg) for 8 w post-surgery |
| Analysis | MRI and histopathology (8 weeks post-surgery) |
| Results/Conclusion | MRI: Pfirrmann grade scores were significantly lower in the icariin treatment group than the saline treatment Histopathology: Icariin treatment reduced histopathological changes (disruption of AF), although some degeneration was still observed Conclusion: Icariin could be utilized as a protective agent to inhibit further degeneration after injury. |
| Resveratrol | |
| Study | Kwon 2013 [91] |
| Aim | To evaluate whether resveratrol had anabolic effects on IDD in a rabbit model |
| Animal/Patient-model | New Zealand white rabbit (G: male, n=24), needle puncture model |
| Intervention | Two times intradiscal injections of 15 µL of 100 µM resveratrol in DMSO, repeat dose administrated 2 weeks after the first injection |
| Analysis | MRI (4, 8, 16 weeks after the initial injection), histopathology (16 weeks after the initial injection) |
| Results/Conclusion | MRI: MRI scores significantly lower in the resveratrol group than the DMSO (vehicle) group Histopathology: Significant higher histological grades are noted in the DMSO group when compared with the resveratrol group Conclusion: icariin may be a promising candidate for the treatment of IDD. |
| Study | Lin et al. 2016 [117] |
| Aim | To assess the effect of resveratrol of on NP-mediated (discogenic) pain |
| Animal/Patient-model | Sprague-Dawley Rat (G: female, n=36), NP-mediated radiculopathy (model) |
| Intervention | Local injection of 0.1ml resveratrol (50 µM) into the underlayer of epineurium |
| Analysis | von Frey filaments test (0, 3, 7, 14, 21 d post-surgery), histopathology and IHC (7 and 14 d post-surgery). |
| Results/Conclusion | von Frey filaments test: significant pain reduction by resveratrol treatment Histopathology and IHC: resveratrol treatment showed improved cell structure, with decreased edema and focal hyperemia compared with the negative control group. The expression level of IL-1 and TNF-α proteins decreased by resveratrol treatment. Conclusion: The results indicate the potential of resveratrol for attenuating discogenic pain. |
| Celecoxib (CXB) | |
| Study | Willems et al. 2015 [141] |
| Aim | To assess the effect of controlled delivery of CXB on IVD regeneration |
| Animal/Patient-model | Dog (G: female, n=18), canine model of spontaneous mild IDD |
| Intervention | a bolus intradiscal injection of CXB (7.7 μM), intradiscal injection CXB loaded hydrogel (77 μM and 770 μM) |
| Analysis | Histopathology and IHC, Q-PCR (4 weeks after the initial injection) |
| Results/Conclusion | Histology and IHC: No significant differences were found between the injected treatments Q-PCR: Only relative gene expression levels of BCL2 and PGE2 were significantly downregulated in the CXB-loaded hydrogel in comparison to the sham Conclusion: The controlled delivery of CXB resulted in limited inhibition of PGE2 production in dogs with spontaneous IDD Limitations: Due to technical limitations, it was impossible to determine the CXB tissue levels, and hence in vivo release profile of CXB. |
| Study | Tellegen et al. 2018 [61] |
| Aim | The effect of control release of CXB on IVD regeneration |
| Animal/Patient-model | Dog (G: male, n=6), canine IDD model |
| Intervention | One month after surgery, Intradiscal delivery of 40 µl CXB loaded microsphere (CXB-M), low (8.4 µg CXB) and high dose (280 µg CXB) |
| Analysis | MRI (0 d, 4 and 12 weeks after injection), histopathology and IHC (12 weeks after the initial injection) |
| Results/Conclusion | MRI: DHI was maintained in the disc treated with either low or high dose CXB-M, Pfirrmann score was lower in CXB-M treated groups compared to the negative control Histopathology and IHC: Controlled release of CXB inhibited progression of IDD, the development of osteophyte formation, and decreased the immunopositivity of nerve growth factor Conclusion: Intradiscal controlled release of CXB inhibited progression of IDD in vivo. |
| Study | Tellegen et al. 2018 [115] |
| Aim | To assess the impact of sustain delivery of CXB on discogenic pain |
| Animal/Patient-model | Dog (G: female, n=10), canine patients with low back pain |
| Intervention | Intradiscal injection loaded hydrogel containing 2.93 μg/mL CXB |
| Analysis | MRI (0 d and 12 weeks after injection), clinical examination of low back pain (12 weeks after the initial injection) |
| Results/Conclusion | MRI: No evident of CXB regenerative effects on MRI Clinical examination: The reduction of back pain achieved in 9 of 10 dogs. In 3 of 10 dogs, back pain recurred after 12 weeks Conclusion: the majority of the treated canine patients, quality of life improved without evident regenerative effects Limitations: small group size, absence of a placebo group. |
| Berberine | |
| Study | Luo et al. 2019 [86] |
| Aim | The effects of berberine on IDD were investigated |
| Animal/Patient-model | Sprague-Dawley Rat (G: female, n=24), needle puncture model |
| Intervention | Intraperitoneal administration of berberine (150 mg/kg/day) for 8 weeks post-surgery |
| Analysis | MRI and histopathology (8 weeks post-surgery) |
| Results/Conclusion | MRI: Pfirrmann scores were significantly lower in the berberine treated animals than the saline treatment Histopathology: The histological scores in the berberine treatment group significantly lower than IDD control group (saline). Conclusion: Berberine could attenuate puncture-induced IDD in animal model. |
| Metformin | |
| Study | Chen et al. 2016 [92] |
| Aim | To assess the effects of Metformin on IDD |
| Animal/Patient-model | Rat (G: NI, n=NI), puncture-induced IDD model |
| Intervention | Intraperitoneal administration of metformin (50 mg/kg/day) for 16 weeks post-surgery |
| Analysis | MRI and histopathology (8-16 weeks post-surgery) |
| Results/Conclusion | MRI: Metformin treated group showed lower Pfirrmann scores compared to the vehicle-treated animals Histopathology: The histologic score of the metformin group was significantly lower than those of negative control both at 8- and 16-weeks post-surgery Conclusion: Metformin showed a protective effect against progression of IDD. |
| SM04690 | |
| Study | Barroga et al. 2017 [109] |
| Aim | To investigate the effects of SM04690 on IDD |
| Animal/Patient-model | Rat (G: NI, n=NI), puncture-induced IDD model |
| Intervention | Single intradiscal of SM04690 (0.066 mg/disc) |
| Analysis | X-ray and histopathology (6 weeks post-surgery) |
| Results/Conclusion | X-ray: % DHI in SM04690 treated animals significantly increased compared to vehicle control Histopathology: Treatment by SM04690 increased number of NP cells and increased ECM vs. vehicle control Conclusion: SM04690 has potential as a modifying therapy for IDD. |
| Gefitinib | |
| Study | Pan et al. 2018 [20] |
| Aim | to investigate the therapeutic potential of gefitinib in ameliorating IDD |
| Animal/Patient-model | Sprague-Dawley Rat (G: female, n=18), puncture-induced IDD model |
| Intervention | Three µl aliquots intradiscal injection of gefitinib (30 mM) |
| Analysis | MRI and histopathology (4 weeks post-surgery) |
| Results/Conclusion | MRI: DHI% values of the gefitinib-treated group were significantly higher than those of the IDD control. The Pfirrmann scores also showed that the degree of disc degeneration was markedly lower in the gefitinib-treated group as well. Histopathology: The gefitinib treatment considerably decreased the histological scores in comparison to IDD control group. Conclusion: The results suggest the potential application of gefitinib for treating IDD. |
| Statin | |
| Study | Than et al. 2014 [142] |
| Aim | To find a new conservative treatment for IDD and related discogenic pain |
| Animal/Patient-model | Sprague-Dawley Rat (G: NI, n=272), puncture-induced IDD model |
| Intervention | Six weeks post-surgery, intradiscal injection of 2μL simvastatin (SIM) at 3 different doses (5, 10, or 15 mg/mL) in either a saline or hydrogel carrier |
| Analysis | MRI and histopathology and IHC (2, 4, 8, 12 and 24 weeks after the initial injection) |
| Results/Conclusion | MRI: MRI analysis showed a higher index (better results) for treatment with 5 mg/ml SIM administered in comparison to the higher doses (15 mg/ml), MRI index: 5 mg/ml hydrogel>5 mg/ml saline>10 mg/ml saline>15 mg/ml saline>15 mg/ml hydrogel Histopathology and IHC: histological grades confirmed the MRI results Conclusion: Intradiscal injection of simvastatin into IDD may result in retardation of degeneration process (5 mg/ml simvastatin in a hydrogel carrier) Limitation: unbalanced time point analysis for all groups, Control group was assessed only histologically. |
| Luteoloside | |
| Study | Lin et al. 2019 [57] |
| Aim | To investigate the protective potential of luteoloside in IDD |
| Animal/Patient-model | Sprague-Dawley Rat (G: NI, n=36), puncture-induced IDD model |
| Intervention | Intraperitoneal injection of 10mg/kg/day luteoloside for 4 and 8 weeks post-surgery |
| Analysis | MRI, X-ray and histopathology (4, and 8 weeks post-surgery) |
| Results/Conclusion | MRI: Pfirrmann MRI grade scores were significantly lower in the luteoloside group than in the IDD group X-ray: DHI was significantly lower in the IDD group than in the luteoloside treatment group Histopathology: Both ECM and NP tissues were better preserved in the luteoloside-treated group when compared to the IDD group Conclusion: Luteoloside only ameliorate IDD progression during long-term follow-up (8 weeks). |
| Curcumin | |
| Study | Ma et al. 2015 [145] |
| Aim | To observe the effect of curcumin on IDD |
| Animal/Patient-model | Sprague-Dawley Rat (G: male, n=60), Surgically induced IDD model in the lumbar area (removal of the spinous processes, the articular processes, the supraspinous ligaments and the interspinous ligaments). |
| Intervention | Intraperitoneal injection of 50mg/kg and 100mg/kg curcumin (single dose) |
| Analysis | MRI, Electron microscopy (EM), RT-PCR, and western blot (WB) (6 weeks post-surgery) |
| Results/Conclusion | MRI: The IVD signals of curcumin-treated animals (L1-6) were slightly lower than those in the normal group but were considerably higher than those of IDD models. EM: The degree of degeneration related to NP, AF and ECM structure of IVD samples was better in curcumin-treated animals in comparison to the IDD models RT-PCR: The expression levels of NF-κB-p65 and TNF-α were significantly lower in curcumin-treated animals than the other groups. WB: curcumin-treated animals had significantly lower NF-κB-p65 and TNF-α expression levels than IDD animal models. Conclusion: curcumin can decelerate the IDD process by blocking the NF-κB-p65 pathway and reducing inflammatory factors Limitation: 1. No statistical analysis was performed on the differences between each group regarding the MRI test. 2. Lack of further verification of the type of lumbar IDD and related IDD categorization. |
G: gender, NI: no information.
Conventional diagnostic approaches and a proposed diagnostic method for detection of IDD. (A) Frequently used diagnostic methods in several in vivo studies for the diagnosis of IVD degeneration and evaluation of treatment responses. (B) Specific antibodies (Ab) may be locally injected into disc areas through the proposed method to attach with the ECM neoepitopes (NE); which produced during early degeneration processes. Then, the whole body animal bioluminescence could be used to track the Abs-neoepitopes complex (rat tail IDD model). PCR: polymerase chain reaction; WB: western blot; MRI: magnetic resonance imaging; IHC: immunohistochemistry; HIVD: healthy intervertebral disc; DIVD: degenerated intervertebral disc; SMs: small molecules; Ab: antibody; NE: neo-epitope; ECM: extracellular matrix; DC: disc cell; MMPs: matrix metalloproteinases.
Small laboratory mammals are the main animal models for IDD [112, 113]; according to Table 3, 16 out of 18 studies utilized rodent and lagomorph animal models (rat and rabbit) to explore the effect of small molecules on IDD regeneration or discogenic pain. However, significant limitations exist with using such animal models for IVD regeneration studies. For instance, in the rat tail models, the mechanism of disc injury is different due to different mechanical loading and persistence of notochordal cells which affect the regeneration processes. Furthermore, due to the smaller size of the disc, the diffusion of nutrition is different in comparison with the human disc. Another limitation is the lack of human-like longitudinal compression in the quadrupedal animal models [114]. In previous studies, chondrodystrophic dogs (beagle) were used to analyse the effect of local delivery of celecoxib (CXB) on IDD [61, 115]. Tellegen et al. studied the therapeutic effect and the safety of celecoxib-loaded microspheres administered in a canine IDD model. This study showed that there was no evidence of adverse effects on MRI or macroscopic evaluation of IVDs. The diagnostic analysis of NP PG content revealed that site-targeted and sustained administration of CXB inhibited IDD. Local delivery of the COX-2 inhibitor showed decreased neuronal growth factor and PGE2 tissue levels, which led to an inhibition of the inflammation and alleviation of pain [61]. In another related study, the biocompatibility, safety and feasibility of poly(ε-caprolactone-co-lactide)-b-poly(ethylene glycol)-b-poly(ε-caprolactone-co-lactide) (PCLA-PEG-PCLA) hydrogel releasing celecoxib was investigated. The biocompatibility was evaluated by administering a subcutaneous injection in rats. The feasibility and safety were evaluated by administering an intradiscal injection to dogs suffering from early spontaneous IDD. Clinical improvement was achieved by reduction of back pain in 9/10 dogs, which was shown by clinical examination and owner questionnaires. The study demonstrated the effectiveness and safety of hydrogel-based celecoxib delivery [115]. Chondrodystrophic dogs, as a large animal model, not only lose the notochordal cells following birth, but they also have a special phenotype (short and curved limbs) predisposing them to spontaneous disc degeneration. With respect to ethical concerns, the number of animals (N ranging from 12 up to 40, Table 3) is another limiting factor for the majority of in vivo studies, which can make it difficult to interpret the results due to high variations.
The behavioral assessment of discogenic pain (i.e., Von Frey filament test) in animal models is still in its infancy [110]. Von Frey filament test provides a quantitative measurement of the paw withdrawal threshold [116]. Krupkova et al. and Lin et al. used this method to analyze the effect of EGCG and resveratrol on IVD regeneration and related discogenic pain [50, 117]. However, in other in vivo studies, it is unknown whether the regeneration of the IVD is correlated with the resolution of discogenic pain.
In a rat tail static compression model, Yurube et al. evaluated the presence of the MMP- and ADAMTS-cleaved aggrecan neoepitopes in vivo, using IHC. These neoepitopes produced by catabolic enzymes in the degeneration process could be used for early detection of IDD [118]. As a proposed diagnostic approach, these antibodies could be modified and labeled with luminescence agents to be detectable via in vivo bioluminescence imaging. This idea might be developed in the future for early diagnosis of IDD and the results could be used for evaluation of the treatment responses in both animal models and clinical cases (Figure 5B).
Until now, there have been no record of clinical trials (clinicaltrails.gov; www.clinicaltrialsregister.eu) and only three clinical studies have been reported regarding the effect of small molecules on IVD regeneration: Five human patients with non-small cell lung cancer and IDD received gefitinib treatment over the past five years, which not only resulted in tumor regression but also ameliorated IDD [20]. Makris et al. conducted a retrospective cohort study to evaluate the use of statins in higher doses for patients suffering from a spinal degenerative joint disease (SDJD). They described an inversely proportional relationship between the dose of statins prescribed to patients suffering from hypercholesterolemia and the risk of SDJD occurrence [119]. Contrary to the previous reports, a higher risk of developing low back pain was associated with the higher dosage of statins, which tended to cause statin-induced myopathy [120]. With respect to the in vivo and clinical studies, the application of biological treatment strategies for the regeneration of IVDs remains a largely undiscovered field which has great potential for further investigations.
Future prospective and Conclusions
Small molecule drugs have shown promising therapeutic potential for the regeneration of IVD tissue and attenuation of the degenerative process. Nowadays, the focus in medicine is on individualized therapies and the factors aggravating the degenerative process in an individual patient need to be considered in more detail. Research towards therapies that induce the intrinsic regenerative potential of the IVD and CEP cells are of high interest due to the long-lasting effects and limited disturbance of the tissue homeostasis which happens with external manipulation. Several in vitro studies have investigated the potential of small molecules to induce autophagy, for stimulating the natural process of IVD cells to adapt and regenerate in a stressful environment [83, 88]. To effectively control the IDD process, emphasis should be put upon an individualized therapeutic approach, like estradiol supplementation which has been advised for menopausal women [121]. Studies on senomorphic drugs show the promising potential of curcumin or o-vanillin in reducing the number of senescent cells. A selective apoptotic effect on senescent cells, while sparing the actively proliferating cells is of high significance and further work must be followed up to translate this promising potential to organ culture or in vivo studies [104]. An important factor which should be highlighted for utilization of different small molecule agents is the limit point of pharmacological or biological treatments. The selection of degeneration severity-based therapeutic options needs to be assessed before the start of any treatment. Biological or molecular treatments including growth factors, cell, gene therapy, and small molecules, could typically be used to repair and regenerate the degenerated disc in the early stages of IDD (Pfirrmann grade I-III). However, these methods are not sufficient to treat the advanced stages of diseases (Pfirrmann grade IV-V) and surgery would be required as the last resort. Therefore, detailed diagnostic measures are indispensable to assess the type and stage of IDD. New diagnostic approaches (i.e., in vivo bioluminescence) should be developed to detect early IDD or to evaluate the prompt tissue responses to different small molecules in vivo.
The promising results of the discussed investigations are only the foundation to answer the more complex questions arising from ex vivo and in vivo models. Small molecules have a huge therapeutic potential, as their application can be directed with controlled release formulas and in many cases, the evidence of their efficacy is already proven in the treatment of other diseases in the clinics [61, 92, 119]. It is of great importance to consider the dosing, the systemic effects of the drugs and interactions of the various compounds with each other and with over the counter and prescribed medicines. Combination therapies may further potentiate the positive effects of the small molecules on the regeneration of the IVD cells. In the search for therapies on the degenerated IVD, delivery of the compound to the IVD tissue is a question that will always complicate the path to clinical translation (Table S4). The small molecules administered systemically may be able to reach the disc in some cases; however, in compromised situations such as CEP damage or sclerosis, it may be a challenge to reach an effective concentration of the compound in the IVD. Local delivery of small molecules (i.e., direct injection) is another strategy to overcome the limitations of systemic delivery, such as diffusion problem due to CEP damage, or systemic side effects. The evolving field of hydrogels, microspheres, nanoparticles, and further substances that allow for a controlled release in situ show promising results to resolve this problem in the future [20, 61, 114]. Nevertheless, needle size should be considered to avoid exacerbating degeneration.
In vitro results have shown the beneficial effects of small molecules on regeneration of degenerated IVDs through attenuation of inflammation, oxidative stress, apoptosis, catabolism, and stimulation of anabolic processes via different signaling pathways. Similarly, most in vivo results also indicate beneficial effects of these small molecules on the amelioration of IDD [20, 57, 63, 92, 116]. However, the number of clinical studies in this regard is very low, and no clinical trials on the effect of these small molecules on IDD have been performed so far. In future, a road map for discovery of new small molecules and their clinical translation for IDD treatments should be provided. To date, the major limitations for clinical translation include the insufficient in vivo evidence, due to a lack of representative animal models; the lack of an adequate delivery system for different small molecules; and the lack of patient stratification methods to identify the patients who would respond to and benefit from a small molecule-based therapy. The potential necessity of long-term treatment should also be emphasized to achieve a successful therapy from small molecule agents for regeneration of degenerated IVD. The avascular nature of the IVD may increase the duration of treatment, particularly for oral administration of small molecules which should first be absorbed by digestive tracts, delivered to the target site via the systemic circulation and finally reach the degenerated disc through diffusion.
To bridge the gap between rudimentary animal models and clinical studies, a more clinically relevant animal model should be used to better replicate the complexity of the human IVD and the pathology of IDD. The modeling of pain or inflammation, systemic response to degeneration and related treatment are only represented to a limited extent in the established in vitro models. Considerations for establishing an appropriate animal model include factors like the absence of notochordal cells, size of IVD tissue, body mass relative to humans, mechanical compression forces upon the IVD, type of injury and ethics [113]. Non-human primates (i.e., baboons and macaques) and chondrodystrophic dogs (i.e., beagle and dachshund) closely match the clinical condition of IDD regarding many of the physical and mechanistic criteria (spontaneous models of IDD) [122, 123]. However, ethical considerations should preclude their widespread use in pre-clinical studies. The ovine IDD model could be one of the best animal models due to desirable characteristics such as the absence of notochordal cells, similar body mass to humans, and mechanical compression forces acting upon the IVD [124]. Other study parameters such as follow-up time and animal numbers are also important factors in study design. With short-term follow-up (8-12 weeks), the study is usually limited to investigating the protective effect of small molecules on IDD progression; while longer-term follow-up studies (6 to 12 months) are necessary to detect any regenerative effects [97]. Moreover, if ethical considerations allow for it, a higher number of animals for in vivo studies could be used to decrease the outcome variation and make it easier to draw conclusions [125].
Behavioral pain assessment should be utilized to assess the effect of small molecules on IDD such as von Frey filament test, gait analysis, weight loading, and hot plate analysis. It should be highlighted that the collaboration of neuroscience, orthopedic, and biomedical sciences is a necessity to extend the current understanding of the complex and multifactorial pathophysiology of IDD and discogenic pain [110]. For in vivo imaging techniques, using equipment with higher sensitivity and resolution is also recommended. Recently, better MRI systems have become available for small animal models that are able to generate magnetic fields of 9.5T or even 21T, offering an excellent tool for small animal preclinical studies [111]. Regarding ethical issues, it should also be noted that all mechanisms, pathways and related beneficial effects of each small molecule on IDD have to be identified as best as possible by in vitro studies, followed by ex-vivo IDD organ culture models to confirm the results. Furthermore, new experimental techniques such as lab-on-a-chip that simulate the activities, mechanics and physiological response of entire organs and organ systems are being developed [126]. Recently, a microfluidic disc-on-a-chip device has been developed that was tailored for laboratory small animal disc organs as a long-term ex-vivo organ culture platform. This device lays groundwork for future studies by simulating the chronic nature of IDD [126].
In conclusion, small molecule therapy is an alternative treatment for conventional therapies and surgical approaches in discogenic pain. Effective regeneration of the degenerated IVD with small molecules has opened an important area of research in IVD regeneration in the last decade. However, it is of great importance to investigate the mechanism of action of small molecules in targeting different signaling pathways towards an effective therapy. Several of these small molecules are effective in inhibiting apoptosis, inflammation, oxidative stress, and senescence, which can prevent degeneration of the disc cells. They also showed anabolic and anti-catabolic effects (intrinsic regeneration) which are critical factors for the regeneration of damaged IVD. Further analysis, especially in large animal and advanced organ culture models and then clinical studies are needed to confirm the preliminary results obtained from in vitro investigations. Moreover, it will be of importance to investigate other regenerative effects of different small molecules, such as the induction of NP-like cell differentiation in endogenous and exogenous stem or progenitor cells.
Abbreviations
LBP: low back pain; IVD: intervertebral disc; IDD: intervertebral disc degeneration; CLBP: chronic low back pain; NP: nucleus pulposus; AF: annulus fibrosus; SP: spinous process; TP: transverse process; SC: spinal cord; VB: vertebral body; ECM: extracellular matrix; PG: proteoglycan; IFN-γ: interferon-gamma; TNF-α: tumor necrosis factor-alpha; IL: interleukin; ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs; MMP: matrix metalloproteinase; CBD: cannabidiol; ECGC: epigallocatechin gallate; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; MAPK: MAP Kinase; JAK3: janus kinase 3; Nrf2: nuclear factor erythroid 2-related factor 2; HO-1: heme oxygenase-1; PGE2: prostaglandin E2; COX2: cyclooxygenase-2; E2: 17 beta-estradiol; Bcl-2: B-cell lymphoma 2; Bax: BCL-associated X; BAK1: BRI1-associated receptor kinase 1; PI3K: phosphatidylinositol 3-kinase; AKT: protein kinase B; ROS: reactive oxygen species; iNOS: inducible nitric oxide synthase; SOX: SRY-Box transcription factor; TGF-β: transforming growth factor beta; EGFR: epidermal growth factor receptor; BMP: bone morphogenetic protein; MRI: magnetic resonance imaging; RT-PCR: real-time polymerase chain reaction; IHC: immunohistochemistry; WB: western blot; HIVD: healthy intervertebral disc; DIVD: degenerated intervertebral disc; SMs: small molecules; CXB: celecoxib; PCLA-PEG-PCLA: poly(ε-caprolactone-co-lactide)-b-poly(ethylene glycol)-b-poly(ε-caprolactone-co-lactide); mTOR: mammalian target of rapamycin; NLRP3: nod-like receptor protein 3; IGF-I: insulin-like growth factor 1; PDGF: platelet-derived growth factor; FGF: fibroblast growth factor; ERK: extracellular signal-regulated kinase; SDJD: spinal degenerative joint disease; UA: urolithin A; T: Tesla; DHI: disc height index; Ab: antibody; NE: neo-epitope; DC: disc cell; IP: intraperitoneal injection; ID: intradiscal injection/delivery.
Supplementary Material
Supplementary figures and tables.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581-85
2. Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95-103
3. Juniper M, Le TK, Mladsi D. The epidemiology, economic burden, and pharmacological treatment of chronic low back pain in France, Germany, Italy, Spain and the UK: a literature-based review. Expert Opin Pharmacother. 2009;10:2581-92
4. Rizvi M. Novel treatment strategies for intervertebral disc degeneration. Saudi J Health Sci. 2015;4:5-15
5. Zhang Y, An HS, Tannoury C, Thonar EJ, Freedman MK, Anderson DG. Biological treatment for degenerative disc disease: implications for the field of physical medicine and rehabilitation. Am J Phys Med Rehabil. 2008;87:694-702
6. Maher C, Underwood M, Buchbinder R. Non-specific low back pain. Lancet. 2017;389:736-747
7. Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88(Suppl 2):10-14
8. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976). 2006;31:2151-61
9. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44-56
10. Lang G, Liu Y, Geries J, Zhou Z, Kubosch D, Sudkamp N. et al. An intervertebral disc whole organ culture system to investigate proinflammatory and degenerative disc disease condition. J Tissue Eng Regen Med. 2018;12:e2051-61
11. Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol (Noisy-le-grand). 2007;53:4-18
12. Kanayama M, Togawa D, Takahashi C, Terai T, Hashimoto T. Cross-sectional magnetic resonance imaging study of lumbar disc degeneration in 200 healthy individuals. J Neurosurg Spine. 2009;11:501-07
13. Patil P, Niedernhofer LJ, Robbins PD, Lee J, Sowa G, Vo N. Cellular senescence in intervertebral disc aging and degeneration. Curr Mol Biol Rep. 2018;4:180-90
14. Feng C, Liu H, Yang M, Zhang Y, Huang B, Zhou Y. Disc cell senescence in intervertebral disc degeneration: Causes and molecular pathways. Cell Cycle. 2016;15:1674-84
15. Dimar JR 2nd, Glassman SD, Carreon LY. Juvenile degenerative disc disease: a report of 76 cases identified by magnetic resonance imaging. Spine J. 2007;7:332-37
16. Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine (Phila Pa 1976). 2007;32:816-23
17. Sheyn D, Ben-David S, Tawackoli W, Zhou Z, Salehi K, Bez M. et al. Human iPSCs can be differentiated into notochordal cells that reduce intervertebral disc degeneration in a porcine model. Theranostics. 2019;9:7506-24
18. Liao Z, Luo R, Li G, Song Y, Zhan S, Zhao K. et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics. 2019;9:4084-100
19. Molinos M, Almeida CR, Caldeira J, Cunha C, Gonçalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12:20141191
20. Pan Z, Sun H, Xie B, Xia D, Zhang X, Yu D. et al. Therapeutic effects of gefitinib-encapsulated thermosensitive injectable hydrogel in intervertebral disc degeneration. Biomaterials. 2018;160:56-68
21. Pattappa G, Li Z, Peroglio M, Wismer N, Alini M, Grad S. Diversity of intervertebral disc cells: phenotype and function. J Anat. 2012;221:480-96
22. Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21-24
23. Paris SV, Lonnemann ME. Chapter 55 - Mechanical and Discogenic Back Pain. In: Placzek JD and Boyce DA (eds) Orthopaedic Physical Therapy Secrets (Third Edition). Elsevier. 2017 pp.444-53
24. Jackson AR, Travascio F, Gu WY. Effect of mechanical loading on electrical conductivity in human intervertebral disk. J Biomech Eng. 2009;131:054505
25. Erwin WM, Hood KE. The cellular and molecular biology of the intervertebral disc: A clinician's primer. J Can Chiropr Assoc. 2014;58:246-57
26. Berthet-Colominas C, Miller A, Herbage D, Ronziere MC, Tocchetti D. Structural studies of collagen fibres from intervertebral disc. Biochim Biophys Acta. 1982;706:50-64
27. Wu Y, Cisewski S, Sachs BL, Yao H. Effect of cartilage endplate on cell based disc regeneration: a finite element analysis. Mol Cell Biomech. 2013;10:159-82
28. Feng Y, Egan B, Wang J. Genetic Factors in Intervertebral Disc Degeneration. Genes Dis. 2016;3:178-85
29. Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13:318-30
30. Vergroesen PP, Kingma I, Emanuel KS, Hoogendoorn RJ, Welting TJ. et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil. 2015;23:1057-70
31. Johnson WE, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658-64
32. Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine (Phila Pa 1976). 2000;25:1625-36
33. Benneker LM, Heini PF, Alini M, Anderson SE, Ito K. Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine (Phila Pa 1976). 2005;30:167-73
34. Monchaux M, Forterre S, Spreng D, Karol A, Forterre F, Wuertz-Kozak K. Inflammatory Processes Associated with Canine Intervertebral Disc Herniation. Front Immunol. 2017;8:1681
35. Binch AL, Cole AA, Breakwell LM, Michael AL, Chiverton N, Creemers LB. et al. Nerves are more abundant than blood vessels in the degenerate human intervertebral disc. Arthritis Res Ther. 2015;17:370
36. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428-435
37. Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010;86:226-35
38. Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435-61
39. Marom B, Rahat MA, Lahat N, Weiss-Cerem L, Kinarty A, Bitterman H. Native and fragmented fibronectin oppositely modulate monocyte secretion of MMP-9. J Leukoc Biol. 2007;81:1466-76
40. Morwood SR, Nicholson LB. Modulation of the immune response by extracellular matrix proteins. Arch Immunol Ther Exp (Warsz). 2006;54:367-374
41. Link W. Principles of Cancer Treatment and Anticancer Drug Development; 1st ed. Switzerlnad:Springer International Publishing. 2019
42. Sandborn W. New Targets for Small Molecules in Inflammatory Bowel Disease. Gastroenterol Hepatol. 2015;11:338-40
43. Hojjat-Farsangi M. Small-Molecule Inhibitors of the Receptor Tyrosine Kinases: Promising Tools for Targeted Cancer Therapies. Int J Mol Sci. 2014;15:13768-13801
44. Efe JA, Ding S. The evolving biology of small molecules: controlling cell fate and identity. Philos Trans R Soc Lond B Biol Sci. 2011;366:2208-21
45. Wuertz K, Vo N, Klestsas D, Boos N. Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-κB and MAP kinases. Eur Cell Mater. 2012;23:103-19
46. Risbud MV, Fertala J, Vresilovic EJ, Albert TJ, Shapiro IM. Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions and resist apoptosis induced by serum withdrawal. Spine. 2005;30:882-89
47. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10:44-56
48. Cao L, Chen X, Xiao X, Ma Q, Li W. Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways. Int J Oncol. 2016;49:735-43
49. Xu L, Botchway BOA, Zhang S, Zhou J, Liu X. Inhibition of NF-κB Signaling Pathway by Resveratrol Improves Spinal Cord Injury. Front Neurosci. 2018;12:690-90
50. Krupkova O, Sekiguchi M, Klasen J, Hausmann O, Konno S, Ferguson SJ. et al. Epigallocatechin 3-gallate suppresses interleukin-1beta-induced inflammatory responses in intervertebral disc cells in vitro and reduces radiculopathic pain in rats. Eur Cell Mater. 2014;28:372-86
51. Hua W, Zhang Y, Wu X, Kang L, Tu J, Zhao K. et al. Icariin Attenuates Interleukin-1beta-Induced Inflammatory Response in Human Nucleus Pulposus Cells. Curr Pharm Des. 2018;23:6071-78
52. Zhu J, Tang H, Zhang Z, Zhang Y, Qiu C, Zhang L. et al. Kaempferol slows intervertebral disc degeneration by modifying LPS-induced osteogenesis/adipogenesis imbalance and inflammation response in BMSCs. Int Immunopharmacol. 2017;43:236-42
53. Lu L, Hu J, Wu Q, An Y, Cui W, Wang J. et al. Berberine prevents human nucleus pulposus cells from IL-1β-induced extracellular matrix degradation and apoptosis by inhibiting the NF-κB pathway. Int J Mol Med. 2019;43:1679-86
54. Liu H, Kang H, Song C, Lei Z, Li L, Guo J. et al. Urolithin A Inhibits the Catabolic Effect of TNFalpha on Nucleus Pulposus Cell and Alleviates Intervertebral Disc Degeneration in vivo. Front Pharmacol. 2018;9:1043
55. Li H, Liang C, Chen Q. et al. Rhein: A potential biological therapeutic drug for intervertebral disc degeneration. Med Hypotheses. 2011;77:1105-07
56. Li Z, Gehlen Y, Heizmann F, Grad S, Alini M, Richards RG. et al. Preclinical ex-vivo testing of antiInflammatory drugs in bovine intervertebral degenerative disc model. Front bioeng biotechnol. 2020;8:583
57. Lin J, Chen J, Zhang Z, Xu T, Shao Z, Wang X. et al. Luteoloside Inhibits IL-1beta-Induced Apoptosis and Catabolism in Nucleus Pulposus Cells and Ameliorates Intervertebral Disk Degeneration. Front Pharmacol. 2019;10:868
58. Chen J, Hou C, Chen X, Wang D, Yang P, He X. et al. Protective effect of cannabidiol on hydrogen peroxideinduced apoptosis, inflammation and oxidative stress in nucleus pulposus cells. Mol Med Rep. 2016;14:2321-27
59. Han Y, Yuan F, Deng C, He F, Zhang Y, Shen H. et al. Metformin decreases LPS-induced inflammatory response in rabbit annulus fibrosus stem/progenitor cells by blocking HMGB1 release. Aging (Albany NY). 2019;11(22):10252-65
60. Suzuki S, Fujita N, Fujii T, Watanabe K, Yagi M, Tsuji T. et al. Potential Involvement of the IL-6/JAK/STAT3 Pathway in the Pathogenesis of Intervertebral Disc Degeneration. Spine (Phila Pa 1976). 2017;42:E817-e824
61. Tellegen AR, Rudnik-Jansen I, Beukers M, Miranda-Bedate A, Bach FC, de Jong W. et al. Intradiscal delivery of celecoxib-loaded microspheres restores intervertebral disc integrity in a preclinical canine model. J Control Release. 2018;286:439-50
62. Zhao C-Q, Jiang L-S, Dai L-Y. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11:2079-88
63. Zhang Z, Wang C, Lin J, Jin H, Wang K, Yan Y. et al. Therapeutic Potential of Naringin for Intervertebral Disc Degeneration: Involvement of Autophagy Against Oxidative Stress-Induced Apoptosis in Nucleus Pulposus Cells. Am J Chin Med. 2018 p: 1-20
64. Deng X, Wu W, Liang H, Huang D, Jing D, Zheng D. et al. Icariin Prevents IL-1β-Induced Apoptosis in Human Nucleus Pulposus via the PI3K/AKT Pathway. Evid Based Complement Alternat Med. 2017;2017:12
65. Li P, Gan Y, Xu Y, Wang L, Ouyang B, Zhang C. et al. 17beta-estradiol Attenuates TNF-α-Induced Premature Senescence of Nucleus Pulposus Cells through Regulating the ROS/NF-κB Pathway. Int J Biol Sci. 2017;13:145-56
66. O'Connell GD, Vresilovic EJ, Elliott DM. Human intervertebral disc internal strain in compression: the effect of disc region, loading position, and degeneration. J Orthop Res. 2011;29:547-55
67. Sun H, Luo G, Chen D, Xiang Z. A A Comprehensive and System Review for the Pharmacological Mechanism of Action of Rhein, an Active Anthraquinone Ingredient. Front Pharmacol. 2016;7:247
68. Jiang Y, Xie Z, Yu J, Fu L. Resveratrol inhibits IL-1β-mediated nucleus pulposus cell apoptosis through regulating the PI3K/Akt pathway. Biosci Rep. 2019;39:BSR20190043
69. Wang D, Hu Z, Hao J, He B, Gan Q, Zhong X. et al. SIRT1 inhibits apoptosis of degenerative human disc nucleus pulposus cells through activation of Akt pathway. Age (Dordr). 2013;35:1741-53
70. Lu L, Hu J, Wu Q, An Y, Cui W, Wang J. et al. Berberine prevents human nucleus pulposus cells from IL-1β-induced extracellular matrix degradation and apoptosis by inhibiting the NF-κB pathway. Int J Mol Med. 2019;43:1679-86
71. Chen Y, Zheng Z, Wang J, Tang C, Khor S, Chen J. et al. Berberine suppresses apoptosis and extracellular matrix (ECM) degradation in nucleus pulposus cells and ameliorates disc degeneration in a rodent model. Int J Biol Sci. 2018;14:682-92
72. He Y, Amer AO. Microbial modulation of host apoptosis and pyroptosis. Front Cell Infect Microbio. 2014;4:83
73. He D, Zhou M, Bai Z, Wen Y, Shen J, Hu Z. Propionibacterium acnes induces intervertebral disc degeneration by promoting nucleus pulposus cell pyroptosis via NLRP3-dependent pathway. Biochem Biophys Res Commun. 2020;525:772-79
74. Zhou L, Zhou J, Sheng B, Li X, Yuan Y. Dexmedetomidine exerts dual effects on human annulus fibrosus chondrocytes depending on the oxidative stress status. Biosci Rep. 2019;39:BSR20190419
75. Zhao Y, Qiu C, Wang W, Peng J, Cheng X, Shangguan Y. et al. Cortistatin protects against intervertebral disc degeneration through targeting mitochondrial ROS-dependent NLRP3 inflammasome activation. Theranostics. 2020;10:7015-33
76. Zhang F, Zhao X, Shen H, Zhang C. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int J Mol Med. 2016;37:1439-48
77. Chen JW, Ni BB, Li B, Yang YH, Jiang SD, Jiang LS. The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: implications for disc degeneration. Cell Physiol Biochem. 2014;34:1175-89
78. Krupkova O, Handa J, Hlavna M, Klasen J, Ospelt C, Ferguson SJ. et al. The Natural Polyphenol Epigallocatechin Gallate Protects Intervertebral Disc Cells from Oxidative Stress. Oxid Med Cell Longev. 2016;2016:7031397
79. Len JS, Koh WSD, Tan SX. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci Rep. 2019 39
80. Chen S, Deng X, Ma K, Zhao L, Huang D, Li Z. et al. Icariin improves the viability and function of cryopreserved human nucleus pulposus-derived mesenchymal stem cells. Oxid Med Cell Longev. 2018;2018:3459612
81. Han X, Leng X, Zhao M, Wu M, Chen A, Hong G. et al. Resveratrol increases nucleus pulposus matrix synthesis through activating the PI3K/Akt signaling pathway under mechanical compression in a disc organ culture. Biosci Rep. 2017;37:BSR20171319
82. Li X, Phillips FM, An HS, Ellman M, Thonar EJ, Wu W. et al. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine (Phila Pa 1976). 2008;33:2586-95
83. Wang XH, Zhu L, Hong X, Wang YT, Wang F, Bao JP. et al. Resveratrol attenuated TNF-alpha-induced MMP-3 expression in human nucleus pulposus cells by activating autophagy via AMPK/SIRT1 signaling pathway. Exp Biol Med (Maywood). 2016;241:848-53
84. Wu JW, Wang JJ, Chen JB, Huang YL, Wang H, Liu GH. et al. Resveratrol could reverse the expression of SIRT1 and MMP-1 in vitro. Genet Mol Res. 2015;14:12386-93
85. Wuertz K, Quero L, Sekiguchi M, Klawitter M, Nerlich A, Konno S. et al. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus-mediated pain in vitro and in vivo. Spine (Phila Pa 1976). 2011;36:E1373-84
86. Luo R, Liao Z, Song Y, Yin H, Zhan S, Li G. et al. Berberine ameliorates oxidative stress-induced apoptosis by modulating ER stress and autophagy in human nucleus pulposus cells. Life Sci. 2019;228:85-97
87. Li N, Whitaker C, Xu Z, Heggeness M, Yang SY. Therapeutic effects of naringin on degenerative human nucleus pulposus cells for discogenic low back pain. Spine J. 2016;16:1231-37
88. Ryu D, Mouchiroud L, Andreux PA, Katsyuba E, Moullan N, Nicolet-Dit-Felix AA. et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22:879-88
89. Yang SD, Ma L, Gu TX, Ding WY, Zhang F, Shen Y. et al. 17beta-Estradiol protects against apoptosis induced by levofloxacin in rat nucleus pulposus cells by upregulating integrin alpha2beta1. Apoptosis. 2014;19:789-800
90. Sheng B, Yuan Y, Liu X, Zhang Y, Liu H, Shen X. et al. Protective effect of estrogen against intervertebral disc degeneration is attenuated by miR-221 through targeting estrogen receptor alpha. Acta Biochim Biophys Sin (Shanghai). 2018;50:345-54
91. Kwon YJ. Resveratrol has anabolic effects on disc degeneration in a rabbit model. J Korean Med Sci. 2013;28:939-45
92. Chen D, Xia D, Pan Z, Xu D, Zhou Y, Wu Y. et al. Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo. Cell Death Dis. 2016;7:e2441
93. Shi C, Wu H, Du D, Im HJ, Zhang Y, Hu B. et al. Nicotinamide Phosphoribosyltransferase Inhibitor APO866 Prevents IL-1beta-Induced Human Nucleus Pulposus Cell Degeneration via Autophagy. Cell Physiol Biochem. 2018;49:2463-82
94. Zhang SJ, Yang W, Wang C, He WS, Deng HY, Yan YG. et al. Autophagy: A double-edged sword in intervertebral disk degeneration. Clin Chim Acta. 2016;457:27-35
95. Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN Jr. Autophagy in the Degenerating Human Intervertebral Disc: In vivo Molecular and Morphological Evidence, and Induction of Autophagy in Cultured Annulus Cells Exposed to Proinflammatory Cytokines-Implications for Disc Degeneration. Spine (Phila Pa 1976). 2015;40:773-82
96. Yang M, Feng C, Zhang Y, Liu C, Li B, Zhu Q. et al. Autophagy protects nucleus pulposus cells from cyclic mechanical tensioninduced apoptosis. Int J Mol Med. 2019;44:750-58
97. Jiang L, Zhang X, Zheng X, Ru A, Ni X, Wu Y. et al. Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: Implications for diabetic intervertebral disc degeneration. J Orthop Res. 2013;31:692-702
98. Miyazaki S, Kakutani K, Yurube T, Maeno K, Takada T, Zhang Z. et al. Recombinant human SIRT1 protects against nutrient deprivation-induced mitochondrial apoptosis through autophagy induction in human intervertebral disc nucleus pulposus cells. Arthritis Res Ther. 2015;17:253
99. Lauerman WC, Platenberg RC, Cain JE, Deeney V. Age-related disk degeneration: preliminary report of a naturally occurring baboon model. J Spinal Disord Tech. 1992;5:170-74
100. Ito M, Yurube T, Kakutani K, Maeno K, Takada T, Terashima Y. et al. Selective interference of mTORC1/RAPTOR protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism with Akt and autophagy induction. Osteoarthr Cartil. 2017;25:2134-46
101. Yurube T, Ito M, Kakiuchi Y, Kuroda R, Kakutani K. Autophagy and mTOR signaling during intervertebral disc aging and degeneration. JOR Spine. 2020;3:e1082
102. Kakiuchi Y, Yurube T, Kakutani K, Takada T, Ito M, Takeoka Y. et al. Pharmacological inhibition of mTORC1 but not mTORC2 protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism through Akt and autophagy induction. Osteoarthr Cartil. 2019;27:965-76
103. Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018;28:436-53
104. Cherif H, Bisson DG, Jarzem P, Weber M, Ouellet JA, Haglund L. Curcumin and o-Vanillin Exhibit Evidence of Senolytic Activity in Human IVD Cells In vitro. J Clin Med. 2019;8:433
105. Nepal M, Li L, Cho HK, Park JK, Soh Y. Kaempferol induces chondrogenesis in ATDC5 cells through activation of ERK/BMP-2 signaling pathway. Food Chem Toxicol. 2013;62:238-45
106. Zhang H, Lin CY. Simvastatin stimulates chondrogenic phenotype of intervertebral disc cells partially through BMP-2 pathway. Spine (Phila Pa 1976). 2008;33:E525-31
107. Hu MH, Hung LW, Yang SH, Sun YH, Shih TT, Lin FH. Lovastatin promotes redifferentiation of human nucleus pulposus cells during expansion in monolayer culture. Artif Organs. 2011;35:411-16
108. Colombier P, Clouet J, Hamel O, Lescaudron L, Guicheux J. The lumbar intervertebral disc: from embryonic development to degeneration. Joint Bone Spine. 2014;81:125-29
109. Barroga C, Deshmukh V, Dellamary L, Stewart J, Hu H, Hood J. et al. Discovery of a Small Molecule Inhibitor of the Wnt Pathway (SM04690) As a Potential Treatment for Degenerative Disc Disease. Arthritis Rheumatol Washington DC. 2016 p: 2120
110. Mosley GE, Evashwick-Rogler TW, Lai A, Iatridis JC. Looking beyond the intervertebral disc: the need for behavioral assays in models of discogenic pain. Ann N Y Acad Sci. 2017;1409:51-66
111. Hoyer C, Gass N, Weber-Fahr W, Sartorius A. Advantages and Challenges of Small Animal Magnetic Resonance Imaging as a Translational Tool. Neuropsychobiology. 2014;69:187-201
112. Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A. et al. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine (Phila Pa 1976). 2002;27:2684-90
113. Norcross JP, Lester GE, Weinhold P, Dahners LE. An in vivo model of degenerative disc disease. J Orthop Res. 2003;21:183-88
114. Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A Review of Animal Models of Intervertebral Disc Degeneration: Pathophysiology, Regeneration, and Translation to the Clinic. Biomed Res Int. 2016;2016:5952165
115. Tellegen AR, Willems N, Beukers M, Grinwis GCM, Plomp SGM, Bos C. et al. Intradiscal application of a PCLA-PEG-PCLA hydrogel loaded with celecoxib for the treatment of back pain in canines: What's in it for humans? J Tissue Eng Regen Med. 2018;12:642-52
116. Muralidharan A, Park TSW, Mackie JT, Gimenez LGS, Kuo A, Nicholson JR. et al. Establishment and Characterization of a Novel Rat Model of Mechanical Low Back Pain Using Behavioral, Pharmacologic and Histologic Methods. Fron Pharmacol. 2017;8:493
117. Lin B, Yu H, He Y, Xu Y, Zhang W, Lu C. et al. Protective effects of resveratrol on autologous nucleus pulposus model of radiculopathy. Exp Ther Med. 2016;12:3917-22
118. Yurube T, Takada T, Suzuki T, Kakutani K, Maeno K, Doita M. et al. Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration. Arthritis Res Ther. 2012;14:R51
119. Cheng YY, Kao CL, Lin SY, Chang ST, Wei TS, Chang SN. et al. Effect of an increased dosage of statins on spinal degenerative joint disease: a retrospective cohort study. BMJ Open. 2018;8:e017442
120. Makris UE, Alvarez CA, Wei W, Mortensen EM, Mansi IA. Association of Statin Use With Risk of Back Disorder Diagnoses. JAMA Intern Med. 2017;177:1044-46
121. Jin L-Y, Lv Z-D, Wang K, Qian L, Song X-X, Li X-F. et al. Estradiol Alleviates Intervertebral Disc Degeneration through Modulating the Antioxidant Enzymes and Inhibiting Autophagy in the Model of Menopause Rats. Oxid Med Cell Longev. 2018;2018:7890291
122. Bergknut N, Rutges JP, Kranenburg HJ, Smolders LA, Hagman R, Smidt HJ. et al. The dog as an animal model for intervertebral disc degeneration? Spine (Phila Pa 1976). 2012;37:351-58
123. Lauerman WC, Platenberg RC, Cain JE, Deeney VF. Age-related disk degeneration: preliminary report of a naturally occurring baboon model. J Spinal Disord. 1992;5:170-74
124. Easley NE, Wang M, McGrady LM, Toth JM. Biomechanical and radiographic evaluation of an ovine model for the human lumbar spine. Proc Inst Mech Eng H. 2008;222:915-22
125. Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother. 2013;4:303-06
126. Dai J, Xing Y, Xiao L. et al. Microfluidic Disc-on-a-Chip Device for Mouse Intervertebral Disc—Pitching a Next-Generation Research Platform To Study Disc Degeneration. ACS Biomater Sci Eng. 2019;5:2041-51
127. Silveira JW, Issy AC, Castania VA, Salmon CE, Nogueira-Barbosa MH, Guimarães FS. et al. Protective effects of cannabidiol on lesion-induced intervertebral disc degeneration. PLoS One. 2014;9:e113161
128. Nan LP, Wang F, Ran D, Zhou SF, Liu Y, Zhang Z. et al. Naringin alleviates H2O2-induced apoptosis via the PI3K/Akt pathway in rat nucleus pulposus-derived mesenchymal stem cells. Connect Tissue Res. 2019 p: 1-14
129. Banala RR, Vemuri S, Madhuri V, Kumar S, Reddy Av G, Gpv S. Evaluation of anti-inflammatory and regenerative efficiency of Naringin and Naringenin in degenerated human nucleus pulposus cells- biological and molecular modelling studies. Asian spine. J. 2019;13:875-89
130. Ishimoto H, Shibata M, Myojin Y, Ito H, Sugimoto Y, Tai A. et al. In vivo anti-inflammatory and antioxidant properties of ellagitannin metabolite urolithin A. Bioorg Med Chem Lett. 2011;21:5901-04
131. Yang SD, Yang DL, Sun YP, Wang BL, Ma L, Feng SQ. et al. 17beta-estradiol protects against apoptosis induced by interleukin-1beta in rat nucleus pulposus cells by down-regulating MMP-3 and MMP-13. Apoptosis. 2015;20:348-57
132. Liu S, Yang SD, Huo XW, Yang DL, Ma L, Ding WY. 17beta-Estradiol inhibits intervertebral disc degeneration by down-regulating MMP-3 and MMP-13 and up-regulating type II collagen in a rat model. Artif Cells Nanomed Biotechnol. 2018;46:182-91
133. Wang H, Ding W, Yang D, Gu T, Yang S, Bai Z. Different concentrations of 17beta-estradiol modulates apoptosis induced by interleukin-1beta in rat annulus fibrosus cells. Mol Med Rep. 2014;10:2745-51
134. Wang T, Zhang L, Huang C, Cheng AG, Dang GT. Relationship between osteopenia and lumbar intervertebral disc degeneration in ovariectomized rats. Calcif Tissue Int. 2004;75:205-13
135. Klawitter M, Quero L, Klasen J, Gloess AN, Klopprogge B, Hausmann O. et al. Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. J Inflamm. 2012;9:29
136. Deng X, Chen S, Zheng D, Shao Z, Liang H, Hu H. Icariin Prevents H2O2-Induced Apoptosis via the PI3K/Akt Pathway in Rat Nucleus Pulposus Intervertebral Disc Cells. Evid Based Complement Alternat Med. 2017;2017:2694261
137. Zhang Q, Li H, Wang S, Liu M, Feng Y, Wang X. Icariin protects rat cardiac H9c2 cells from apoptosis by inhibiting endoplasmic reticulum stress. Int J Mol Sci. 2013;14:17845-60
138. Hua W, Zhang Y, Wu X, Kang L, Tu J, Zhao K. et al. Icariin attenuates interleukin-1β-induced inflammatory response in human nucleus pulposus cells. Curr Pharm Des. 2017;23:6071-78
139. Wang W, Li P, Xu J, Wu X, Guo Z, Fan L. et al. Resveratrol attenuates high glucose-induced nucleus pulposus cell apoptosis and senescence through activating the ROS-mediated PI3K/Akt pathway. Biosci Rep. 2018;38:BSR20171454
140. Liu H, Guo R, Shang J, Zhang B, Dai M. Resveratrol inhibits TNF-α-induced matrix degradation via the p38/MAPK and Akt pathways in human nucleus pulposus cells. Int J Clin Exp Med. 2017;10:2764-72
141. Willems N, Yang H-y, Langelaan ML, Tellegen AR, Grinwis GC, Kranenburg H-JC. et al. Biocompatibility and intradiscal application of a thermoreversible celecoxib-loaded poly-N-isopropylacrylamide MgFe-layered double hydroxide hydrogen in a canine model. Arthritis Res Ther. 2015;17:214
142. Than KD, Rahman SU, Wang L, Khan A, Kyere KA, Than TT. et al. Intradiscal injection of simvastatin results in radiologic, histologic, and genetic evidence of disc regeneration in a rat model of degenerative disc disease. Spine J. 2014;14:1017-28
143. Gimenez-Bastida JA, Gonzalez-Sarrias A, Larrosa M, Tomas-Barberan F, Espin JC, Garcia-Conesa MT. Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-alpha-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol Nutr Food Res. 2012;56:784-96
144. Klawitter M, Quero L, Klasen J, Gloess AN, Klopprogge B, Hausmann O. et al. Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. J Inflamm (Lond). 2012;9:29
145. Ma T, Guo CJ, Zhao X, Wu L, Sun SX, Jin QH. The effect of curcumin on NF-kappaB expression in rat with lumbar intervertebral disc degeneration. Eur Rev Med Pharmacol Sci. 2015;19:1305-14
146. Hua W, Li S, Luo R, Wu X, Zhang Y, Liao Z. et al. Icariin protects human nucleus pulposus cells from hydrogen peroxide-induced mitochondria-mediated apoptosis by activating nuclear factor erythroid 2-related factor 2. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165575
Author contact
![]() Corresponding author: Dr. Sibylle Grad, Ph.D. AO Research Institute Davos, Davos, Switzerland; E-mail: Sibylle.gradorg, Phone: +41 81 414 24 80.
Corresponding author: Dr. Sibylle Grad, Ph.D. AO Research Institute Davos, Davos, Switzerland; E-mail: Sibylle.gradorg, Phone: +41 81 414 24 80.
 Global reach, higher impact
Global reach, higher impact