13.3
Impact Factor
Theranostics 2020; 10(22):9984-10000. doi:10.7150/thno.47044 This issue Cite
Research Paper
PARK2 promotes mitochondrial pathway of apoptosis and antimicrotubule drugs chemosensitivity via degradation of phospho-BCL-2
1. Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Medical Research Center, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China.
2. Division of Life Science, Applied Genomics Centre and Centre for Statistical Science, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China.
3. Cancer Science Institute of Singapore, National University of Singapore, Singapore.
4. Division of Hematology/Oncology, Cedars-Sinai Medical Center, University of California Los Angeles School of Medicine, Los Angeles, CA, USA.
5. Department of Breast Oncology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, 510120, China.
*These authors contributed equally to this work.
Abstract
Rationale: Neoadjuvant chemotherapy has become the standard treatment of locally advanced breast cancer. Antimicrotubule drugs and DNA-damaging drugs are the most popular medicines used for neoadjuvant chemotherapy. However, we are unable to predict which chemotherapeutic drug will benefit to an individual patient. PARK2 as a tumor suppressor in breast cancer has been reported. While the role of PARK2 in chemotherapy response remains unknown. In this study, we explore the impact of PARK2 on chemosensitivity in breast cancer.
Methods: PARK2 expression in breast cancer patients with different neoadjuvant chemotherapeutic regimens was studied using immunohistochemistry. Data was correlated to disease-free survival (DFS), overall survival and pathologic complete response (pCR). The functional roles of PARK2 were demonstrated by a series of in vitro and in vivo experiments. Including mass spectrometry, Co-immunoprecipitation, isolation of subcellular fractionation, fluorescence microscopy, in vivo ubiquitination assay and luciferase analyses.
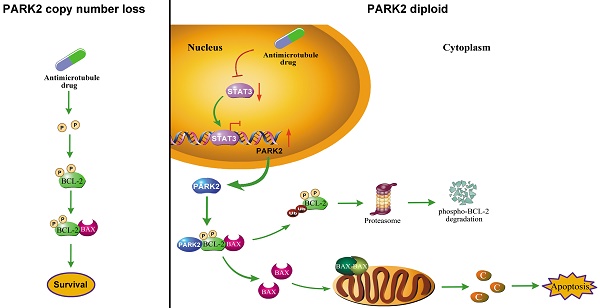
Results: Highly expressed PARK2 predicted better response to antimicrotubule drugs-containing regimen associated with higher rate of pathologic complete response (pCR). In contrast, PARK2 expression did not predict response to the DNA-damaging drugs regimen. Following antimicrotubule drugs treatment, levels of PARK2 was upregulated due to the repression of STAT3-mediated transcriptional inhibition of PARK2. Moreover, overexpression of PARK2 specifically rendered cells more sensitive to antimicrotubule drugs, but not to DNA-damaging drugs. Depletion of PARK2 enhanced resistance to antimicrotubule drugs. Mechanistically, PARK2 markedly activated the mitochondrial pathway of apoptosis after exposure to antimicrotubule drugs. This occurred through downregulating the antiapoptotic protein, phospho-BCL-2. BCL-2 phosphorylation can be specifically induced by antimicrotubule drugs, whereas DNA-damaging drugs do not. Notably, PARK2 interacted with phospho-BCL-2 (Ser70) and promoted ubiquitination of BCL-2 in an E3 ligase-dependent manner. Hence, PARK2 significantly enhanced the chemosensitivity of antimicrotubule drugs both in vitro and in vivo, while loss-of-function PARK2 mutants did not.
Conclusions: Our findings explained why PARK2 selectively confers chemosensitivity to antimicrotubule drugs, but not to DNA-damaging drugs. In addition, we identified PARK2 as a novel mediator of antimicrotubule drugs sensitivity, which can predict response of breast cancer patients to antimicrotubule drugs-containing regime.
Keywords: PARK2, antimicrotubule drug, docetaxel, phospho-BCL-2, breast cancer
 Global reach, higher impact
Global reach, higher impact