13.3
Impact Factor
Theranostics 2020; 10(21):9644-9662. doi:10.7150/thno.47651 This issue Cite
Research Paper
HDAC3 inhibition ameliorates ischemia/reperfusion-induced brain injury by regulating the microglial cGAS-STING pathway
1. The Brain Science Center, Beijing Institute of Basic Medical Sciences, No. 27 Taiping Road, Haidian District, Beijing 100850, China.
2. Center on Translational Neuroscience, College of Life & Environmental Science, Minzu University of China, Beijing 100081, China.
3. Department of Neurology, Affiliated Drum Tower Hospital of Nanjing University, Medical School, Nanjing 210008, China.
4. Beijing Institute for Brain Disorders, Capital Medical University, Beijing, 100069, China.
5. Department of Biochemistry, Medical College, Qingdao University, Qingdao, Shandong, 266071, China.
*Co-first authors.
Abstract
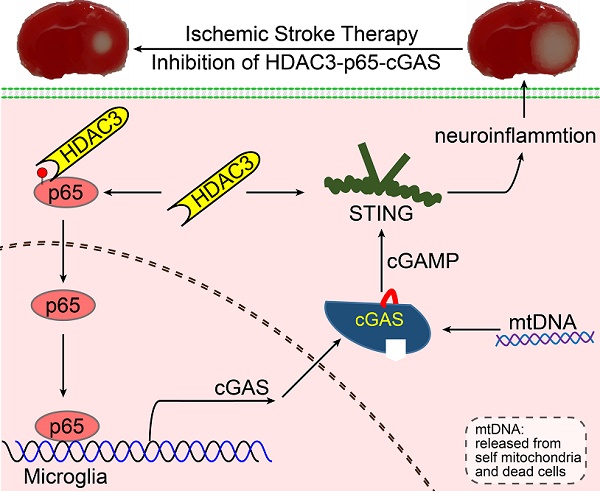
Rationale: It is known that neuroinflammation plays a critical and detrimental role in the development of cerebral ischemia/reperfusion (I/R), but the regulation of the cyclic GMP-AMP synthase (cGAS)-mediated innate immune response in I/R-induced neuroinflammation is largely unexplored. This study aimed to investigate the function and regulatory mechanism of cGAS in I/R-induced neuroinflammation and brain injury, and to identify possible strategies for the treatment of ischemic stroke.
Methods: To demonstrate that microglial histone deacetylase 3 (HDAC3) regulates the microglial cGAS-stimulator of interferon genes (cGAS-STING) pathway and is involved in I/R-induced neuroinflammation and brain injury, a series of cell biological, molecular, and biochemical approaches were utilized. These approaches include transient middle cerebral artery occlusion (tMCAO), real-time polymerase chain reaction (PCR), RNA sequencing, western blot, co-immunoprecipitation, chromosome-immunoprecipitation, enzyme-linked immunosorbent assay (ELISA), dual-luciferase reporter assay, immunohistochemistry, and confocal imaging.
Results: The microglial cGAS- STING pathway was activated by mitochondrial DNA, which promoted the formation of a pro-inflammatory microenvironment. In addition, we revealed that HDAC3 transcriptionally promoted the expression of cGAS and potentiated the activation of the cGAS-STING pathway by regulating the acetylation and nuclear localization of p65 in microglia. Our in vivo results indicated that deletion of cGAS or HDAC3 in microglia attenuated I/R-induced neuroinflammation and brain injury.
Conclusion: Collectively, we elucidated that the HDAC3-p65-cGAS-STING pathway is involved in the development of I/R-induced neuroinflammation, identifying a new therapeutic avenue for the treatment of ischemic stroke.
Keywords: Microglia, HDAC3, cGAS, Neuroinflammation, Ischemia/reperfusion
 Global reach, higher impact
Global reach, higher impact