13.3
Impact Factor
Theranostics 2020; 10(21):9477-9494. doi:10.7150/thno.45763 This issue Cite
Research Paper
Molecular signatures of BRCAness analysis identifies PARP inhibitor Niraparib as a novel targeted therapeutic strategy for soft tissue Sarcomas
1. Department of Musculoskeletal Oncology, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
2. Guangdong Provincial Key Laboratory of Orthopedics and Traumatology, Guangzhou, China.
3. OrigiMed, Co., Ltd., Shanghai, China.
4. Department of Integrative Biology & Pharmacology, McGovern Medical School, University of Texas Health Science Center at Houston.
5. Department of Anesthesiology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, P. R. China.
#These authors contributed equally to this work.
Abstract
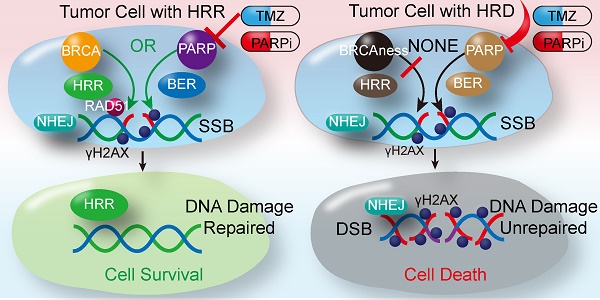
Background: Patients with advanced soft tissue sarcomas (STS) have a dismal prognosis with few effective therapeutic options. A defect in the homologous recombination repair (HRR) pathway can accumulate DNA repair errors and gene mutations, which can lead to tumorigenesis. BRCAness describes tumors with an HRR deficiency (HRD) in the absence of a germline BRCA1/2 mutation. However, the characteristics of BRCAness in STS remain largely unknown. Thus, this study aimed to explore the genomic and molecular landscape of BRCAness using whole exome sequencing (WES) in STS, aiming to find a potential target for STS treatment.
Methods: WES was performed in 22 STS samples from the First Affiliated Hospital of Sun Yat-sen University to reveal the possible genomic and molecular characteristics. The characteristics were then validated using data of 224 STS samples from The Cancer Genome Atlas (TCGA) database and in vitro data. The analysis of the potential biomarker for BRCAness was performed. Targeted drug susceptibility and combination therapy screening of chemotherapeutics for STS were evaluated in STS cell lines, cell-line-derived xenografts (CDX), and patient-derived xenografts (PDX).
Results: Compared with 30 somatic mutation signatures of cancers, high cosine-similarity (0.75) was identified for HRD signatures in the 22 STS samples using nonnegative matrix factorization. Single nucleotide polymorphism indicated a low mutation rate of BRCA1/2 in the 22 STS samples (11.76% and 5.88%, respectively). However, copy number variation analyses demonstrated widespread chromosomal instability; furthermore, 54.55% of STS samples (12/22) carried BRCAness traits. Subsequently, similar genomic and molecular characteristics were also detected in the 224 STS samples from TCGA and in vitro. Poly (ADP-ribose) polymerases (PARP)-1 could be a promising reflection of HRD and therapeutic response. Furthermore, the level of PAR formation was found to be correlated with PARP-1. Subsequently, STS cell lines were determined to be sensitive to PARP inhibitor (PARPi), niraparib. Moreover, based on the screening test of the five common PARPis and combination test among doxorubicin, ifosfamide, dacarbazine, and temozolomide (TMZ), niraparib and TMZ were the most synergistic in STS cell lines. The synergistic effect and safety of niraparib and TMZ combination were also shown in CDX and PDX.
Conclusions: BRCAness might be the common genomic and molecular characteristics of majority of STS cases. PARP-1 and PAR could be potential proper and feasible theranostic biomarkers for assessing HRD in patients. STSs were sensitive to PARPi. Moreover, the combination of niraparib and TMZ showed synergistic effect. Niraparib and TMZ could be a promising targeted therapeutic strategy for patients with STS.
Keywords: BRCAness, Genomic characteristic, PARP inhibitor, PDX models, Soft tissue sarcomas
 Global reach, higher impact
Global reach, higher impact