13.3
Impact Factor
Theranostics 2020; 10(20):9172-9185. doi:10.7150/thno.45994 This issue Cite
Research Paper
Pancreatic Cancer detection via Galectin-1-targeted Thermoacoustic Imaging: validation in an in vivo heterozygosity model
1. MOE Key Laboratory of Laser Life Science & Institute of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou 510631, China.
2. Guangdong Provincial Key Laboratory of Laser Life Science, College of Biophotonics, South China Normal University, Guangzhou 510631, China.
#These authors contributed equally to this work.
Abstract
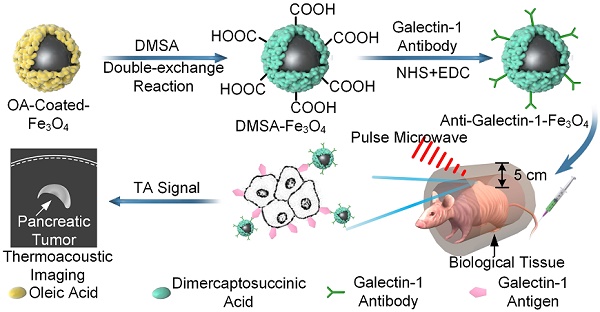
Purpose: To investigate the feasibility of microwave-induced thermoacoustic imaging (MTAI) in detecting small pancreatic tumors (< 10 mm in diameter) and to complement the limitation of current clinical imaging methods.
Methods: A home-made MTAI system composed of a portable antenna and pulsed microwave generator was developed. The thermoacoustic nanoparticles were composed of the galectin-1 antibody for targeting pancreatic tumors and Fe3O4 nanoparticles as microwave absorbers (anti-Gal1-Fe3O4 nanoparticles). The microwave absorption properties of the nanoparticles were measured with a vector network analyzer and the resolving power of MTAI was investigated by imaging excised pancreatic tumors of different sizes (diameters of 1.0 mm, 3.1 mm, 5.0 mm, 7.2 mm). To simulate actual imaging scenarios, an in vivo heterozygosity model was constructed by covering the pancreatic tumors (~ 3 mm in diameter) in BALB/c nude mice with biologic tissue (~ 5 cm in depth). MTAI images of the heterozygosity model were acquired with/without the injection of the anti-Gal1-Fe3O4 nanoparticles and the thermoacoustic contrast from pancreatic tumors was evaluated with Student's paired t test. The data were analyzed with analysis of variance and nonparametric statistics.
Results: Following intravenous infusion, anti-Gal1-Fe3O4 nanoparticles efficiently accumulated in the tumor. The MTAI contrast enhancement in pancreatic tumors with anti-Gal1-Fe3O4 nanoparticles was verified in vitro and in vivo. The pancreatic tumors were visible in nude mice examined with MTAI with a mean contrast enhancement ratio of 2.3 ± 0.15 (standard error of the mean) (P =. 001) at 6 h post-injection of the nanoparticles. MTAI identified tiny pancreatic tumors in deep tissues with high fidelity.
Conclusion: MTAI offers deep imaging depth and high contrast when used with anti-Gal1-Fe3O4 nanoparticles. It can identify pancreatic tumors smaller than 5 mm, which is beyond the identification limit size (~10 mm) of other nondestructive clinical imaging methods. Thus, MTAI has great potential as an alternative imaging modality for early pancreatic cancer detection.
Keywords: thermoacoustic imaging, small pancreatic tumors, nanoparticles
 Global reach, higher impact
Global reach, higher impact