13.3
Impact Factor
Theranostics 2020; 10(20):9153-9171. doi:10.7150/thno.43502 This issue Cite
Research Paper
A novel nucleolin-binding peptide for Cancer Theranostics
1. School of Life Sciences, Gwangju Institute of Science and Technology, Gwangju 61005, Republic of Korea.
2. Molecular Physiology and Biophysics Section, Porter Neuroscience Research Center, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, United States.
3. Pilot Plant, Anygen, Gwangju, Technopark, 333 Cheomdankwagi-ro, Buk-gu, Gwangju, 61008, Republic of Korea.
Abstract
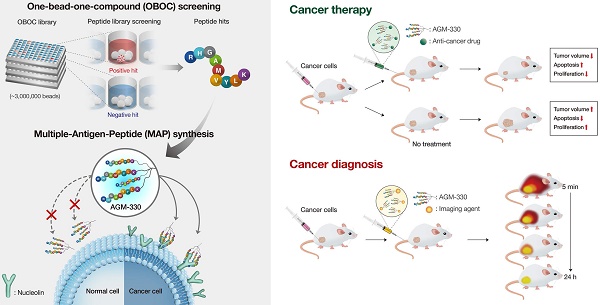
Background: Cancer-specific ligands have been of great interest as pharmaceutical carriers due to the potential for site-specific delivery. In particular, cancer-specific peptides have many advantages over nanoparticles and antibodies, including high biocompatibility, low immunogenicity, and the formation of nontoxic metabolites. The goal of the present study was the development of a novel cancer-specific ligand.
Methods: Cancer-specific peptide ligands were screened using a one-bead-one-compound (OBOC) combinatorial method combined with a multiple-antigen-peptide (MAP) synthesis method. The specificity of the peptide ligands toward cancer cells was tested in vitro using a whole-cell binding assay, flow cytometry, and fluorescence confocal microscopy. The tissue distribution profile and therapeutic efficacy of a paclitaxel (PTX)-conjugated peptide ligand was assessed in vivo using xenograft mouse models.
Results: We discovered that AGM-330 specifically bound to cancer cells in vitro and in vivo. Treatment with PTX-conjugated AGM-330 dramatically inhibited cancer cell growth in vitro and in vivo compared to treatment with PTX alone. The results of pull-down assay and LC-MS/MS analyses showed that membrane nucleolin (NCL) was the target protein of AGM-330. Although NCL is known as a nuclear protein, we observed that it was overexpressed on the membranes of cancer cells. In particular, membrane NCL neutralization inhibited growth in cancer cells in vitro.
Conclusions: In summary, our findings indicated that NCL-targeting AGM-330 has great potential for use in cancer diagnosis and targeted drug delivery in cancer therapy.
Keywords: peptide, one-bead-one-compound, multiple-antigen-peptide, nucleolin, paclitaxel
 Global reach, higher impact
Global reach, higher impact