13.3
Impact Factor
Theranostics 2020; 10(19):8648-8664. doi:10.7150/thno.48291 This issue Cite
Review
Emerging role of exosomes in craniofacial and dental applications
The State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST) & Key Laboratory of Oral Biomedicine Ministry of Education, School & Hospital of Stomatology, Wuhan University, Wuhan, China.
Received 2020-5-17; Accepted 2020-6-22; Published 2020-7-9
Abstract
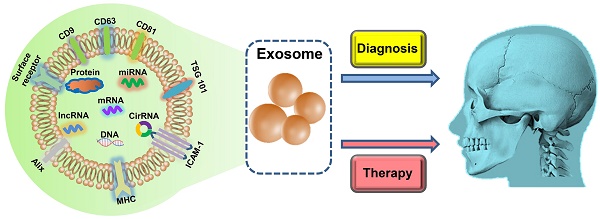
Exosomes, a specific subgroup of extracellular vesicles that are secreted by cells, have been recognized as important mediators of intercellular communication. They participate in a diverse range of physiological and pathological processes. Given the capability of exosomes to carry molecular cargos and transfer bioactive components, exosome-based disease diagnosis and therapeutics have been extensively studied over the past few decades. Herein, we highlight the emerging applications of exosomes as biomarkers and therapeutic agents in the craniofacial and dental field. Moreover, we discuss the current challenges and future perspectives of exosomes in clinical applications.
Keywords: exosome, craniofacial, dental, biomarker, therapy
Introduction
Exosomes, which were firstly introduced in the 1980s [1, 2], are nanoscale extracellular lipid bilayer vesicles that are secreted by various cells under physiological and pathological conditions [3]. Based on their size and release mechanism, extracellular vesicles (EVs) are classified into three primary types, namely apoptotic bodies, microvesicles, and exosomes. Exosomes are membrane vesicles, with a diameter of 30-150 nm. They are intraluminal vesicles formed by the inward budding of the endosomal membranes during the maturation of multivesicular endosomes [4]. The fusion of the multivesicular endosome with the plasma membrane results in the release of individual exosomes. Apoptotic bodies and microvesicles are considered to be larger than 100 nm in size and are released directly from the plasma membrane into extracellular fluid [5]. Therefore these two groups will not be further discussed here.
Initially, exosomes were regarded as a simple means for the disposal of unwanted cellular debris. In the past decade, these “waste bags” and their crucial roles in cell communication attracted mounting research attention [6]. The nanoscale lipid bilayer exosomes contain various cargos, including proteins, lipids, miRNAs, mRNA, and many other noncoding RNAs. Notably, the presence of these cargo biomolecules depends on their parent cells and organismal status. Through the intercellular transfer of their cargo molecules, exosomes participate in fundamental physiological processes or pathological disorders by regulating the properties of their target cells; their effects can be beneficial or detrimental [7]. They are widely distributed throughout bodily fluids, including blood, saliva, breast milk, and urine [8, 9]. The functional states of exosome origin cells can be estimated by analyzing the contents of easily accessible exosomes. This approach lays the foundation for exosome-based diagnosis. Except for disease diagnosis, exosomes could be applied as therapeutic tools in various fields, including tissue regeneration, drug delivery, and cancer treatment [10-12]. Presently, a total of 167 clinical trials involving exosome-related treatments and diagnoses of various diseases are registered at Clinicaltrials.gov. The speed of the clinical translation of exosome-based diagnosis and therapeutics has far exceeded initial expectations.
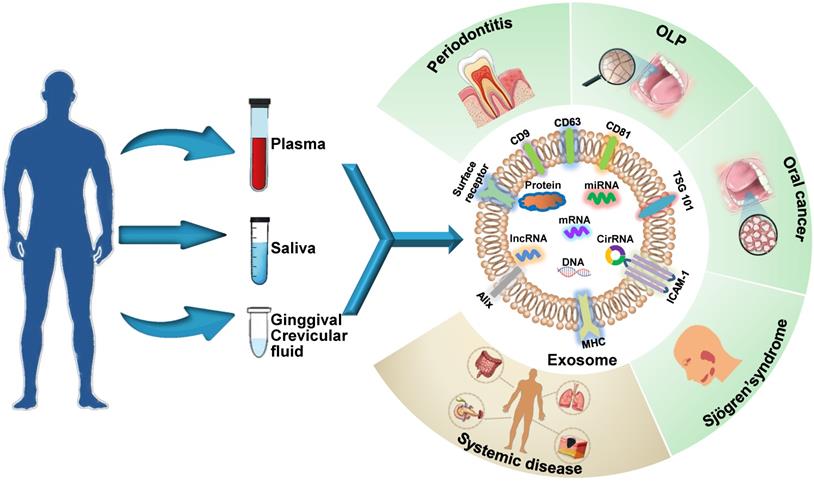
Schematic presentation of the use of exosomes as diagnostic biomarkers in craniofacial and dental applications. Exosomes carry multiple proteins and nucleic acids that are specific to the type and condition of their parent cells. The analysis of the concentration, morphology, molecular make-up, and cargos of exosomes circulating in bodily fluids reflects parental cell status and provides insights into disease diagnosis. Exosomes have been shown to act as potential diagnostic biomarkers for periodontitis, oral lichen planus (OLP), oral cancers, and Sjögren's syndrome in the craniofacial and dental field. Additionally, systemic diseases can be diagnosed with the help of exosomes derived from oral liquid.
In this review, we discuss and summarize up to date and comprehensive literature on the applications of exosomes in the dental and craniofacial field, with particular focus on exosome-based diagnostics and therapeutics. Moreover, the challenges and future development prospects of exosome applications are discussed.
Exosomes as Diagnostic Biomarkers
Exosomes are ideal non-invasive biomarkers for disease diagnosis. They exist extensively in various bodily fluids, their molecular contents are specific to their parental cell type, and their levels of components depend primarily on the functional states of these cells, i.e., whether the cells are in normal physiological state or a pathological state, such as oxidative stress, transformation, apoptosis and abnormal division [13]. Therefore, an analysis of exosomal cargos circulating in bodily fluids reflects the altered state of parental cells and provides insights into the diagnosis of systemic and oral diseases (Figure 1). Exosome biomarkers for craniofacial and dental diseases are summarized in Table 1. The potential oral exosome biomarkers detected in systemic diseases are shown in Table 2.
Exosome biomarkers for craniofacial and dental diseases
Periodontitis
Periodontitis is defined as a chronic inflammatory disease that is initiated by the accumulation of microbial plaque and characterized by the progressive destruction of tooth-supporting tissues [14]. Periodontitis has a high global prevalence and has become a major public health concern. One of the goals of research on periodontology is the development of high-impact diagnostic biomarkers that have a considerable effect on clinical decision-making, patient outcomes, and healthcare providers [15]. This could be attained through the application of protein-containing exosomes. A previous study indicated that reduced salivary levels of CD9/CD81 exosomes are associated with the pathogenesis of the periodontal disease [16]. In another study comparing the differences between salivary exosomal proteins in young adults with severe periodontitis and healthy individuals through mass spectrometry (MS) and gene ontology analysis, 26 immune-related proteins were unique to severe periodontitis [17]. Besides, exosome-associated nucleic acids could act as potential biomarkers of periodontal disease status. PD-L1 plays an essential role in various cancers and inflammation-etiologic diseases, including periodontitis [18, 19]. PD-L1 mRNA in salivary exosomes is enriched in periodontitis, and its level is associated with the severity of periodontitis [20]. A recent study revealed three significantly elevated miRNAs (hsa-miR-140-5p, hsa-miR-146a-5p, and hsa-miR-628-5p) in only salivary exosomes in periodontitis patients but not in healthy controls [21]. In an additional exploratory study, Chaparro et al. detected the total concentration of EVs in gingival crevicular fluid (GCF) and saliva samples from patients with healthy gums/gingivitis or periodontitis. The authors did not detect significant differences in salivary EVs but found significant increases in the total concentration of medium/large EVs in GCF [22]. Since GCF consists of serum and locally generated components, including tissue breakdown products, inflammatory mediators, and antibodies in response to oral microorganisms, we speculate that there could be significant differences in GCF exosome levels between periodontitis patients and healthy individuals. However, ascertaining this is experimentally difficult owing to the limited available volume of GCF (microliter level), which could be partially responsible for the lack of literature in this area. Unlike GCF, which is easily contaminated with saliva, blood, and plaque, saliva is a more desirable source of exosome for periodontitis diagnosis. Moreover and notably, the efficacy of plasma exosome biomarkers for periodontitis has not been studied. Therefore, these need further investigations.
Oral lichen planus
Oral lichen planus (OLP) is a chronic immune-mediated inflammatory disease of the oral mucosal characterized by various clinical manifestations with keratotic or erythematous and ulcerative lesions [23].
Exosome biomarkers for craniofacial and dental diseases
| Disease | Biofluid | Biomarker | Type | Methods | Ref |
|---|---|---|---|---|---|
| Periodontitis | Saliva | CD9, CD81 | Protein | ELISA | [16] |
| Saliva | CA2, Histone H2A type 1, Ig kappa chain V-I region AU, HBG1, HIST1H2BJ HIST1H1D, CCL28, HIST1H1B, CPN2, C8A, PGLYRP2, SAA1, C1S, SELL, Ig kappa chain V-II region FR, BLVRB, C6, BPGM, CPB2, Ig heavy chain V-III region KOL, EHD2, Ig lambda chain V-II region TRO, MDK, APOL1, C8B, Ig lambda chain V-I region VOR | Protein | LC-MS/MS | [17] | |
| Saliva | PD-L1 | mRNA | qRT-PCR | [20] | |
| Saliva | hsa-miR-140-5p, hsa-miR-146a-5p, hsa-miR-628-5p | microRNA | qRT-PCR | [21] | |
| OLP | Saliva | miR-4484 | microRNA | MicroRNA microarray analysis, qRT-PCR | [26] |
| Plasma | miR-34a-5p | microRNA | MicroRNA microarray analysis, qRT-PCR | [27] | |
| Oral cancer | Oral fluid | CD81, CD9, CD63, total concentration, size | Protein | AFM, ELISA | [31] |
| Saliva | CD63, populations, morphologies, size | — | AFM | [32] | |
| Saliva | Spectral signature | — | FTIR, machine learning | [33] | |
| Plasma | CD63, CAV-1 | Protein | Immunocapture-based analysis | [34] | |
| Plasma | PF4V1, CXCL7, F13A1, ApoA1 | Protein | LC-MS/MS | [35] | |
| Plasma | PD-L1 | Protein | Flow cytometry | [36] | |
| Plasma | Laminin-332 | Protein | ELISA | [37] | |
| Saliva | mucin 5B, galectin-3-binding protein, immunoglobulin alpha-1 chain c region, prolactininducible protein, alpha-2-macroglobulin, haptoglobin alpha chain, pyruvate kinase isozymes M1/M2, glyceraldehyde-3-phosphate dehydrogenase | Protein | LC-MS/MS | [38] | |
| Plasma | hsa-miR-19a, hsa-miR-512-3p, hsa-miR-27b, hsa-miR-20a, hsa-miR-28-3p, hsa-miR-200c, hsa-miR-151-3p, hsa-miR-223, hsa-miR-20b, hsa-miR-22, hsa-miR-516-3p, hsa-miR-370, hsa-miR-139-5p, hsa-let-7e, hsa-miR-145-3p, hsa-miR-30c | microRNA | MicroRNA microarray analysis, qRT-PCR | [39] | |
| Saliva | miR-302b-3p, miR-517b-3p, miR-512-3p, miR-412-3p | microRNA | MicroRNA microarray analysis | [40] | |
| Saliva | miR-24-3p | microRNA | MicroRNA microarray analysis | [41] | |
| Sjögren's syndrome | Saliva | hsa-miR-150, hsa-miR-23a, hsa-miR-27b, hsa-miR-29b, hsa-miR-29c, hsa-miR-335, hsa-miR-379, hsa-miR-433, hsa-miR-454, hsa-miR-483-3p, hsa-miR-584, hsa-miR-621, hsa-miR-652, hsa-miR-760, hsa-miR-888, miRPIus_17824, miRPIus_17841, miRPIus_17848, miRPIus_17858 | microRNAs | MicroRNA microarray analysis | [44] |
| Saliva | HLA, S100A9, HLA-B, CAT, LY6D, TYROBP, DEFA1, RHOA, GNA13, SLPI, ANXA6, SIRPA, NCF1B, WDR1, MUC5AC, ARPC1B, LSP1, LV302, CD59, FCER1G, RP2, LCN2, HVCN1, KV312, GPI, PTPRJ, HLA-A, SNAP23, HV102, BASP1, APMAP, GNAI3, RAB5C, ICAM3, CA4, MUC2, HIDE, PTPRC, SLC6A14, ACTN1, PPP1CA, CD9, SPRR1A, CALM1,FERMT3, BSG, CNP, UBA52, TLN1, CAPZB, CORO1A, DSTN, SLC9A3R2, KV303, SIGLEC5, OLFM4, GNB2 ARRB2, PGLYRP1, NCF2 | Protein | LC-MS | [45] | |
| Tear | CPNE1, CALM | Protein | LC-MS | [45] |
LC‐MS/MS: liquid chromatography-tandem mass spectrometry; ELISA,:enzyme-linked immunosorbent assay; OLP: oral lichen planus; AFM: atomic force microscopy; FTIR: Fourier transform infrared spectroscopy; LC-MS: liquid chromatography-mass spectrometry.
Oral exosome biomarkers for the detection of systemic diseases
| Systemic Disease | Oral Biofluid | Biomarker | Type | Methods | Ref |
|---|---|---|---|---|---|
| Inflammatory bowel disease | Saliva | PSMA7 | Protein | LC-MS/MS | [46] |
| Ageing | Saliva | miR-24-3p | microRNAs | MicroRNA microarray analysis | [47] |
| Pancreatic cancer | Saliva | Apbb1ip, Daf2, Foxp1, Incenp, Aspn, BC031781, Gng2 | mRNA | Microarray | [48] |
| Pancreatobiliary tract cancer | Saliva | miR-1246, miR-4644 | microRNAs | qRT-PCR | [49] |
| Lung cancer | Saliva | Annexin Al, A2, A3, A5, A6, A11, NPRL2, CEACAM1, HIST1H4A, MUC1, PROM1, TNFAIP3 | Protein | LC-MS/MS | [50] |
| Lung cancer | Saliva | BPIFA1, CRNN, MUC5B, IQGAP | Protein | LC-MS/MS | [51] |
| Melanoma | Saliva | Human melan-A | RNA | qRT-PCR | [52] |
| Gestational diabetes mellitus | Gingival crevicular fluid | Total concentration | — | Qubit protein assay kit | [53] |
LC‐MS/MS: liquid chromatography-tandem mass spectrometry.
The WHO categorizes OLP as an oral potentially malignant disorder (OPMD) given its malignant tendency, unclear etiology and the lack of a unified therapy [24]. A recent study indicated that exosomes are involved in the pathogenesis of OLP [25]. Can exosomes be a valuable tool for the diagnosis of OLP? A comparison of salivary exosomal miRNA from 16 patients with OLP and 8 healthy controls revealed that miR-4484 is significantly upregulated in patients with OLP [26]. In addition to salivary exosomes, circulating plasma exosomes could serve as potential diagnostic biomarkers for OLP. Peng et al. compared the exosomal miRNA profiles isolated from the plasma of patients with OLP with those of healthy individuals by miRNA array analysis. They discovered that circulating exosomal miR-34a-5p is significantly upregulated in patients with OLP and positively correlated with the severity of OLP [27]. In general, a biopsy is recommended for definite OLP diagnosis. The above reports suggest that exosome biomarkers are expected to be a superior alternative for the diagnosis of OLP. However, it is difficult to state which one could serve as the most effective biomarker for OLP. The plasma exosomal miR-34a-5p seems to have a significant reference value beacause of its direct association with OLP severity. In contrast, salivary exosomal miR-4484 has distinctive advantages over plasma because saliva sampling is simple, non-invasive, with minimal training requirements compared with blood sampling. Future research should aim at elucidating salivary exosomal biomarkers that are positively correlated with OLP severity as an optimal tool for diagnosis.
Oral cancer
Oral cancer is preventable and curable in its early stages. However, considerable cases of oral squamous cell carcinomas (OSCC) are not diagnosed until progressed stages, which are associated with poor therapeutic responsiveness and prognosis [28]. Generally, cancer diagnostics rely on tissue biopsies. Nowadays, endeavors have been made to discover novel, non-invasive methods for cancer diagnosis. For instance, liquid biopsy based on the detection of circulating tumor cells (CTCs), circulating tumor DNA (ctDNA) and circulating tumor RNA (ctRNA), and exosomes [29]. In squamous cell carcinomas, exosomes have been shown to be crucial components in the tumor microenvironment, suggesting their significance in tumorigenesis, tumor invasion, and metastasis [30]. Growing research evidence shows that the characteristics of exosomal morphology, proteins (surface and cargo), and miRNAs serve as potential biomarkers for the diagnosis of OSCC. Sharma et al. and Zlotogorski et al. attempted to perform atomic force microscopy on exosomes collected from saliva and reported that the morphological features of exosomes differ between patients with oral cancer and healthy individuals [31, 32]. Similarly, fourier-transform infrared spectroscopy coupled with computational-aided discriminating analysis was used to assess the diagnostic potential of salivary exosomes from oral cancer patients and healthy individuals. The results of this analysis showed that oral cancer exosomes can be accurately differentiated from their benign counterparts by detecting subtle changes in the conformations of proteins, lipids and nucleic acids [33]. The expression of exosomal surface proteins, including CD63, CD9, and CD81, is moreover different in salivary exosomes from patients with oral cancer and healthy individuals in the above-mentioned study by Sharma et al. and Zlotogorski et al. [31, 32]. Besides, a pilot clinical study by Rodriguez et al. evaluated the relationship between CD63- and CAV1-positive exosome levels in patients with OSCC before and after surgical treatment and correlated this relationship with overall survival. They found that CD63-positive exosome levels have decreased after surgery, whereas CAV-1 levels have increased most likely due to postsurgery inflammatory response [34]. Examining exosomal cargo proteins through proteomic analysis provides a useful diagnostic tool for detecting malignant changes in oral cancers. A study involving quantitative proteomics analysis of serum exosomes in OSCC patients with lymph node metastasis (LNM) identified ApoA1, CXCL7, PF4V1, and F13A1 as potential diagnostic biomarkers but not prognostic biosignatures [35]. Research evidence indicated circulating PD-L1 on the surfaces of exosomes isolated from plasma is a useful metric that is associated with disease progression in patients with OSCC [36]. Additionally, laminin-332 levels in plasma exosomes from OSCC patients with LNM are markedly higher compared with OSCC patients without lymphatic metastasis; implying that plasma exosomal laminin-332 is a potential and non-invasive biomarker for the detection of lymph node metastasis in OSCC [37]. Winck et al. measured salivary exosomal proteins of OSCC patients through liquid chromatography-tandem MS (LC-MS/MS) analyses. Through bioinformatics analysis, the authors obtained a priority list of 139 proteins identified from salivary exosomes in patients with OSCC and healthy controls. Statistical analysis revealed that 8 proteins are differentially expressed between the two groups; these proteins may serve as biomarkers of oral cancer [38]. Furthermore, exosomal miRNAs, which act as regulatory gatekeepers of coding genes, are potential minimally invasive diagnostic biomarkers that could be used to screen for oral cancer. Rabinowits and colleagues comparatively analyzed miRNA expression in the benign and malignant tissues and plasma of patients with tongue cancer. They found that 16 miRNAs are differentially expressed between tumors and their matched benign tissue. Amongst these miRNAs, 9 upregulated and 7 downregulated miRNAs can be found in circulating exosomes [39]. In addition to plasma miRNAs, the application of salivary exosomal miRNAs as diagnostic biomarkers for oral cancer has been extensively studied. In a study comparing the miRNA content of exosomes from the saliva of patients and healthy controls, Gai et al. showed that miR-302b-3p and miR-517b-3p are expressed only in OSCC patients and two other miRNAs, miR-512-3p and miR-412-3p, are upregulated in OSCC compared with the healthy individuals [40]. Moreover, He et al. demonstrated that oral cancer-derived salivary exosomal miR-24-3p may also serve as a potential detective biomarker for OSCC screening [41]. Although numerous studies reveal the significant association of exosome biomarkers with oral cancers, the results of these individual studies show poor concordance. Inconsistent isolation strategies of exosomes and stage diversity of cancers may contribute to this mismatch. Additionally, compared with other cancer types for which various diagnostic tests are registered according to Clinicaltrials.gov, the application of exosomal biomarkers in the diagnosis of oral cancer in clinical settings remains absent and requires further research for effective implementation.
Sjögren's syndrome
Sjögren's syndrome (SS) is a chronic autoimmune disease that is characterized by lymphocyte infiltration and inflammation in the exocrine glands, particularly the salivary and lacrimal. The disease causes oral and ocular dryness (xerostomia and keratoconjunctivitis sicca) [42]. Numerous SS biomarkers have been identified in saliva, tears, and plasma [43]. However, research on the application of exosomal biomarkers in SS diagnosis is limited. Michael et al. studied salivary exosomal miRNAs using TaqMan quantitative PCR and miRNA microarrays in a patient with SS and healthy individuals [44]. Although the author presented their obtained miRNA patterns only as a proof of concept without drawing any disease-specific conclusions, they provided the first report describing the correlation between salivary exosomes and SS. Aqrawi et al. performed a proteomic analysis of EVs isolated from the saliva and tears of patients with SS by using liquid chromatography-mass spectrometry (LC-MS). They found that dozens of proteins are significantly upregulated in the salivary EVs of patients with SS compared with the control group. Only 2 proteins from tears are upregulated in patients with SS because of the low tear fluid volumes collected [45]. Overall, saliva and tear sample collection is an example of a non-invasive sampling method. Besides the two studies above, additional evidence, such as plasma-derived exosomes, could validate potential exosomal biomarkers for SS. Nonetheless, identifying biomarkers in tears and saliva is desirable since their sampling is manageable, inexpensive, and non-invasive. The possibility of combining salivary and tear exosomal cargos might provide a more precise detection approach for SS; therefore, future research should be focused on this.
Oral exosome biomarkers for systemic diseases
The role of exosomal biomarkers in oral disease diagnosis is an example of the current applications of exosomes in dentistry. Exosomes isolated from oral fluids can additionally serve as effective biomarkers for systemic disease diagnosis. A previous study revealed high levels of PSMA7 in salivary exosomes in patients with inflammatory bowel disease, suggesting that PSMA7 is a promising biomarker alternative to colonoscopy [46]. Machida et al. utilised samples from 13 elderly individuals and 15 young healthy volunteers to examine the correlation between salivary exosomal miRNAs and ageing. Through microarray analysis and real-time PCR validation, they identified miR-24-3p as a novel candidate biomarker of ageing [47]. Besides oral cancers, salivary exosomes have been used as diagnostic biomarkers in other cancer types, such as pancreatic cancer, pancreatobiliary tract cancer, lung cancer, and melanomas [48-52]. Monteiro et al., in a pilot study, demonstrated significantly different concentrations of EVs in the GCF between women with gestational diabetes mellitus and normoglycemic pregnant women; hence, they could be used as an early biomarker for the prediction of gestational diabetes mellitus in pregnant women [53].
Collectively, exosomal morphology, counts, and the levels of exosome-incorporated contents reflect the pathological state of the disease. Exosomes protect their cargos, making them more stable in biological fluids and reliable biomarkers compared to freely circulating biomarkers. These features, as well as their extensive availability in various bodily fluids, make them promising candidates as diagnostic biomarkers of disease. Among all the parental exosome biofluids, the collection of saliva constitutes the most commonly recommended approach for prospective clinical applications based on the advantages discussed earlier. Moreover, the limited sample size and methodological differences in exosome enrichment in the current studies could result in biased estimates and inconsistent results. Therefore, large-scale, prospective multicenter preclinical studies and clinical trials under well-defined conditions (e.g., uniform purification method, standardized detection procedure, and specific pathological stage of disease) are prerequisites for the application of exosomes as precise diagnostic tools in clinical practice. Besides, to achieve higher sensitivity and specificity of exosome-based diagnostics, a combinatorial and multicomponent approach, including combinations of multiple exosomal markers (e.g., nucleic acids in combination with proteins) should also be considered.
Exosome-based therapeutics for craniofacial and dental applications
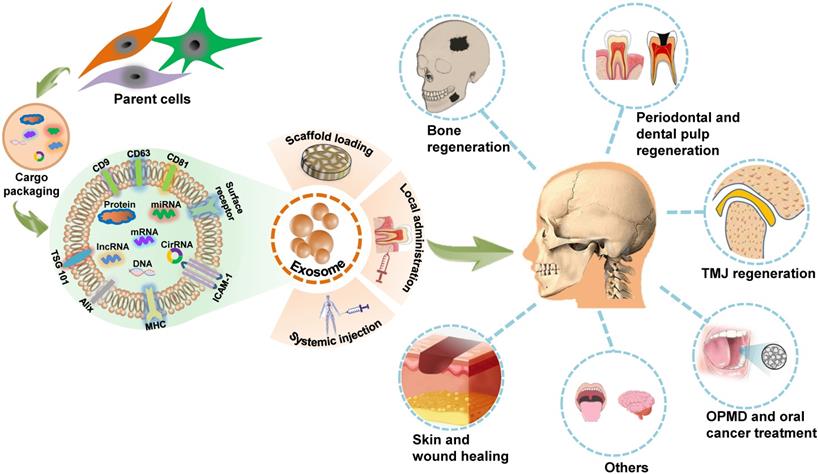
The innate attributes of exosomes indicate that they could be applied in the design of potential therapeutic agents. The nanoscale size, excellent immunocompatibility, rapid endocytosis, nontoxicity, stability, and accessibility to biological barriers of exosomes render them as novel cell-free therapy agents with attractive advantages over their parent cells in disease treatment [54, 55]. There is increasing research evidence regarding the in vitro exploration of exosome biology and utility in controlling cell activity. The direct applications of exosomes in living organisms, including animal models, or clinical trials, provide an easy-to-access and convincing paradigm for exosome-based therapies. The role of MSCs-derived exosomes in craniofacial tissue engineering and regeneration has been summarized in detail in a previous review [56]. Here, we provide a broad overview of in vivo applications of exosomes as potential cell-free therapeutic agents in the regeneration of craniofacial bone, skin, temporomandibular joint (TMJ), periodontal tissue and dental pulp, as well as the treatment of oral cancer, OPMD, and other craniofacial and dental diseases (Figure 2). A list of studies focusing on in vivo exosome-based therapeutic applications in the craniofacial and dental field is provided in Table 3.
Craniofacial bone regeneration
Craniofacial bone defects after trauma, infection, tumor resection and congenital deformities lead to different degrees of deformity and dysfunction in patients. The traditional clinical approaches for repair involve autologous, allogeneic bone grafting, and distraction osteogenesis, which may provide positive results but suffer from shortcomings, including donor site morbidity, immune complications, and cosmetic concerns [57]. Bone tissue engineering has emerged as a promising solution to overcome these shortcomings with the controlled application of cells, combined with biocompatible materials, for therapy [58]. Angiogenesis and osteogenesis are critical stages in bone regeneration [59]. Numerous in vitro studies have demonstrated that exosomes act in a paracrine manner to regulate osteogenesis and angiogenesis of recipient cells. Mesenchymal stem cells (MSCs) are currently the most established promising parental sources of exosomes for tissue engineering and regeneration. Exosomes derived from human-induced pluripotent stem cell-derived MSCs (hiPS-MSCs), human adipose MSCs (hADSCs), and human perivascular stem cells (hPSCs) induce naive stem cells into to an osteogenic linage [60-63]. Additionally, MSCs-derived exosomes can be used as biomimetic tools to regulate osteoblast proliferation and activity directly [64, 65]. Angiogenesis, which provides nutrition and oxygen to the surrounding cells, is a vital step in bone healing. Exosomes derived from multiple types of cells, including the bone marrow-derived MSCs (BMSCs) [66, 67], ADSCs [68-70], human placenta-derived MSCs (hP-MSCs) [71, 72], periodontal ligament stem cells (PDLSCs) [73] promote in vitro proliferation, migration, and tube formation of endothelial cells.
In addition to MSCs, exosomes harvested from other cells are potential pro-osteogenic and pro-angiogenic factors. Exosomes from mineralizing osteoblast cells [74], myoblasts [75], periodontal ligament fibroblasts [76], monocytes [77] and macrophages [78] activate osteogenic differentiation in cell culture.
Summary of in vivo studies on exosome-based therapy in craniofacial and dental application
| Tissue | Model/Species | Exosome Origin | Involved Pathway | Administration Methods | Functional Effects | Ref |
|---|---|---|---|---|---|---|
| Bone regeneration | Calvarial bone defect/ Osteoporotic rats | hiPSC-MSCs | — | β-TCP scaffold | Promote osteogenesis and angiogenesis | [60] |
| Calvarial bone defect/ Mice | hADSCs | — | PLGA scaffold | Promote osteogenesis | [62] | |
| Calvarial bone defect/ Rats | hiPSC-MSCs | PI3K/Akt | β-TCP scaffold | Promote osteogenesis | [81] | |
| Calvarial bone defect/ Rats | miR-375- overexpressed hADSCs | — | Hydrogel | Promote osteogenesis | [61] | |
| Calvarial bone defect/ Rats | DMOG-stimulated hBMSCs | AKT/mTOR | Porous HA scaffold | Stimulate angiogenesis | [67] | |
| Calvarial bone defect/ Mice | hPSCs | SAPK/JNK, HGF, Sirtuin, FGF, PDGF, AMPK, PTEN | Percutaneous injection | Promote osteogenesis | [63] | |
| Alveolar bone defect/ Rats | SHEDs | AMPK | β-TCP scaffold | Promote osteogenesis and angiogenesis | [82] | |
| BRONJ/Rats | hBMMSCs | — | Tail vein injection | Promote angiogenesis and bone regeneration | [83] | |
| TMJ regeneration | TMJ osteoarthritis/Rats | hESC-MSCs | AKT, ERK, AMPK | Intra-articular injection | Suppress inflammation and pain , reduce apoptosis, enhance matrix synthesis | [88] |
| Periodontal regeneration | Periodontitis/Rat | rADSCs | — | Pocket local injection | Anti-inflammatory effect, enhance osteoid tissues and blood vessels formation | [93] |
| Periodontal intrabony defects/Rats | hESC-MSCs | AKT, ERK | Collagen sponge | Enhance osteogenesis and periodontal ligament formation | [94] | |
| Periodontitis/Human clinical trial | hADSCs | — | Local injection into periodontal pockets | — | NCT04270006 | |
| Dental pulp regeneration | Tooth root slice model/Mice | hDPSCs under odontogenic conditions | P38 MAPK | Collagen membrane | Promote stem cell differentiation and blood vessels formation | [105] |
| Skin regeneration | Full-thickness skin wounds/ Diabetic mice | mADSCs | — | FEP hydrogel | Stimulate angiogenesis and enhance cell proliferation, granular tissue formation, collagen deposition, remodeling and re-epithelialization | [106] |
| Full-thickness skin wounds/ Diabetic mice | mADSCs | — | FHE hydrogel | Promote angiogenesis, re- epithelialization and collagen deposition | [107] | |
| Full-thickness skin wounds/ Rat | rADSCs | — | Alginate hydrogel | Enhance re-epithelialization, collagen deposition and angiogenesis | [108] | |
| Full-thickness skin wounds/ Diabetic rats | hGMSCs | — | Chitosan/silk hydrogel sponge | Promote re-epithelialization, deposition and ECM remodeling and enhance angiogenesis and neuronal ingrowth | [109] | |
| Full-thickness skin wounds/ Mice | HucMSCs | TGF-β/SMAD2 | HydroMatrix hydrogel | Suppress myofibroblast aggregation and scar formation | [110] | |
| Full-thickness skin wounds/ Mice | hADSCs | PI3K/Akt | subcutaneous and intradermal injection | Promote collagen synthesis and optimize collagen deposition | [111] | |
| Full-thickness skin wounds/ Mice | hMenSCs | NF- kB | Intradermal injection | Induce M1-M2 macrophage polarization, enhance neoangiogenesis and re-epithelialization | [112] | |
| Full-thickness skin wounds/ Mice | hBMMSCs | — | Intravenous injections | Promote macrophages towards M2 polarization | [113] | |
| Full-thickness skin wounds/ Diabetic mice | miR-21-5p overexpressed hADSCs | Wnt/β-catenin | Direct addition, coverage with alginate gel | Enhance re-epithelization, collagen remodeling, angiogenesis and vessel maturation | [114] | |
| Full-thickness skin wounds/Diabetic mice | mmu_circ_0000250 overexpressed mADSCs | miR-128-3p/SIRT1 | Subcutaneous injection | Promote angiopoiesis and suppress apoptosis by autophagy | [115] | |
| Full-thickness skin wounds/ Diabetic rat | hPRP | RhoA/YAP, PI3K/Akt, Erk1/2 | Sodium alginate hydrogel | Promote angiogenesis | [116] | |
| Skin wound model/ Mice | mM2 Mφs | — | Subcutaneous injection | Enhance angiogenesis, re-epithelialization, and collagen deposition | [117] | |
| Full-thickness skin wounds/ Rats | hOMECs | — | Direct addition, coverage with TegaDerm and gauze | Reduce fibroblast proliferation and stimulate the release of growth factors | [118] | |
| Full-thickness skin wounds/ Mice | hUCB | PTEN, SPRY1 | Subcutaneous injection | Promote re-epithelization and angiogenesis | [119] | |
| Deep second-degree burn wound/ Rats | HucMSCs | Yap, Wnt/β-Catenin | Subcutaneous injection | Restrict stem cell expansion and collagen deposition | [120] | |
| Deep second-degree burn wound/ Rats | HucMSCs | Wnt/β-Catenin, AKT | Subcutaneous injection | Enhance proliferation of skin cells and promote re-epithelialization | [121] | |
| UVB-Induced skin photoaging/ Mice | HDFs | TGF-β | Dermo-jet model G (needleless injection) | Regulate dermal fibroblasts to induce efficient collagen biosynthesis and ameliorate inflammation | [122] | |
| Full-thickness gingival wound/ Mice | mGMSCs | Fas/Fap-1/Cav-1 | Submucosally injection | Produce high amounts of IL-1RA | [124] | |
| Oral Cancer | DMBA-induced OSCC/ Hamsters | hMenSCs | — | Injection into the base of the tumor | Reduce tumor vasculature | [131] |
| OPMD | DMBA-induced OPMD/ Hamsters | microRNA-185 overexpressed mBM-MSCs | Akt | Solution painting | Alleviate inflammation, inhibit cell proliferation and angiogenesis and induce apoptosis | [133] |
| Others | Free-falling induced TBI/ Rats | SHEDs | — | Injection into rat brains | Shift microglia M1/M2 polarization | [135] |
| Critical-sized tongue defect/ Rats | hGMSCs | — | SIS-ECM | Promote reepithelialization, reinnervation and taste bud regeneration | [136] |
hiPSC-MSC: human induced pluripotent stem cells-derived mesenchymal stem cells; β-TCP: β-tricalcium phosphate; hADSCs: human adipose-derived mesenchymal stem cells; PLGA: poly(lactic-co-glycolic acid); DMOG: dimethyloxaloylglycine; hBMSCs: human bone mesenchymal stem cells; HA: hydroxyapatite; hPSCs: human perivascular stem cells; SHEDs: stem cells from human exfoliated deciduous teeth; TMJ: temporomandibular joint; hESC-MSCs: human embryonic stem cell-derived MSCs; rADSCs: rat adipose-derived stem/stromal cells; hDPSCs: human dental pulp stem cells; mADSCs: mice adipose-derived mesenchymal stem cells; FEP: F127 (F127) grafting polyethylenimine (PEI) and aldehyde pullulan (APu); FHE: multifunctional hydrogel composed of Pluronic F127, oxidative hyaluronic acid and Poly-ε-L-lysine; hGMSCs: human gingival mesenchymal stem cells; ECM: extracellular matrix; HucMSCs: human umbilical cord mesenchymal stem cells; hMenSCs: human menstrual blood-derived mesenchymal stem cells; hBMMSCs: human bone marrow-derived mesenchymal stem cells; hPRP: human platelet-rich plasma; mM2 Mφs: mice M2 phenotype macrophages; hOMECs: human oral mucosa epithelial cells; hUCB: human umbilical cord blood; HDFs: human dermal fibroblasts; mGMSCs: mice gingiva-derived mesenchymal stem cells; DMBA: dimethylbenzanthracene; OPMD: oral potentially malignant disorders; mBM-MSCs: mice bone marrow-derived mesenchymal stem cells; TBI: traumatic brain injury; BRONJ: bisphosphonate-related osteonecrosis of the jaw; SIS-ECM: small intestinal submucosa-extracellular matrix.
Schematic presentation of exosome-based therapeutic applications in the craniofacial and dental field. Exosomes are secreted by many types of cells and modulate biofunctions by conveying unique signals obtained from their parental cells to recipient cells. Through scaffold loading or application via local administration or systemic injection, exosomes can function as a novel potential therapeutic tool for regenerating the craniofacial bone, the temporomandibular joint (TMJ), the skin, the periodontium and dental pulp; treating oral potentially malignant disorders (OPMD), oral cancer, and several other craniofacial and dental diseases.
Several in vitro studies have also demonstrated that exosomes derived from skeletal muscle [79] and leukemia [80] regulate endothelial cell function and stimulate angiogenesis.
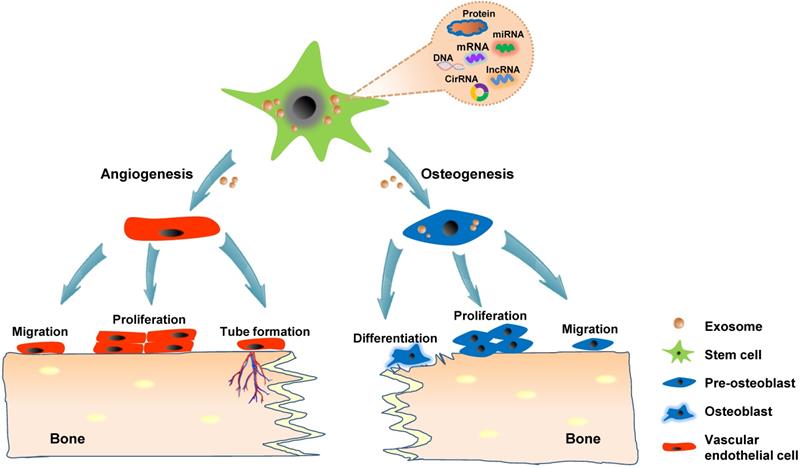
Although numerous in vitro studies have indicated the osteogenic and angiogenic capacity of exosomes, direct in situ application in animal models is highly convincing and intuitive for proving the effect of exosomes on bone regeneration. The calvarial bone defect model is the most widely used animal model for studying in vivo bone regeneration potential of exosomes in craniofacial and dental applications. The strategies for loading exosomes derived from hiPS-MSCs and hADSCs into β-tricalcium phosphate (β-TCP) and polydopamine-coating poly (lactic-co-glycolic acid) (PLGA) scaffolds, respectively have resulted in successful calvarial bone formation [60, 62, 81]. Modified exosomes moreover exhibit potential in stimulating bone regeneration; for instance, the patching of hydrogel containing exosomes harvested from miR-375-overexpressing hADSCs over calvarial wounds results in significantly accelerated healing [61]. Liang et al. showed that loading exosomes derived from dimethyloxaloylglycine-stimulated human bone marrow MSCs into porous hydroxyapatite scaffolds improve bone healing at six weeks after implantation into the critical-sized calvarial defects [67]. In addition to the incorporation into a delivery system, exosomes could be applied through direct injection. Recently, exosomes derived from hPSCs were percutaneously injected into the tissue directly overlying a mouse calvarial bone defect, which accelerated bone defect healing [63]. Alveolar bone defect models have also been shown as suitable experimental models for verifying the role of exosomes in craniofacial and dental bone regeneration. Wu et al. indicated that exosomes secreted via stem cells from human exfoliated deciduous teeth (SHEDs) enhance osteogenesis and angiogenesis through the AMPK signaling pathway. By using a rat model of alveolar bone defects, they showed that exosome-loaded β-TCP scaffolds significantly promote bone formation compared with β-TCP or the control treatment [82]. Watanabe et al. created a bisphosphonate-related osteonecrosis of the jaw (BRONJ) model by administering zoledronic acid to rats and extracting the teeth. Their results indicated that the administration of MSCs-derived EVs prevents senescence of cells involved in wound healing and the spread of chronic inflammation around senescent cells, thereby promoting angiogenesis and bone regeneration and preventing BRONJ [83]. Collectively, exosomes, especially stem cells-derived exosomes, could be potentially applied in treating various bone diseases and promoting bone regeneration because of their proangiogenesis ability and stimulatory effects on osteogenic cells (Figure 3).
Exosomes promote bone regeneration through the regulation of angiogenesis and osteogenesis. Stem cells-derived exosomes convey functional cargos, including various proteins and nucleic acids, into the recipient cells existing in the bone microenvironment. The angiogenesis mechanism involves the activation of the vascular endothelial cells, resulting in migration, proliferation, and the formation of new blood vessels. The potential exosomal osteogenic ability possibly stimulates naive stem cells into to an osteogenic linage or directly regulating osteoblast.
TMJ regeneration
TMJ is a complex articulation covered by dense fibrocartilage formed between the mandibular condyle and the temporal bone. TMJ diseases have attracted mounting research interests in regenerative strategies that combine stem cells, scaffolds, and bioactive molecules, including exosomes [84]. A previous study indicated that plasma-derived exosomes loaded with miR-140 induce BMSCs differentiation into chondrocytes [85]. Further, Luo et al. showed that miR-100-5p-carrying exosomes derived from SHEDs suppress inflammation in TMJ chondrocytes by activating the mTOR signaling pathways [86]. These in vitro exosome treatments had positive outcomes and revealed exosomes as potential therapeutic agents for in joint and cartilage repair. Indeed, the various studies that yielded these results were conducted in animal models. However, most studies on the application of exosomes in joint and cartilage regeneration in vivo focused on arthritic knees [87]. In a recent study, human embryonic stem cell-derived exosomes injected into the compartment of the TMJ in a rat model with monosodium-iodoacetate-induced TMJ osteoarthritis promoted TMJ repair and regeneration by regulating inflammatory responses and healing condylar cartilage and subchondral bone [88]. To our knowledge, this is the first in vivo study demonstrating the translational potential of an exosome-based therapy for TMJ repair and regeneration. The anatomical, structural, and functional regeneration of TMJ is highly challenging and specific owing to its uniqueness and complexity. Additional research evidence for the appropriate animal and human models is needed to rationalize the application of exosomes in the regeneration of TMJ structures, including the cartilage, subchondral bone, and even the TMJ disc.
Periodontal and dental pulp regeneration
The periodontium is a hierarchically organized tissue that consists of the gingiva, periodontal ligament, cementum, and the alveolar bone. It provides physical and mechanical support to the teeth. The ultimate objective of periodontal treatment is the regeneration of periodontium, which involves the functional reattachment of the periodontal ligament to the new cementum and the alveolar bone [89]. Novel approaches, such as biomaterials and cell-based therapy for periodontal regeneration, have been explored. Although these strategies have shown positive outcomes, they possess the challenges of maintaining cell vitality, immune compatibility, and safety. Exosomes have emerged as a cell-free therapeutic approach with low immunogenicity and increased safety, given their endogenous origins. In vitro studies have shown that exosomes involved in inflammatory signal transfer and periodontitis progression, which may provide therapeutic targets for periodontal regeneration [76, 90, 91]. Royo et al. demonstrated that exosomes derived from PDLSCs regulate angiogenesis via the exosome-mediated transfer of miR-17-5p-targeted VEGFA [73]. Additionally, SHEDs-derived exosomes enhance PDLSCs osteogenic differentiation partly due to the presence of Wnt3a and BMP2 in the exosomes. This effect provides new insights into the therapeutic use of SHEDs exosomes in treating periodontitis-induced bone defects [92]. Mohammed et al. firstly investigated the therapeutic effect of exosomes in periodontal regeneration with animal models [93]. They injected ADSCs exosomes locally into pockets in a ligature-induced periodontitis rat model. Their results showed remarkable new periodontal tissue formation and provided direct support for the potential application of exosomes for periodontal regeneration. Chew et al. reported another study that further verified the in vivo periodontal application of exosomes. This report demonstrated that MSCs exosomes enhance periodontal regeneration possibly by increasing periodontal ligament cell migration and proliferation and suggested that MSCs exosomes are an available ready-to-use and cell-free therapy for periodontal defects [94]. Notably, according to Clinicaltrials.gov, a human clinical trial (NCT04270006) entitled “Effect of adipose-derived stem cells exosomes as an adjunctive therapy to scaling and root planning in the treatment of periodontitis” is being conducted by an Egyptian researcher. To our knowledge, this is the first and only clinical trial involving the application of exosomes in the craniofacial and dental field.
The dental pulp is richly vascularized and innervated tissue that maintains a wide range of biological and physiological functions, including responding to bacterial insult and injury, providing neuronal sensitivity and transmitting mechanical stimuli for repair and regeneration [95]. Infection or necrosis of dental pulp constitutes the most common endodontic disease. The typical treatment of pulp diseases involves root canal therapy to remove diseased dental pulp tissue, followed by filling the root canal with inorganic material. The loss of dental pulp tissue results in loss of tooth vitality. Tissue engineering approaches, including stem cell-mediated functional therapy aimed at regenerating dental pulp, can readily address this issue [96]. Recently, the use of exosomes as tools in regenerative medicine has gained prominence. Exosomes derived from dental pulp stem cells (DPSCs) have been shown to suppress inflammation, reduced edema, and promote angiogenesis [97, 98]. Moreover, stem cells derived from the dental pulp of human exfoliated deciduous teeth have unique anti-apoptotic and neurogenic properties [99]. Ivica et al. revealed that exosomes secreted by SHEDs activate the recruitment and proliferation of human MSCs [100]. Similarly, Lim and colleagues reported that exosomes derived from human DPSCs under odontogenic conditions promote the odontogenic differentiation of human DPSCs through the TGFβ1/smads signaling pathway via the transfer of microRNAs [101]. Schwann cells are an essential cellular source of dental MSCs that migrate to injured sites and differentiate into odontoblasts and dental pulp cells [102]. Moreover, Schwann cells are nerve-associated glial cells capable of axonal regeneration and reconnection establishment after peripheral nerve injury [103]. A recent study showed that exosomes from hDPSCs, particularly from lipopolysaccharide-preconditioned hDPSCs, promote the proliferation, migration, and odontogenic differentiation of Schwann cells [104]. The author declared that their findings might provide new insights into the regulatory capability of exosomes from hDPSCs for Schwann cells involved in pulp regeneration. The above studies have provided only in vitro evidence for exosome-mediated dental pulp regeneration. However, little information is available regarding the effects of exosomes in the regeneration of dental pulp in situ. A previous study that utilized a tooth root slice model that was implanted subcutaneously in the back of athymic nude mice to test in vivo dental pulp regeneration [105]. The results of this study indicated that exosomes isolated either from normal pulp cells or odontogenic pulp cells can trigger the regeneration of dental pulp-like tissue, and the latter shows better regenerative efficiency than the former.
Unlike the numerous therapeutic applicaitions of stem cells, in vivo data on the use of exosomes as potent cell-free therapeutic agent in the field of periodontal and dental diseases are scarce. Research on exosome applications for periodontal and dental regeneration is still in its early stage. Further research on exosomes, especially in vivo applications that include animal models and clinical trials, should be conducted to harness the potential of exosomes as therapeutics agents.
Skin and wound healing
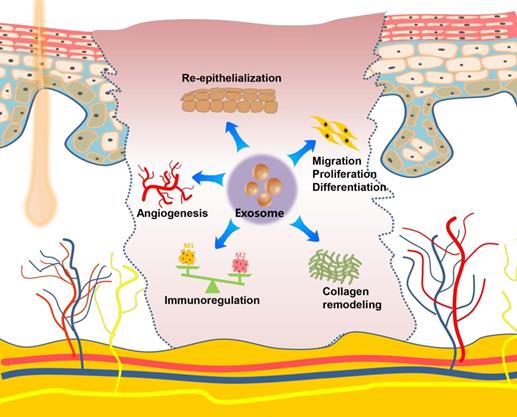
The skin is the largest organ in the human body and plays an important role in the defense against the invasion of pathogenic microorganisms. Skin damage, which is frequently caused by extensive burns, trauma, or diabetic ulcers, result in a broad spectrum of complications with functional and cosmetic repercussions. Numerous studies show that exosomes derived from MSCs (ADSCs, gingival MSCs, and umbilical cord-derived MSCs), incorporated into a hydrogel scaffold, have therapeutic effects when used as a potential cell-free therapeutic tool for in situ full-thickness cutaneous wound healing [106-110]. Along with the scaffold-loading exosome strategies discussed above, local injection (subcutaneously and intradermally) [111, 112] or systemic administration, such as the intravenous injection [113] of MSCs-derived exosomes into the wound sites of skin, presents another commonly used strategy for evaluating the effects of exosomes on cutaneous wound healing. Researchers have also developed a gene-modified MSCs-derived exosome-based miRNA delivery strategy to enhance therapeutic efficacy [114, 115]. The engineered exosomes exhibit excellent effects on accelerating diabetic wound healing by increasing re-epithelization and stimulation of angiogenesis. Except for MSCs, bone marrow derived-macrophages, platelet-rich plasma (PRP), umbilical cord blood plasma, and oral mucosal epithelial cells are possible sources of exosomes for skin wound repair [116-119]. Zhang et al. established a rat model of skin deep second-degree burn wound and demonstrated that the subcutaneous injection of human umbilical cord MSCs-derived exosomes significantly promotes cutaneous wound healing [120, 121]. Although almost all of these studies used a skin defect or burn model on the back, these models are also applicable for craniofacial skin, given that the fundamental steps of the typical wound-healing process are conserved in the skins at different anatomical sites. Recently, a UVB-induced skin photoaging model was created and used to investigate the effects of exosomes on anti-ageing properties, in which exosomes derived from the three-dimensional human dermal fibroblast spheroids reduced skin ageing by regulating dermal fibroblast proliferation, migration, and protein expression effectively [122]. Oral gingival/mucosal wounds, which mostly heal with minimal to no scarring similar to fetal wounds and heal faster than cutaneous wounds [123], have been studied to a lesser extent than cutaneous healing. A study involving a palatal gingival wound model indicated that exosomes secreted by gingival MSCs have therapeutic effects on wound healing [124]. Overall, exosomes, mostly from stem cells, promote the regeneration of skin wounds by enhancing angiogenesis, stimulating the migration, proliferation, and differentiation, facilitating re-epithelialization and collagen remodeling, as well as regulating the immune activity. Exosome-mediated therapy could provide a multifaceted strategy for promoting cutaneous regeneration and repair (Figure 4).
Oral cancer treatment
Head and neck cancer, with oral carcinoma as its major subtype, is one of the most widespread malignancies worldwide. Despite the continuous progress in its treatment and diagnosis, the 5-year overall survival rate of oral cancer remains low at approximately 50% [125, 126]. Growing evidence demonstrates that exosomes shuttle different agents, including small interfering RNAs (siRNAs), miRNAs, and targeted drugs as therapeutic molecules into recipient cells, thereby attenuating the bioactivity for OSCC cells [127-130]. A previous review discussed and summarized various aspects of exosome biology and functions in head and neck squamous cell carcinoma well [12]. In contrast to studies involving animal testing and certain clinical trials on exosome application as treatment agents for other cancer types, preclinical studies involving in vivo exosome-based therapy for oral cancer are limited. A recent study involving the use of the hamster buccal pouch carcinoma model, a preclinical model that closely mimics human OSCC, assessed the effects of exosome treatment on oral cancer in live animals and showed that the antitumor effect of the intra-tumoral injection of stem cell exosomes is associated with the loss of tumor vasculature [131]. As mentioned earlier, stem cell-derived exosomes have been shown to promote angiogenesis in tissue regeneration. However, in tumor treatment, they present as anti-angiogenic agents. The underlying mechanisms responsible for the pro- or anti-angiogenic property of stem cell exosomes remain unclear. This could possibly be attributed to receptor-mediated specific molecules intercellular transportation. Exosome-based oral cancer therapy remains at its infancy in contrast to its considerable application in oral cancer diagnostic. More studies using relevant preclinical models are required to validate the potential value of exosome in oral cancer treatment.
Role and regulatory mechanism of exosomes on the skin and wound healing. Exosomes exert their repair capacity in skin injury by promoting skin cell migration, proliferation and differentiation, enhancing re-epithelization, stimulating angiogenesis, remodeling collagen, and regulating the immune function.
OPMD treatment
OPMD includes oral leukoplakia, erythroplakia and oral submucous fibrosis, and its malignant transformation into oral cancer is highly associated with chronic inflammation [24]. MSCs-derived exosomes and exosomal miR-8485 have been proved to be involved in premalignant lesions and carcinogenesis, indicating that intervention with the secretion of MSCs-derived exosomes could be an innovative strategy to prevent carcinogenesis [132]. Wang et al. applied the MSCs-derived exosomes on buccal lesions in a dimethylbenzanthracene (DMBA)-induced OPMD model and demonstrated the feasibility of exosome-carried miR-185 as a novel therapeutic option for treating OPMD [133]. Numerous treatments have been recommended for OPMDs, ranging from medical and surgical interventions, lasers, and photodynamic therapy [134]. Multicenter randomized clinical trials with larger sample sizes should be conducted to ascertain whether exosome therapies have an advantage over these traditional remedies.
Others
In addition to its application in commonly occurring diseases discussed above, exosome-based therapy has been utilized in relatively rare diseases in the craniofacial and dental domain. Investigators reported that SHEDs-derived exosome could be administered to manage traumatic brain injury [135]. Most interestingly, Zhang et al. used a critical-sized tongue defect model in rats and showed that combinatory transplantation of small intestinal submucosa-extracellular matrix with gingival mesenchymal stem cells-derived exosomes promotes tongue lingual papilla recovery and taste bud regeneration [136]. These studies provide more insights on exosome-based therapeutic applications in craniofacial and dental diseases and show that exosome-based strategies are not merely applicable in existing demonstrations.
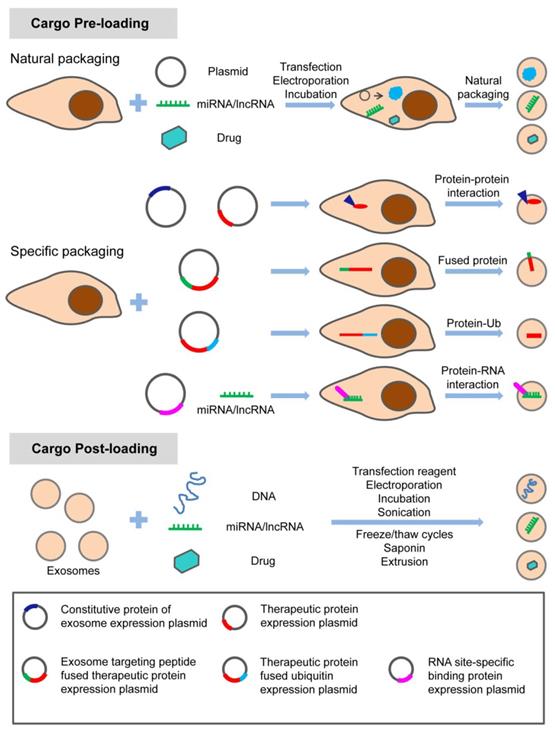
In summary, exosome-based therapy has great application potential from regenerative medicine to oncology in the craniofacial and dental field. As shown in Table 3, natural exosomes were utilized in a considerable number of studies, with few studies adopting engineered exosomes loading specific nucleic acids. Strategies with specific modifications maximize the therapeutic potential of exosomes in the craniofacial and dental fields. Different options are considered to meet this purpose. There are various modification methods for loading the specific treating molecules (proteins, nucleic acids, and small molecule drugs) into exosomes (Figure 5). Apart from loading, targeting strategy is also a potential enhancer for the therapeutic application of exosomes. Targeting exosomes could be acquired by the assembly of specific ligands on the exosome surface that recognize the target receptor of recipient cells. For instance, regarding the pro- or anti-angiogenic characteristic of stem cells, a novel and powerful engineered exosome with anti-tumor effect could be constructed by loading it with anti-angiogenic proteins, miRNAs, and equipping it with iRGD peptide (targeting tumor cells) on the surface. The recent emergence of potential exosome-mimetics with similar structure and biomarkers of exosomes and the ability to overcome drawbacks, such as low loading efficiency and low production yields, significantly promotes the development of conventional exosome-based therapy [137, 138]. In our recent study, we fabricated a specific exosome-mimetics by serial mechanical extrusion and encapsulated it with the plasmid gene of vascular endothelial growth factor. Then the engineered exosome-mimetics was integrate into a biotin-avidin modified coaxial electrospun through covalent bonding. This well-designed, functional exosome-mimetics-mediated compound sustainably delivers the VEGF gene and significantly enhances osteogenesis and angiogenesis in a cranial defect model [139]. The potential therapeutic effects of exosomes provide a great opportunity for developing exosome-related biomedical applications in various fields along with chemical, cellular, and genetic engineering techniques.
Modification strategy for loading therapeutic molecules into exosomes. The specific treating molecules (proteins, nucleic acids, and small chemicals) can be loaded into exosomes before or after exosome production through natural or specific packaging. Adapted with permission from [54], copyright 2019 Ivyspring International Publisher.
Challenges and Perspectives
Considerable progress in the field of exosomes has increased our understanding of their biogenesis, molecular content, and biological function over the last decade. Exosomes have received considerable research attention as mediators of intercellular communication given their potential role as biomarkers and therapeutics. Using exosomes as biomarkers and therapeutic agents in the clinical application has several advantages. Exosomes-based diagnosis has minimal trauma and wide availability in various bodily fluids. The diversity of exosomal cargos, which provides multiple diagnostic parameters, could enhance the diagnostic sensitivity and specificity. The encapsulated analyte is protected from degradation, and it is stable due to the exosome protection by their bilayer lipid membrane. Regarding therapeutic applications, exosomes have been shown relatively free of ethical issues, excellent immune-compatibility, lower toxicity, efficient cellular entry, intrinsic ability to traverse biological barriers, and potential targeting ability through the surface-specific domain. Besides, since exosomes house multiple biomolecule types, they exert different therapeutic mechanisms simultaneously. Furthermore, exosomes can be modified, including internal loading or surface modification, due to their unique structure and physicochemical characteristics.
Despite the advances discussed herein, there are numerous limitations and challenges to be overcome before exosomes could be translated successfully into clinical applications. This extends beyond the craniofacial and dental field to the entire biomedical field. The major challenge prohibiting exploring exosomes in clinical applications is the lack of reliable and standardized methods for large-scale production and distinguishing them from other EVs. This is the first critical issue that requires to be addressed. Various separation strategies, including ultra-speed centrifugation, ultrafiltration, immunoaffinity capture and charge neutralization-based polymer precipitation, have been reported [140]. However, it is difficult to identify which isolation and purification strategy produce optimum results. We speculate that ultracentrifugation is as one of the most common methods for exosome isolation before the emergence of high specific markers for exosome. Besides, exosomes are cell-derived vesicles that contain distinct bioactive cargos and present in the conditioned media of cultured cells and almost all bodily fluids. Considerable attention must be accorded to the stability and storage strategies necessary to translate the striking preclinical consequences of exosomes into clinical and commercial success as off-the-shelf diagnostic and therapeutic tools. Cryopreservation methods have drawn growing interest in exosome storage. However, controversy exists on whether the freeze-thaw cycles affect the stability of exosomes [141]. Therefore, further preclinical testing should be designed to develop novel preservation strategies tailored for exosomes before clinical applications on a large scale. Additionally, there are controversies regarding defining exosome dosage, i.e., the concentration of exosomal proteins or the number of particles. This optimization is particularly crucial for clinical trials and requires relevant criteria to be proposed by reputable industry associations. Moreover, an appropriate cell source for exosomes based on their intended therapeutic use is essential. At present, the well-studied stem cells are widely used in certain diseases owing to their inherent attributes. The list of additional cell sources that require further characterization and detection continues to grow.
Despite the current challenges, the idea of using exosomes as a diagnostic and therapeutic tool is promising and inspiring. It is highly expected that, with adequate research, the additional exciting applications of exosomes in clinical practice are expected in the near future.
Acknowledgements
This work was supported by Natural Science Foundation of China (No.81771051, 81970912 and 81100735) and was partially supported by the Fundamental Research Funds for the Central Universities of China (no. 2042019kf0120).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942-8
2. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412-20
3. Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11-5
4. Van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-28
5. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17
6. Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170
7. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193-208
8. Lasser C, Alikhani VS, Ekstrom K, Eldh M, Paredes PT, Bossios A. et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9
9. Street JM, Koritzinsky EH, Glispie DM, Yuen PST. Urine exosome isolation and characterization. Methods Mol Biol. 2017;1641:413-23
10. Basu J, Ludlow JW. Exosomes for repair, regeneration and rejuvenation. Expert Opin Biol Ther. 2016;16:489-506
11. Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T. et al. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019;86:1-14
12. Xiao C, Song F, Zheng YL, Lv J, Wang QF, Xu N. Exosomes in head and neck squamous cell carcinoma. Front Oncol. 2019;9:894
13. Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44:2060-4
14. Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809-20
15. Ghallab NA. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases: review of the current evidence. Arch Oral Biol. 2018;87:115-24
16. Tobon-Arroyave SI, Celis-Mejia N, Cordoba-Hidalgo MP, Isaza-Guzman DM. Decreased salivary concentration of CD9 and CD81 exosome-related tetraspanins may be associated with the periodontal clinical status. J Clin Periodontol. 2019;46:470-80
17. Huang X, Hu X, Zhao M, Zhang Q. Analysis of salivary exosomal proteins in young adults with severe periodontitis. Oral Dis. 2020;26:173-81
18. Zhang J, Wang CM, Zhang P, Wang X, Chen J, Yang J. et al. Expression of programmed death 1 ligand 1 on periodontal tissue cells as a possible protective feedback mechanism against periodontal tissue destruction. Mol Med Rep. 2016;13:2423-30
19. Xie F, Xu M, Lu J, Mao L, Wang S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Molecular Cancer. 2019;18:146
20. Yu J, Lin Y, Xiong X, Li K, Yao Z, Dong H. et al. Detection of exosomal PD-L1 RNA in saliva of patients with periodontitis. Front Genet. 2019;10:202
21. Han P, Bartold PM, Salomon C, Ivanovski S. Salivary small extracellular vesicles associated mirnas in periodontal status-a pilot study. Int J Mol Sci. 2020;21:2809
22. Chaparro Padilla A, Weber Aracena L, Realini Fuentes O, Albers Busquetts D, Hernandez Rios M, Ramirez Lobos V. et al. Molecular signatures of extracellular vesicles in oral fluids of periodontitis patients. Oral Dis. 2020;00:1-8
23. Mutafchieva MZ, Draganova-Filipova MN, Zagorchev PI, Tomov GT. Oral lichen planus - known and unknown: a review. Folia Med (Plovdiv). 2018;60:528-35
24. Warnakulasuriya S. Clinical features and presentation of oral potentially malignant disorders. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:582-90
25. Peng Q, Zhang J, Zhou G. Circulating exosomes regulate T-cell-mediated inflammatory response in oral lichen planus. J Oral Pathol Med. 2019;48:143-50
26. Byun JS, Hong SH, Choi JK, Jung JK, Lee HJ. Diagnostic profiling of salivary exosomal microRNAs in oral lichen planus patients. Oral Dis. 2015;21:987-93
27. Peng Q, Zhang J, Zhou G. Differentially circulating exosomal microRNAs expression profiling in oral lichen planus. Am J Transl Res. 2018;10:2848-58
28. Thomson PJ. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J Oral Pathol Med. 2018;47:803-7
29. Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531-48
30. Languino LR, Singh A, Prisco M, Inman GJ, Luginbuhl A, Curry JM. et al. Exosome-mediated transfer from the tumor microenvironment increases TGFβ signaling in squamous cell carcinoma. Am J Transl Res. 2016;8:2432-7
31. Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Salo T, Vered M. Morphological and molecular features of oral fluid-derived exosomes: oral cancer patients versus healthy individuals. J Cancer Res Clin Oncol. 2016;142:101-10
32. Sharma S, Gillespie BM, Palanisamy V, Gimzewski JK. Quantitative nanostructural and single-molecule force spectroscopy biomolecular analysis of human-saliva-derived exosomes. Langmuir. 2011;27:14394-400
33. Zlotogorski-Hurvitz A, Dekel BZ, Malonek D, Yahalom R, Vered M. FTIR-based spectrum of salivary exosomes coupled with computational-aided discriminating analysis in the diagnosis of oral cancer. J Cancer Res Clin Oncol. 2019;145:685-94
34. Rodriguez Zorrilla S, Perez-Sayans M, Fais S, Logozzi M, Gallas Torreira M, Garcia Garcia A. A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers (Basel). 2019 11
35. Li C, Zhou Y, Liu J, Su X, Qin H, Huang S. et al. Potential markers from serum-purified exosomes for detecting oral squamous cell carcinoma metastasis. Cancer Epidemiol Biomarkers Prev. 2019;28:1668-81
36. Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1+ exosomes in plasma of head and neck cancer patients. Clin Cancer Res. 2018;24:896-905
37. Wang SH, Liou GG, Liu SH, Chang JS, Hsiao JR, Yen YC. et al. Laminin γ2-enriched extracellular vesicles of oral squamous cell carcinoma cells enhance in vitro lymphangiogenesis via integrin α3-dependent uptake by lymphatic endothelial cells. Int J Cancer. 2019;144:2795-810
38. Winck FV, Prado Ribeiro AC, Ramos Domingues R, Ling LY, Riano-Pachon DM, Rivera C. et al. Insights into immune responses in oral cancer through proteomic analysis of saliva and salivary extracellular vesicles. Sci Rep. 2015;5:16305
39. Rabinowits G, Bowden M, Flores LM, Verselis S, Vergara V, Jo VY. et al. Comparative analysis of microRNA expression among benign and malignant tongue tissue and plasma of patients with tongue cancer. Front Oncol. 2017;7:191
40. Gai C, Camussi F, Broccoletti R, Gambino A, Cabras M, Molinaro L. et al. Salivary extracellular vesicle-associated miRNAs as potential biomarkers in oral squamous cell carcinoma. BMC Cancer. 2018;18:439
41. He L, Ping F, Fan Z, Zhang C, Deng M, Cheng B. et al. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed Pharmacother. 2020;121:109553
42. Chen X, Wu H, Wei W. Advances in the diagnosis and treatment of sjogren's syndrome. Clin Rheumatol. 2018;37:1743-9
43. Baldini C, Ferro F, Elefante E, Bombardieri S. Biomarkers for sjogren's syndrome. Biomark Med. 2018;12:275-86
44. Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG. et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34-8
45. Aqrawi LA, Galtung HK, Vestad B, Ovstebo R, Thiede B, Rusthen S. et al. Identification of potential saliva and tear biomarkers in primary sjogren's syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res Ther. 2017;19:14
46. Zheng X, Chen F, Zhang Q, Liu Y, You P, Sun S. et al. Salivary exosomal PSMA7: a promising biomarker of inflammatory bowel disease. Protein Cell. 2017;8:686-95
47. Machida T, Tomofuji T, Ekuni D, Maruyama T, Yoneda T, Kawabata Y. et al. MicroRNAs in salivary exosome as potential biomarkers of aging. Int J Mol Sci. 2015;16:21294-309
48. Lau C, Kim Y, Chia D, Spielmann N, Eibl G, Elashoff D. et al. Role of pancreatic cancer-derived exosomes in salivary biomarker development. J Biol Chem. 2013;288:26888-97
49. Machida T, Tomofuji T, Maruyama T, Yoneda T, Ekuni D, Azuma T. et al. MiR-1246 and miR-4644 in salivary exosome as potential biomarkers for pancreatobiliary tract cancer. Oncol Rep. 2016;36:2375-81
50. Sun Y, Xia Z, Shang Z, Sun K, Niu X, Qian L. et al. Facile preparation of salivary extracellular vesicles for cancer proteomics. Sci Rep. 2016;6:24669
51. Sun Y, Huo C, Qiao Z, Shang Z, Uzzaman A, Liu S. et al. Comparative proteomic analysis of exosomes and microvesicles in human saliva for lung cancer. J Proteome Res. 2018;17:1101-7
52. Kim J, Shin H, Park J. RNA in salivary extracellular vesicles as a possible tool for systemic disease diagnosis. J Dent Res. 2017;96:938-44
53. Monteiro LJ, Varas-Godoy M, Monckeberg M, Realini O, Hernandez M, Rice G. et al. Oral extracellular vesicles in early pregnancy can identify patients at risk of developing gestational diabetes mellitus. PLoS One. 2019;14:e0218616
54. Liu C, Su C. Design strategies and application progress of therapeutic exosomes. Theranostics. 2019;9:1015-28
55. He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237-55
56. Cooper LF, Ravindran S, Huang CC, Kang M. A role for exosomes in craniofacial tissue engineering and regeneration. Front Physiol. 2019;10:1569
57. Confalonieri D, Schwab A, Walles H, Ehlicke F. Advanced therapy medicinal products: a guide for bone marrow-derived MSCs application in bone and cartilage tissue engineering. Tissue Eng Part B Rev. 2018;24:155-69
58. Tian T, Zhang T, Lin Y, Cai X. Vascularization in craniofacial bone tissue engineering. J Dent Res. 2018;97:969-76
59. Qin Y, Sun R, Wu C, Wang L, Zhang C. Exosome: a novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int J Mol Sci. 2016 17
60. Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X. et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12:836-49
61. Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X. et al. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52:e12669
62. Li W, Liu Y, Zhang P, Tang Y, Zhou M, Jiang W. et al. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces. 2018;10:5240-54
63. Xu J, Wang Y, Hsu CY, Gao Y, Meyers CA, Chang L. et al. Human perivascular stem cell-derived extracellular vesicles mediate bone repair. Elife. 2019 8
64. Zhao P, Xiao L, Peng J, Qian YQ, Huang CC. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur Rev Med Pharmacol Sci. 2018;22:3962-70
65. Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng Part A. 2017;23:1212-20
66. Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu W. et al. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11:38
67. Liang B, Liang JM, Ding JN, Xu J, Xu JG, Chai YM. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res Ther. 2019;10:335
68. Xu F, Xiang Q, Huang J, Chen Q, Yu N, Long X. et al. Exosomal miR-423-5p mediates the proangiogenic activity of human adipose-derived stem cells by targeting Sufu. Stem Cell Res Ther. 2019;10:106
69. Chen B, Cai J, Wei Y, Jiang Z, Desjardins HE, Adams AE. et al. Exosomes are comparable to source adipose stem cells in fat graft retention with up-regulating early inflammation and angiogenesis. Plast Reconstr Surg. 2019;144:816e-27e
70. Xue C, Shen Y, Li X, Li B, Zhao S, Gu J. et al. Exosomes derived from hypoxia-treated human adipose mesenchymal stem cells enhance angiogenesis through the PKA signaling pathway. Stem Cells Dev. 2018;27:456-65
71. Du W, Zhang K, Zhang S, Wang R, Nie Y, Tao H. et al. Enhanced proangiogenic potential of mesenchymal stem cell-derived exosomes stimulated by a nitric oxide releasing polymer. Biomaterials. 2017;133:70-81
72. Komaki M, Numata Y, Morioka C, Honda I, Tooi M, Yokoyama N. et al. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther. 2017;8:219
73. Zhang Z, Shuai Y, Zhou F, Yin J, Hu J, Guo S. et al. PDLSCs regulate angiogenesis of periodontal ligaments via VEGF transferred by exosomes in periodontitis. Int J Med Sci. 2020;17:558-67
74. Cui Y, Luan J, Li H, Zhou X, Han J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016;590:185-92
75. Xu Q, Cui Y, Luan J, Zhou X, Li H, Han J. Exosomes from C2C12 myoblasts enhance osteogenic differentiation of MC3T3-E1 pre-osteoblasts by delivering miR-27a-3p. Biochem Biophys Res Commun. 2018;498:32-7
76. Zhao M, Dai W, Wang H, Xue C, Feng J, He Y. et al. Periodontal ligament fibroblasts regulate osteoblasts by exosome secretion induced by inflammatory stimuli. Arch Oral Biol. 2019;105:27-34
77. Ekstrom K, Omar O, Graneli C, Wang X, Vazirisani F, Thomsen P. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS One. 2013;8:e75227
78. Wei F, Li M, Crawford R, Zhou Y, Xiao Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019;86:480-92
79. Nie Y, Sato Y, Garner RT, Kargl C, Wang C, Kuang S. et al. Skeletal muscle-derived exosomes regulate endothelial cell functions via reactive oxygen species-activated nuclear factor-κB signalling. Exp Physiol. 2019;104:1262-73
80. Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288:34343-51
81. Zhang J, Liu X, Li H, Chen C, Hu B, Niu X. et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7:136
82. Wu J, Chen L, Wang R, Song Z, Shen Z, Zhao Y. et al. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. ACS Biomater Sci Eng. 2019;5:3561-71
83. Watanabe J, Sakai K, Urata Y, Toyama N, Nakamichi E, Hibi H. Extracellular vesicles of stem cells to prevent BRONJ. J Dent Res. 2020;99:552-60
84. Lee YH, Park HK, Auh QS, Nah H, Lee JS, Moon HJ. et al. Emerging potential of exosomes in regenerative medicine for temporomandibular joint osteoarthritis. Int J Mol Sci. 2020 21
85. Won Lee G, Thangavelu M, Joung Choi M, Yeong Shin E, Sol Kim H, Seon Baek J. et al. Exosome mediated transfer of miRNA-140 promotes enhanced chondrogenic differentiation of bone marrow stem cells for enhanced cartilage repair and regeneration. J Cell Biochem. 2020;121:3642-52
86. Luo P, Jiang C, Ji P, Wang M, Xu J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res Ther. 2019;10:216
87. Zhou QF, Cai YZ, Lin XJ. The dual character of exosomes in osteoarthritis: antagonists and therapeutic agents. Acta Biomater. 2020;105:15-25
88. Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35-47
89. Liu J, Ruan J, Weir MD, Ren K, Schneider A, Wang P. et al. Periodontal bone-ligament-cementum regeneration via scaffolds and stem cells. Cells. 2019;8:537
90. Zheng Y, Dong C, Yang J, Jin Y, Zheng W, Zhou Q. et al. Exosomal microRNA-155-5p from PDLSCs regulated Th17/Treg balance by targeting sirtuin-1 in chronic periodontitis. J Cell Physiol. 2019;234:20662-74
91. Wang R, Ji Q, Meng C, Liu H, Fan C, Lipkind S. et al. Role of gingival mesenchymal stem cell exosomes in macrophage polarization under inflammatory conditions. Int Immunopharmacol. 2020;81:106030
92. Wang M, Li J, Ye Y, He S, Song J. SHED-derived conditioned exosomes enhance the osteogenic differentiation of PDLSCs via Wnt and BMP signaling in vitro. Differentiation. 2020;111:1-11
93. Mohammed E, Khalil E, Sabry D. Effect of adipose-derived stem cells and their exo as adjunctive therapy to nonsurgical periodontal treatment: a histologic and histomorphometric study in rats. Biomolecules. 2018 8
94. Chew JRJ, Chuah SJ, Teo KYW, Zhang S, Lai RC, Fu JH. et al. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019;89:252-64
95. Sui B, Chen C, Kou X, Li B, Xuan K, Shi S. et al. Pulp stem cell-mediated functional pulp regeneration. J Dent Res. 2019;98:27-35
96. Ahmed GM, Abouauf EA, AbuBakr N, Dorfer CE, El-Sayed KF. Tissue engineering approaches for enamel, dentin, and pulp regeneration: an update. Stem Cells Int. 2020;2020:5734539
97. Pivoraite U, Jarmalaviciute A, Tunaitis V, Ramanauskaite G, Vaitkuviene A, Kaseta V. et al. Exosomes from human dental pulp stem cells suppress carrageenan-induced acute inflammation in mice. Inflammation. 2015;38:1933-41
98. Xian X, Gong Q, Li C, Guo B, Jiang H. Exosomes with highly angiogenic potential for possible use in pulp regeneration. J Endod. 2018;44:751-8
99. Jarmalaviciute A, Tunaitis V, Pivoraite U, Venalis A, Pivoriunas A. Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy. 2015;17:932-9
100. Ivica A, Ghayor C, Zehnder M, Valdec S, Weber FE. Pulp-derived exosomes in a fibrin-based regenerative root filling material. J Clin Med. 2020 9
101. Hu X, Zhong Y, Kong Y, Chen Y, Feng J, Zheng J. Lineage-specific exosomes promote the odontogenic differentiation of human dental pulp stem cells (DPSCs) through TGFβ1/smads signaling pathway via transfer of microRNAs. Stem Cell Res Ther. 2019;10:170
102. Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A. et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. 2014;513:551-4
103. Webber C, Zochodne D. The nerve regenerative microenvironment: Early behavior and partnership of axons and Schwann cells. Exp Neurol. 2010;223:51-9
104. Li J, Ju Y, Liu S, Fu Y, Zhao S. Exosomes derived from lipopolysaccharide-preconditioned human dental pulp stem cells regulate Schwann cell migration and differentiation. Connect Tissue Res. 2019:1-10
105. Huang CC, Narayanan R, Alapati S, Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103-15
106. Wang M, Wang C, Chen M, Xi Y, Cheng W, Mao C. et al. Efficient angiogenesis-based diabetic wound healing/skin reconstruction through bioactive antibacterial adhesive ultraviolet shielding nanodressing with exosome release. ACS Nano. 2019;13:10279-93
107. Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W. et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. 2019;9:65-76
108. Shafei S, Khanmohammadi M, Heidari R, Ghanbari H, Taghdiri Nooshabadi V, Farzamfar S. et al. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: an in vivo study. J Biomed Mater Res A. 2020;108:545-56
109. Shi Q, Qian Z, Liu D, Sun J, Wang X, Liu H. et al. GMSC-derived exosomes combined with a chitosan/silk hydrogel sponge accelerates wound healing in a diabetic rat skin defect model. Front Physiol. 2017;8:904
110. Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi H. et al. Umbilical cord-derived mesenchymal stem cell-derived exosomal microRNAs suppress myofibroblast differentiation by inhibiting the transforming growth factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med. 2016;5:1425-39
111. Zhang W, Bai X, Zhao B, Li Y, Zhang Y, Li Z. et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp Cell Res. 2018;370:333-42
112. Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13:555-68
113. He X, Dong Z, Cao Y, Wang H, Liu S, Liao L. et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019;2019:7132708
114. Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J. Engineered human adipose stem-cell-derived exosomes loaded with miR-21-5p to promote diabetic cutaneous wound healing. Mol Pharm. 2020;17:1723-33
115. Shi R, Jin Y, Hu W, Lian W, Cao C, Han S. et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318:C848-C56
116. Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81-96
117. Kim H, Wang SY, Kwak G, Yang Y, Kwon IC, Kim SH. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing. Adv Sci (Weinh). 2019;6:1900513
118. Sjoqvist S, Ishikawa T, Shimura D, Kasai Y, Imafuku A, Bou-Ghannam S. et al. Exosomes derived from clinical-grade oral mucosal epithelial cell sheets promote wound healing. J Extracell Vesicles. 2019;8:1565264
119. Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J. et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8:169-84
120. Zhang B, Shi Y, Gong A, Pan Z, Shi H, Yang H. et al. HucMSC exosome-delivered 14-3-3ζ orchestrates self-control of the Wnt response via modulation of YAP during cutaneous regeneration. Stem Cells. 2016;34:2485-500
121. Zhang B, Wang M, Gong A, Zhang X, Wu X, Zhu Y. et al. HucMSC-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells. 2015;33:2158-68
122. Hu S, Li Z, Cores J, Huang K, Su T, Dinh PU. et al. Needle-free injection of exosomes derived from human dermal fibroblast spheroids ameliorates skin photoaging. ACS Nano. 2019;13:11273-82
123. Hakkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontol 2000. 2000;24:127-52
124. Kou X, Xu X, Chen C, Sanmillan ML, Cai T, Zhou Y. et al. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci Transl Med. 2018 10
125. Kareemaghay S, Tavassoli M. Clinical immunotherapeutic approaches for the treatment of head and neck cancer. Int J Oral Maxillofac Surg. 2019;48:419-36
126. Vigneswaran N, Williams MD. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac Surg Clin North Am. 2014;26:123-41
127. Li L, Li C, Wang S, Wang Z, Jiang J, Wang W. et al. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Res. 2016;76:1770-80
128. Higaki M, Shintani T, Hamada A, Rosli SNZ, Okamoto T. Eldecalcitol (ED-71)-induced exosomal miR-6887-5p suppresses squamous cell carcinoma cell growth by targeting heparin-binding protein 17/fibroblast growth factor-binding protein-1 (HBp17/FGFBP-1). In Vitro Cell Dev Biol Anim. 2020;56:222-33
129. Xie C, Du LY, Guo F, Li X, Cheng B. Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Mol Cell Biochem. 2019;458:11-26
130. Li L, Cao B, Liang X, Lu S, Luo H, Wang Z. et al. Microenvironmental oxygen pressure orchestrates an anti- and pro-tumoral γδ T cell equilibrium via tumor-derived exosomes. Oncogene. 2019;38:2830-43
131. Rosenberger L, Ezquer M, Lillo-Vera F, Pedraza PL, Ortuzar MI, Gonzalez PL. et al. Stem cell exosomes inhibit angiogenesis and tumor growth of oral squamous cell carcinoma. Sci Rep. 2019;9:663
132. Li W, Han Y, Zhao Z, Ji X, Wang X, Jin J. et al. Oral mucosal mesenchymal stem cell-derived exosomes: a potential therapeutic target in oral premalignant lesions. Int J Oncol. 2019;54:1567-78
133. Wang L, Yin P, Wang J, Wang Y, Sun Z, Zhou Y. et al. Delivery of mesenchymal stem cells-derived extracellular vesicles with enriched miR-185 inhibits progression of OPMD. Artif Cells Nanomed Biotechnol. 2019;47:2481-91
134. Nadeau C, Kerr AR. Evaluation and management of oral potentially malignant disorders. Dent Clin North Am. 2018;62:1-27
135. Li Y, Yang YY, Ren JL, Xu F, Chen FM, Li A. Exosomes secreted by stem cells from human exfoliated deciduous teeth contribute to functional recovery after traumatic brain injury by shifting microglia M1/M2 polarization in rats. Stem Cell Res Ther. 2017;8:198
136. Zhang Y, Shi S, Xu Q, Zhang Q, Shanti RM, Le AD. SIS-ECM laden with GMSC-derived exosomes promote taste bud regeneration. J Dent Res. 2019;98:225-33
137. Antimisiaris SG, Mourtas S, Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics. 2018 10
138. Nasiri Kenari A, Kastaniegaard K, Greening DW, Shambrook M, Stensballe A, Cheng L. et al. Proteomic and post-translational modification profiling of exosome-mimetic nanovesicles compared to exosomes. Proteomics. 2019;19:e1800161
139. Zha Y, Lin T, Li Y, Zhang X, Wang Z, Li Z. et al. Exosome-mimetics as an engineered gene-activated matrix induces in-situ vascularized osteogenesis. Biomaterials. 2020;247:119985
140. Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L. et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684-707
141. Kusuma GD, Barabadi M, Tan JL, Morton DAV, Frith JE, Lim R. To protect and to preserve: novel preservation strategies for extracellular vesicles. Front Pharmacol. 2018;9:1199
Author contact
![]() Corresponding authors: E-mail: zhiliedu.cn; Tel/Fax: 86-027-87686216 (Zhi Li) or lizubingedu.cn; Tel/Fax: 86-027-87686217 (Zubing Li)
Corresponding authors: E-mail: zhiliedu.cn; Tel/Fax: 86-027-87686216 (Zhi Li) or lizubingedu.cn; Tel/Fax: 86-027-87686217 (Zubing Li)
 Global reach, higher impact
Global reach, higher impact