13.3
Impact Factor
Theranostics 2020; 10(18):8446-8467. doi:10.7150/thno.31711 This issue Cite
Research Paper
Lentivirus-mediated IL-10-expressing Bone Marrow Mesenchymal Stem Cells promote corneal allograft survival via upregulating lncRNA 003946 in a rat model of corneal allograft rejection
Tianjin Key Laboratory of Retinal Functions and Diseases, Tianjin International Joint Research and Development Centre of Ophthalmology and Vision Science, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin, 300384, China.
#These authors contributed equally to this work.
Abstract
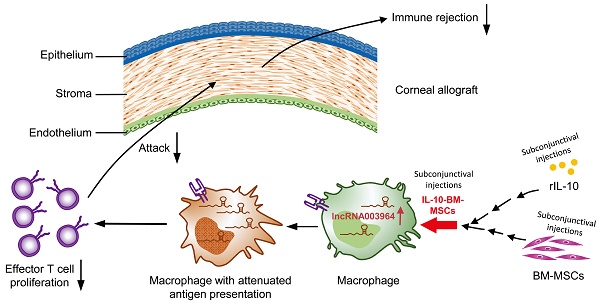
Rationale: Corneal transplantation is an effective treatment to corneal blindness. However, the immune rejection imperils corneal allograft survival. An interventional modality is urgently needed to inhibit immune rejection and promote allograft survival. In our previous study, subconjunctival injections of bone marrow-derived mesenchymal stem cells (BM-MSCs) into a rat model of corneal allograft rejection extended allograft survival for 2 d. In this study, we sought to generate IL-10-overexpressing BM-MSCs, aiming to boost the survival-promoting effects of BM-MSCs on corneal allografts and explore the molecular and cellular mechanisms underlying augmented protection.
Methods: A population of IL-10-overexpressing BM-MSCs (designated as IL-10-BM-MSCs) were generated by lentivirus transduction and FACS purification. The self-renewal, multi-differentiation, and immunoinhibitory capabilities of IL-10-BM-MSCs were examined by conventional assays. The IL-10-BM-MSCs were subconjunctivally injected into the model of corneal allograft rejection, and the allografts were monitored on a daily basis. The expression profiling of long noncoding RNA (lncRNA) in the allografts was revealed by RNA sequencing and verified by quantitative real-time PCR. The infiltrating immune cell type predominantly upregulating the lncRNA expression was identified by RNAscope in situ hybridization. The function of the upregulated lncRNA was proved by loss- and gain-of-function experiments both in vivo and in vitro.
Results: The IL-10-BM-MSCs possessed an enhanced immunoinhibitory capability and unabated self-renewal and multi-differentiation potentials as compared to plain BM-MSCs. The subconjunctivally injected IL-10-BM-MSCs reduced immune cell infiltration and doubled allograft survival time (20 d) as compared to IL-10 protein or plain BM-MSCs in the corneal allograft rejection model. Further, IL-10-BM-MSCs significantly upregulated lncRNA 003946 expression in CD68+ macrophages infiltrating corneal allografts. Silencing and overexpressing lncRNA 003946 in macrophage cultures abolished and mimicked the IL-10-BM-MSCs' suppressing effects on the macrophages' antigen presentation, respectively. In parallel, knocking down and overexpressing the lncRNA in vivo abrogated and simulated the survival-promoting effects of IL-10-BM-MSCs on corneal allografts, respectively.
Conclusion: The remarkable protective effects of IL-10-BM-MSCs support further developing them into an effective interventional modality against corneal allograft rejection. IL-10-BM-MSCs promote corneal allograft survival mainly through upregulating a novel lncRNA expression in graft-infiltrating CD68+ macrophages. LncRNA, for the first time, is integrated into an IL-10-BM-MSC-driven immunomodulatory axis against the immune rejection to corneal allograft.
Keywords: corneal transplantation, allograft rejection, bone marrow-derived mesenchymal stem cell, Interleukin-10, long noncoding RNA
 Global reach, higher impact
Global reach, higher impact