13.3
Impact Factor
Theranostics 2020; 10(16):7260-7272. doi:10.7150/thno.46332 This issue Cite
Research Paper
Bioenergetic Crosstalk between Mesenchymal Stem Cells and various Ocular Cells through the intercellular trafficking of Mitochondria
1. Laboratory for Stem Cell and Retinal Regeneration, Institute of Stem Cell Research, Division of Ophthalmic Genetics, the Eye Hospital, Wenzhou Medical University; National Center for International Research in Regenerative Medicine and Neurogenetics, Wenzhou, 325027 China.
2. State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, 100101 China.
3. Beijing Institute of Ophthalmology, Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University, Beijing Ophthalmology and Visual Science Key Laboratory, Beijing, 100730 China.
Abstract
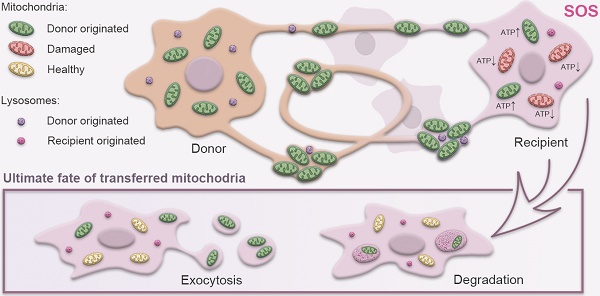
Rationale: Mitochondrial disorders preferentially affect tissues with high energy requirements, such as the retina and corneal endothelium, in human eyes. Mesenchymal stem cell (MSC)-based treatment has been demonstrated to be beneficial for ocular degeneration. However, aside from neuroprotective paracrine actions, the mechanisms underlying the beneficial effect of MSCs on retinal and corneal tissues are largely unknown. In this study, we investigated the fate and associated characteristics of mitochondria subjected to intercellular transfer from MSCs to ocular cells.
Methods: MSCs were cocultured with corneal endothelial cells (CECs), 661W cells (a photoreceptor cell line) and ARPE-19 cells (a retinal pigment epithelium cell line). Immunofluorescence, fluorescence activated cell sorting and confocal microscopy imaging were employed to investigate the traits of intercellular mitochondrial transfer and the fate of transferred mitochondria. The oxygen consumption rate of recipient cells was measured to investigate the effect of intercellular mitochondrial transfer. Transcriptome analysis was performed to investigate the expression of metabolic genes in recipient cells with donated mitochondria.
Results: Mitochondrial transport is a ubiquitous intercellular mechanism between MSCs and various ocular cells, including the corneal endothelium, retinal pigmented epithelium, and photoreceptors. Additionally, our results indicate that the donation process depends on F-actin-based tunneling nanotubes. Rotenone-pretreated cells that received mitochondria from MSCs displayed increased aerobic capacity and upregulation of mitochondrial genes. Furthermore, living imaging determined the ultimate fate of transferred mitochondria through either degradation by lysosomes or exocytosis as extracellular vesicles.
Conclusions: For the first time, we determined the characteristics and fate of mitochondria undergoing intercellular transfer from MSCs to various ocular cells through F-actin-based tunneling nanotubes, helping to characterize MSC-based treatment for ocular tissue regeneration.
Keywords: mitochondrial transfer, mesenchymal stem cell, corneal endothelium, photoreceptor, retinal pigment epithelium
 Global reach, higher impact
Global reach, higher impact