13.3
Impact Factor
Theranostics 2020; 10(15):6898-6914. doi:10.7150/thno.42347 This issue Cite
Research Paper
Homozygous MESP1 knock-in reporter hESCs facilitated cardiovascular cell differentiation and myocardial infarction repair
Center for Stem Cell Biology and Regenerative Medicine, School of Medicine, Tsinghua University, Beijing, 100084, China.
#These authors contributed equally to this work.
Abstract
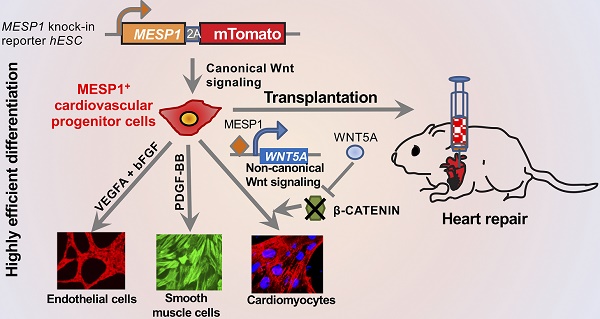
Different populations of cardiovascular progenitor cells have been shown to possess varying differentiation potentials. They have also been used to facilitate heart repair. However, sensitive reporter cell lines that mark the human cardiovascular progenitors are in short supply.
Methods: MESP1 marks the earliest population of cardiovascular progenitor cells during embryo development. Here, we generated a homozygous MESP1 knock-in reporter hESC line where mTomato gene joined to the MESP1 coding region via a 2A peptide, in which both MESP1 alleles were preserved. We performed transcriptome and functional analysis of human MESP1+ cardiovascular progenitor cells and tested their therapeutic potential using a rat model of myocardial infarction.
Results: MESP1-mTomato knock-in reporter faithfully recapitulated the endogenous level of MESP1. Transcriptome analysis revealed that MESP1+ cells highly expressed early cardiovascular genes and heart development genes. The activation of MESP1 relied on the strength of canonical Wnt signaling, peak MESP1-mTomato fluorescence correlated with the window of canonical Wnt inhibition during in vitro differentiation. We further showed that MESP1 bound to the promoter of the WNT5A gene and the up-regulation of WNT5A expression suppressed canonical Wnt/β-CATENIN signaling. Moreover, induced MESP1 expression could substitute the canonical Wnt inhibition step and promote robust cardiomyocyte formation. We used a configurable, chemically defined, tri-lineage differentiation system to obtain cardiomyocytes, endothelial cells, and smooth muscle cells from MESP1+ cells at high efficiency. Finally, we showed that the engraftment of MESP1+ cells repaired rat myocardial infarction model.
Conclusions: MESP1-mTomato reporter cells offered a useful platform to study cardiovascular differentiation from human pluripotent stem cells and explore their therapeutic potential in regenerative medicine.
Keywords: Human embryonic stem cells, MESP1, Cardiomyocyte differentiation, Myocardial infarction, Transplantation
 Global reach, higher impact
Global reach, higher impact